Abstract
Three visual event-related potential components to the second of two sequentially presented words that rhymed or not discriminated children who improved (AR) from those who failed following (IR) reading intervention. Right hemisphere P100 amplitudes discriminated Typically Developing (TD) children from AR children but IR from AR children over left hemisphere sites. N200 amplitudes across hemispheres discriminated TD from IR children and AR from IR children. P300 hemisphere differences differentiated TD from AR and IR children. P300 amplitudes discriminated rhyming from non-rhyming words across children. Results extend prior work asserting that normalization and compensatory mechanisms are active during successful interventions.
The reauthorization of the Individuals with Disabilities Education Act (IDEA; 2004) permitted schools to identify children with learning disabilities (LD) based, in part, on a child’s response to intervention (RTI). As part of the RTI framework (Fuchs & Fuchs, 2006; Fletcher, Lyon, Fuchs, & Barnes, 2007; Justice, 2006), children who are at-risk for reading disabilities (RD) are identified, and those who do not attain grade-level benchmarks with classroom reading instruction alone are placed into progressively more intensive instructional settings, such as small group or one-on-one interventions. To date, much of the research has looked at how children improve in reading abilities through focused, layered reading interventions (Compton, Fuchs, Fuchs, & Bryant, 2006; Fletcher, Lyon, Fuchs, & Barnes, 2007; Mathes et al., 2005; Torgesen et al., 2001; Vellutino, Scanlon, Small, & Fanuele, 2006). Through these interventions, some children respond adequately and improve their reading to grade-level benchmarks, while others do not and continue to struggle. Documentation of a child’s response to generally effective and relatively intensive intervention is considered in the determination of whether the child has a learning disability.
There are many studies published on the cognitive and behavioral correlates of RTI including several extensive reviews and meta-analyses (Al Otaiba & Fuchs; 2002; Nelson, Benner, & Gonzalez, 2003; Vellutino, Scanlon, Zhang, & Schatschneider, 2008). However, fewer studies examined the neural correlates of instructional responses. Of particular interest in these studies are the brain areas thought to be related to reading which are composed of three systems: (1) a dorsal system composed of the temporo-parietal area (TMP), responsible for slow-processing of words; (2) a ventral system composed of the occipito-temporal area (Oc-T), responsible for fast, automated word recognition or pattern matching; and (3) an anterior system, composed of the inferior frontal gyrus (IFG), which is responsible for phonological recoding during reading; for a review see Pugh et al. (2001, 2008). Among these few studies are a series of studies conducted by Simos and colleagues (2002, 2005, 2006, 2007) using magnetoencephalography (MEG), a measure of the magnetic fields corresponding to the electrical activity of the brain. In these studies, children with and without dyslexia were engaged in different tasks, including pseudo-word reading, letter-sound identification, and real-word reading, during MEG recording; pronounced differences between typically developing (TD) and RD children are reported across studies. Children with RD showed less activation in the left temporo-parietal (TMP) areas compared to TD children and significantly more activation in right TMP region. The TMP includes areas of the posterior Superior Temporal Gyrus (STGp), the middle temporal gyrus (MTGp), the angular (ANG) and supramarginal gyri (SMG). In addition, a laterality effect was found, with TD children showing greater activation in the left hemisphere compared to the right hemisphere, while children with RD did not show this lateralization. This differential, hemispheric pattern of responsiveness of children with RD compared to TD is a well-established finding.
Simos et al. also identified neural changes in children who showed improvement in reading skills through participation in reading intervention and showed changes in brain activation that have been characterized as “normalization” of activation. Specifically, children (7 to 17 years of age) in Simos et al. (2002) who were previously poor readers showed normalization of activation in generating left temporo-parietal brain activation patterns at the completion of intervention that were similar to those of the TD comparison group. The subsequent studies in the series, Simos et al. (2005: children aged 5.6 to 7.2), Simos et al. (2006: children aged 8.6), and Simos et al. (2007: children aged 7–9.8), report changes in activation in three of the major cortical regions frequently found to be involved in reading: (1) ventral pathway (Oc-T); 2) dorsal pathway (TMP, Wernicke’s area); and (3) left frontal (Broca’s area, IFG, insular cortex). These areas are believed to be involved in visual-orthographic recognition, phonological decoding, and phonological and articulation tasks reflecting processes involved in word reading (Perfetti & Bolger, 2004; Pugh et al., 2001). In Simos et al. (2005), activation was also found in the right hemisphere after intervention in the previously poor reading children that remained unchanged and that may reflect compensatory processing in these children.
Consistent with research using MEG, research using functional magnetic resonance imaging (fMRI) has shown evidence of normalization of activation in poor readers who complete a reading intervention. In these fMRI studies, Temple et al. (2003: children aged 8–12), Aylward et al (2003: children aged 10–12.5), Shaywitz et al. (2004: children aged 6.1–9.4), Meyler, Keller, Cherkassky, Gabrieli, and Just (2008: children aged 9–10) report normalizing changes following intervention in select brain regions (Left IFG, superior and inferior parietal, angular gyrus, and left superior temporal sulcus) such that children with RD no longer differed from TD children. There were additional brain areas that remained active after interventions that were not active in TD children (putamen, right insula, right IFG/MFG/SFG, Right STG), which were classified as compensatory areas. These findings, like those of Simos et al. (2005), support the notion that both normalization and compensatory changes occur in the brains of poor readers following intervention.
NEURAL CORRELATES OF ADEQUATE AND INADEQUATE RESPONSE
While previous investigators have identified changes in the neural correlates of reading following a reading intervention, few imaging studies have focused on what differentiates Inadequate Responders (IR) from Adequate Responders (AR) and Typically Developing (TD) children. Simos et al. (2005, 2007) presented case examples showing little change in the activation patterns of IR following intervention based on MEG results. Odegard, Ring, Smith, Biggan, and Black (2008) reported differences between AR and IR children 10–14 years of age following a reading intervention using fMRI and a phoneme–grapheme mapping task. After intervention, IR children showed less activation in the left inferior parietal lobe than TD children, although differences in this region between AR and IR were not reliable. Additional differences were found in the right inferior frontal gyrus, where AR showed more activation than both IR and TD children. Davis et al. (2011) also identified differences between AR and IR and TD children (N = 16, 7.5 years) using fMRI and a letter-sound matching task. Differences among all three groups were found in the left superior temporal gyrus, while differences between the TD children and AR were found in the left angular gyrus. Farris et al. (2011) found group differences (N = 15, aged 10–14 years) using active-state functional connectivity. They reported that AR children, TD children, and IR children differed in the right inferior frontal lobe connectivity (triangularis and precentral gyrus). Connectivity of these areas correlated with phonological decoding and word reading performance.
Together, these studies all point to differences between AR and IR children in both hemispheres, with left areas including language centers (left IPL, Left STG), previously found to differentiate TD children from RD children. These findings indicate that AR children are differentiating themselves from IR children in these regions by resembling the TD children. Differences in the right hemisphere areas (Right IFG) are found in MEG studies to differentiate TD children from RD children and in fMRI studies as a compensatory area active in AR children.
To our knowledge, there have been no published studies of the neural correlates of response to reading intervention utilizing event-related potentials (ERPs). This is a curious omission because ERPs have a long history of use in identifying and differentiating children with RD (including dyslexia) from TD children. Examples of these studies using letter, word and/or rhyming tasks with children are summarized in Table 1.
TABLE 1.
Comparison of ERP Studies Investigating Differences Between Children With Dyslexia, Reading Disabilities, and TD Children
| Author | Year | Paradigm | Age | Sample Size | LD/RD Discrim | ERP Peakss |
|---|---|---|---|---|---|---|
| Ackerman, Dykman, & Oglesby | 1994 | Rhyming Tasks | 7.5–12 years | 119 | WISC–R and WRAT–R | N450, P500, Negative slow wave |
| Bergmann, Hutzler, Klimesch, & Wimmer | 2005 | Letter & Word Reading | 13–14 years | 40 (20 RD) | Sentence Reading Task | N220, P310, N400 |
| Bonte & Blomert | 2004 | Rhyming Tasks | 7.5–9.5 years | 23 (12 RD) | Assessment at Regional Institute of Dyslexia | N150, N300 |
| Dainer et al. | 1981 | Letter & Word Identification | 11.1–15.3 | 38 (19LD) | Psychoeducational battery, 1.4 years below grade norms on reading or math | Late Positive Component |
| Holcomb, Ackerman, & Dykman | 1985 | Letter & Word Identification | 8.0–12 years | 93 (24 TD) | UAMS Child Study Center evaluation | P300 |
| Lovrich, Cheng, & Velting | 2003 | Rhyming Tasks | 11.1–13.6 years | 12 | WJ Word Attack or Passage Comp at least 1 SD below | N270, P450 |
| Molfese et al. | 2006 | Letter & Word Identification | 9–12 years | 27 | Word Recognition and Reading Skill | N200, P300 |
| Symann-Louett, Gascon, Matsumiya, & Lombroso | 1977 | Letter & Word | Average age 12.3 years | 22 (12 TD) | Two grade levels below on reading | # peaks between 0–200 msec and # of peaks between 200–350 msec |
Note. RD = reading disabilities; TD = typically developing; UAMS = University of Arkansas Medical Sciences; WISC–R = Wechsler Intelligence Scale for Children–Revised; WJ = Woodcock & Johnson; WRAT–R = Wide Range Achievement Test–Revised.
The present study sought to identify differences in brain processing of AR and IR children who completed a reading intervention program compared to TD children using ERPs. Following previous work in ERP (Ackerman, Dykman, & Olgesby, 1994; Bonte & Blomert, 2004; Lovrich, Cheng, & Velting, 2003) and MEG (Simos et al., 2002, 2005), we employed a word-rhyming task to specifically engage areas of the brain involved in phonological processing (e.g., IFG, TMP, and Oc-T; Frost et al., 2009), a core cognitive deficit in struggling readers as well as a cognitive correlate of intervention response (Fletcher et al., 2011). Using this task and examining data from electrodes over the tempero-parietal regions of each hemisphere, it was expected that differences between AR, IR and TD children would be detectable.
Based on previously published work (Bonte & Blomert, 2004; Lovrich et al, 2003; Molfese et al., 2006; Simos et al. 2002, 2005, 2006, 2007) linking rhyming tasks with developmental studies of RD, we targeted three peaks a priori (P100, N200, and P300). For these peaks, we hypothesized: (1) Each peak (P100, N200, P300) would differentiate the three groups of children (TD, AR, IR) by a group × hemisphere interaction. Based on previous findings from ERP (Bonte and Blomert, 2004; Molfese et al., 2006), MEG (Simos et al. 2002, 2005, 2006, 2007), and fMRI (Aylward et al., 2003; Meyler et al., 2008; Shaywitz et al., 2004; Temple et al., 2003), we expected that TD and AR would be differentiated from IR children in the left hemisphere, due in part to the normalization of the brain following successful reading intervention. Additionally, we believed that the TD and AR groups would differ in the right hemisphere, possibly due to compensatory mechanisms. (2) We also hypothesized that children in the TD and AR groups would show a hemisphere effect (LH > RH) in the normalization scheme displayed by Simos et al. (2002, 2005, 2006, 2007) and by Molfese et al. (2006); we did not anticipate a laterality effect for children in the IR group. (3) Finally, we hypothesized that a stimulus condition × group interaction would be present in the P300, where children in the TD and AR groups would generate larger evoked responses to non-rhyming trials compared to rhyming trials, similar to Bonte and Blomert (2004). The functional significance is that all children regardless of group will show condition effects due to priming, which signifies that the children can read the words but read them differently.
METHODS
Participants
A subset of children participating in an ongoing reading intervention study was recruited to participate in this study (Denton, Cirino, et al., 2011; Denton, Tolar, et al., under review). Prior to testing, the parents provided consent for their children to participate in this study and children gave assent to participate. Children who were struggling readers had been classified as IR to enhanced classroom reading instruction and participated in a small-group supplemental reading intervention in first grade (Denton, Cirino, et al., 2011). At the end of first grade, children were classified as IR if they failed to meet year-end benchmarks on standardized reading achievement and word reading assessments and were enrolled during second grade in a more intensive reading intervention. At the end of second grade, children were categorized as AR or IR based on their performance on standardized reading achievement and word reading assessments. Participants in the current study were either categorized as AR based on performance at or above the 25th percentile on the TOWRE composite score or IR if reading was below the 25th percentile (see Table 2). All participants had IQ scores above the range associated with intellectual disabilities (i.e., >70) as measured by the Kaufman Brief Intelligence Test (K-BIT).
TABLE 2.
Performance of Participants on Reading Assessments: Means (Standard Deviations)
| Group | n | WJ LW | WJ WA | TOWRE SW | TOWRE PDE |
|---|---|---|---|---|---|
| TD | 7 | 112 (9.06) | 109.57 (11.41) | 113.86 (10.71) | 110.14 (11.33) |
| AR | 10 | 99.80 (9.24) | 100.60 (6.42) | 101.44 (7.45) | 102.56 (7.02) |
| IR | 7 | 82 (9.81) | 83 (6.63) | 82 (8.79) | 82.43 (8.73) |
Note. TD = Typically Developing; AR = Adequate Responders; IR = Inadequate Responders; WJ LW = Woodcock & Johnson (1989)—Letter Word Identification; WJ WA = Woodcock Johnson—Word Attack; TOWRE SW = Test of Word Reading Efficiency—Sight Words; TOWRE PDE = Test of Word Reading Efficiency—Phonetic Decoding Efficiency.
The TD children participating in the ERP study were grade-matched to the AR and IR children. They also attended the same schools. Of the 29 participants recruited, data from three TD participants were excluded because their reading score on one of the reading assessments was below the 25th percentile, one AR participant was excluded due to equipment failure, and one IR participant was excluded due to lack of a sufficient number of trials due to eye movements and eye blinks during the experiment. The final sample included 24 second grade children (15 males, 9 females) who were divided into three groups: 7 TD children (5 male, 2 female), 10 AR children (7 male, 3 female), and 7 IR children (3 male, 4 female). While the group sizes are small, they are comparable in size to those of previous investigations of AR and IR (Alyward et al., 2003; Simos et al., 2002, 2005, 2007; Temple et al., 2003), reflecting the difficulty many studies experience in recruiting post intervention participants to imaging and neurophysiology studies.
Equipment
This study utilized an EEG/ERP system from Electrical Geodesics, Inc. (EGI, Inc.); the system included a 300 series 128 high impedance amplifier, several electrode nets (each with 128 electrodes) for different head sizes, and two laptop computers. EEG/ERP was recorded using a Apple Macintosh “MacBook Pro” computer operating EGI’s proprietary Net Station software, version 4.3.1. The visual stimuli were presented using E-PRIME 1.2.1 software (PST, Inc.) on a 15″ monitor (Dell) positioned centered directly in front of the seated participant at a distance of one meter.
Data were collected unfiltered, with sampling rate of 250 Hz. Eye movements and blinks were monitored with built-in electrodes on the electrode net corresponding to EOG channels both vertically above and below the eye as well as channels horizontal and adjacent to each eye. All data were recorded using a Cz reference.
Procedures
Children were tested during the school day in quiet rooms located at their schools, during the months of April and May of second grade. ERP recordings were made using a 128-electrode Geodesic Sensor Net (GSN200; Electrical Geodesics, Inc., Eugene, OR). The net was soaked in warm saline prior to application. The head circumference of each child was measured to ensure proper net fit. Following net application, electrodes were adjusted until electrical impedances levels were below 40,000 ohms. Once electrode impedance and net placement were corrected, the child received instructions and then began the experimental task. These data were collected in concert with another experimental task, not reported here. However, experiment order was counterbalanced across participants. Short breaks were provided between each task. Testing time was approximately 30 minutes. Impedance measurements were tested before and after each task to ensure data quality. Children received a small gift for participation.
Task
This study employed an equal probable design with a rhyming task similar to that used in Simos et al. (2002) and using age-appropriate words selected from the Dolch word list (see Appendix). Words either rhymed or did not rhyme. Visual presentation was used to elicit the ERPs and consisted of white letters comprising a word presented on a black background in order to reduce eyestrain while maximizing contrast (shown in Figure 1). A plus sign (+) used as a fixation point was presented immediately before the first word of a pair appeared on the screen. The plus sign disappeared and reappeared after each word was presented. The first word presentation was followed by the second word of a pair, which was then followed by a question mark prompt. At this point participants had been instructed to respond to the question mark using one of two buttons (one for rhyme, one for non-rhyme; buttons were counterbalanced across subjects). The question mark was displayed until the participant responded. Following the response, both words were presented on the screen, and the participant read the words aloud.
FIGURE 1.
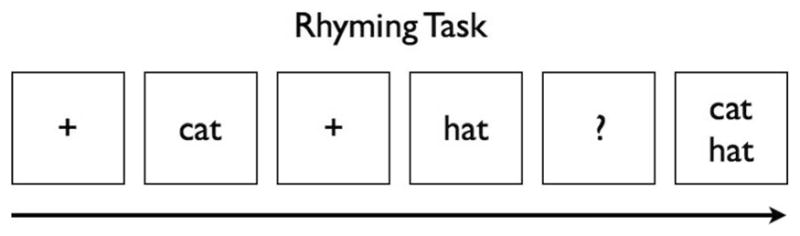
The rhyming task order of stimuli.
Each word appeared for 1,800 msec and each plus sign appeared for between 800 and 1,400 msec. The inter-stimulus interval (ISI) between words varied randomly from 800 to 1,400 msec. The variable length ISI was important for reducing habituation and slow wave baseline shifts associated with contingent negative variation (CNV). A total of 56 trials were presented (28 rhyme, 28 non-rhyme), each trial consisting of two words presented consecutively. All stimuli were presented in a random order. The ERPs were time locked to the onset of the second word in the word pair.
Data Processing
Following data collection, the ERP data were pre-processed using Net Station 4.4.2. The same processing criteria were used for all participants and conditions. Data were first filtered from the original recording using a bandpass of 0.1–30 Hz. The EEG was then segmented into epochs of 1 sec, with 100 msec pre-stimulus and 900 msec post-stimulus time windows. Analyses took place in two steps, the first step analyzed differences between groups for the first word in each presentation. The second step of analysis analyzed differences between groups for the second word in each presentation. In this second step, trials were split into rhyming and non-rhyming trials. All trials were submitted to a semi-automated artifact rejection algorithms that identified eye-blinks and eye movements based on EOG channels that were positioned horizontally and vertically to the eyes. Voltage spikes in the EEG greater than 150 μV in the vertical leads were marked as eye blinks, while the slower changing shifts in the EEG of 130 μV in horizontal leads were marked as eye movements. Epochs containing (1) eye blinks, (2) eye movements, or (3) more than 10 bad electrode channels were discarded from further processing. Epochs containing nine or fewer bad electrode channels were modified so that each bad electrode was interpolated using the spherical-spline technique (Perrin, Pernier, Bertrand, & Echallier, 1989), as recommended in Picton et al. (2000). Following artifact rejection, data were baseline corrected to the first 100 msec pre-stimulus period and then re-referenced to the average reference as recommended by Picton et al. (2000) and Dien (1998). Finally, the individual epochs were averaged together by category (rhyme, or non-rhyme). Each participant had a minimum of 10 trials per average, with most participants contributing substantially more trials per condition (mean = 18.06; standard deviation = 5.29). There were no significant differences in the number of accepted trials between groups, F(2, 21) = 0.172, p = .843.
To address our hypotheses, we used measures of peak amplitude and latency. Amplitude and latency measures were averaged (similar to Molfese et al., 2006) from 10 electrodes. The Sensor Layout (or electrode montage) is in Figure 2. The 10 electrodes are located over the temporo-parietal region of each hemisphere (left: 50, 51, 52, 56, 57, 58, 59, 60, 63, 64; right: 86, 92, 93, 96, 97, 98, 100, 101, 102, 108) for each of three ERP components, the P100 (100–125 msec; henceforth P1), the N200 (190–210 msec; henceforth N2), and the P300 (290–350 msec; henceforth P3). Peaks latency ranges were identified by inspection of a grand average and confirmed using automated peak detection software. Adaptive mean amplitudes were calculated comprising the peak of the waveform within these windows and the average of 2 data point on either side of the peak for five data points in all. All data points were input to SAS 9.3 for Windows for all further analyses (SAS Institute, Inc.).
FIGURE 2.
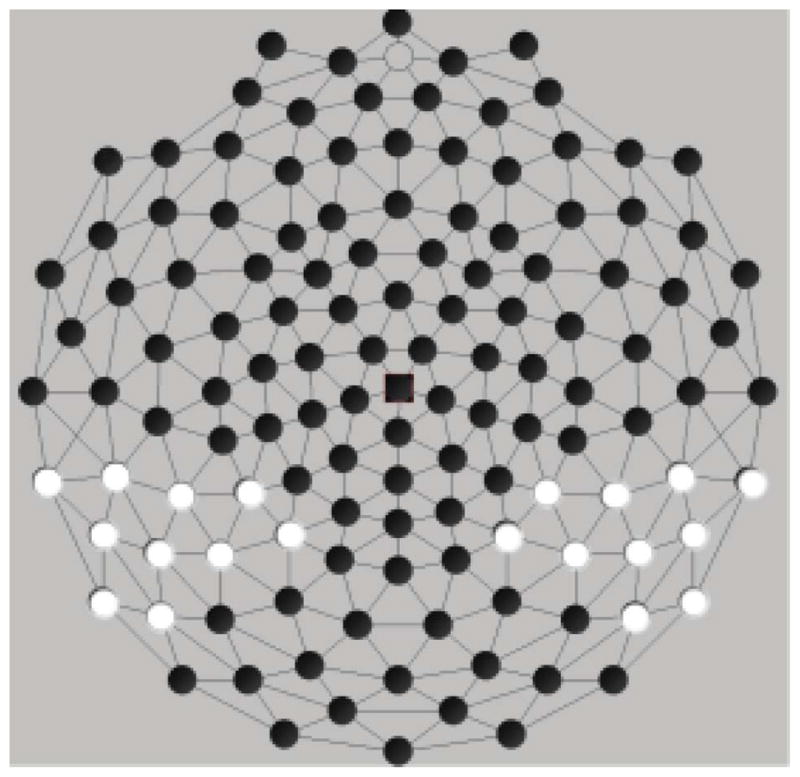
Sensor layout—Peak amplitude/latency analysis.
RESULTS
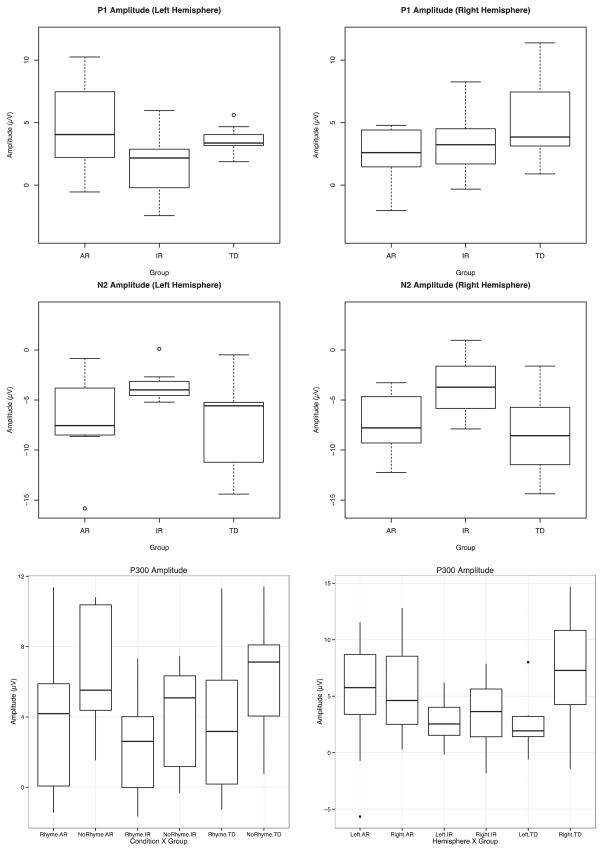
PROC MIXED was used to conduct separate ANOVAs for amplitude and latency measures at each peak (P1, N2, P3) for a total of six separate models comprised of within-subjects factor for hemisphere and condition, and a between-factor for group alongside their respective interactions. Models were fit using a compound symmetric covariance matrix and estimated with Reduced Maximum Likelihood (REML), correction for multiple comparisons was performed using Fischer’s LSD correction for effects involving group, conducting them all at p = .05, as outlined in Maxwell and Delaney (2004). All peaks adhered to a normal distribution (Figure 4), with the exception of the left hemisphere N2 for rhyming words, Shapiro-Wilks W(24) = 0.88, p = .0093.
FIGURE 4.
Boxplots of event-related potential amplitude for second word in rhyming pair.
The analysis of the P1 peak amplitude identified as hypothesized a significant group × hemisphere interaction, F(2, 21) = 4.76, p = .019. Post-hoc comparisons were investigated along the hemisphere axis of the 2 × 3 interaction. Fischer’s LSD correction applied to the three group comparisons within each hemisphere. Significant differences were found as hypothesized between the TD and AR children from electrodes positioned over the right hemisphere, t(21) = 2.33, p = .03 (TD 5.33 μv, AR 2.34 μV; Cohen’s d = 0.86) and as hypothesized a difference was found between AR and IR from electrodes over the left hemisphere, t(21) = 2.43, p = .024 (AR 4.70 μV, IR 1.57 μV; Cohen’s d = 0.98). Additionally, hemisphere differences were identified in TD children, t(21) = 2.32, p = .031 (Left 3.61 μV, Right 5.33 μV; Cohen’s d = 0.62). No main effects or interactions were found.
Peak amplitude analyses of the N2 component identified a group effect F(2, 21) = 3.70, p = .042. Post-hoc comparisons using Fisher’s LSD indicated that differences occurred between AR and IR children, t(21) = 2.16, p = .042, Cohen’s d = 1.41. Additional differences were identified between the IR and TD children, t(21) = 2.56, p = .018, Cohen’s d = 1.26. No significant difference was found between the TD and AR children, t(21) = 0.62, p = .54, Cohen’s d = 0.26 (TD = −8.02μV; AR = −7.02 μV; IR = −3.57 μV). No other main effects or interactions were found.
Analyses of the P3 component identified a significant condition effect, F(1, 21) = 9.51, p = .005, driven by larger peaks that occurred in response to non-rhyming than the rhyming stimuli (3.36 μV < 5.55 μV, Cohen’s d = 0.57); and a significant hemisphere effect, F(1, 21) = 6.70, p = .017 (left = 3.54 μV, right = 5.38 μV), clarified by a significant group × hemisphere interaction, F(2, 21) = 3.62, p = .0446. This effect was driven by hemisphere differences in TD children (LH < RH), t(21) = 3.54, p = .002, Cohen’s d = 1.04; neither of the other two groups demonstrated significant hemisphere differences (IR Cohen’s d = 0.02; AR Cohen’s d = 0.06). No other main effects or interactions were found.
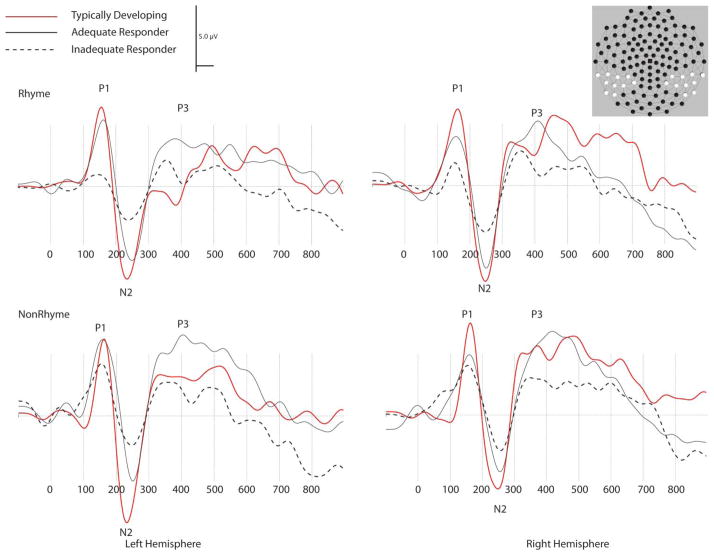
Analyses of peak latency found only a hemisphere difference for the N2 component, F(1, 21) = 5.77, p = .025, in which the left hemisphere N2 reached its maximum peak earlier than over the right hemisphere (204.73 msec vs. 207.05 msec). No other main effects or interactions were significant for the N2, or for the other peaks (P1 and P3). Average ERP waveforms representing each peak are shown in Figure 3.
FIGURE 3.
Event-related potential waveforms in milliseconds for left and right temporo-parietal area region by group. (color figure available online)
EXPLORATORY ANALYSES
Based on finding that the ERP data reflected at least some differences between the three groups (AR, IR, TD) at the three peaks (P1, N2 and P3), we investigated whether ERPs at these peaks could be used to differentiate group membership. Data from the left hemisphere rhyming condition (P1, N2, P3) were used in a discriminant function analysis with uniform priors of 0.33 per group probability definition. The discriminant function analysis yielded significant Wilks’ Lambda F(6, 38) = 4.06, p = .0031. Canonical correlations were 0.73 and 0.45, with the first reaching significance F(6, 38) = 4.06, p = .0031, while the second was non-significant F(2, 20) = 2.60, p = .0989. For the IR group, all seven children were correctly classified (100% accurate); of the 10 children in the AR group, six were correctly classified as AR, three were classified as TD, and one was classified as IR (60% accurate); of the seven children in the TD group, five were correctly classified as TD, and two were classified as AR (71.43% accurate). Evaluating the classification using groups collapsed into those reading at grade level (TD and AR) and those reading below grade level (IR), only one child is misclassified (one classified as AR in the IR group). The inclusion of other variables in the discriminant function analysis (e.g., non-rhyming peaks, right hemisphere) did not improve accuracy of the classification model.
DISCUSSION
This study addressed differences in the neural correlates of reading intervention using an ERP paradigm. To our knowledge, this is the first such study evaluating ERP differences between AR and IR, complimenting a small, but growing research base using MEG and fMRI. While it is understood that scalp recorded ERPs may not reflect brain-based activity at sites directly underlying electrodes placement, nevertheless the ERP differences found in this study are related as hypothesized. As such, we believe that this study represents an important advancement in the continued study of neural correlates of response to intervention.
Support for Hypothesis 1
The findings from this study replicated those of previous studies reporting differences between children who respond adequately (AR) and inadequately (IR) to a reading intervention and typically developing (TD) children using MEG (Simos et al., 2002, 2005, 2007) and fMRI (Alyward et al., 2003; Davis, et al., 2011; Meyler et al., 2008; Odegard, et al., 2008; Shaywitz et al., 2004; Temple et al., 2003). In this study, we found group differences in the amplitude of the first positive peak (P1), where TD children generated larger amplitudes compared to AR over the right hemisphere, while AR showed larger amplitudes than IR over the left hemisphere. Additional group differences were found in the first negative peak (N2), identifying a difference between TD children and IR as well as differences between AR and IR. Previous neuroimaging work on RTI hypothesized that instructional response, when successful, contributes to two mechanisms: normalization and compensation. While this study did not use a pre/post design, the pattern of differences reported here could be relevant to these two mechanisms. For the P1 component, AR children differed from IR children over left hemisphere electrode sites, the same hemisphere identified by previous MEG and fMRI as more activated in proficient readers than in ineffective readers—a possible normalization in brain processing during reading. At the same time, AR differed from TD children over the right hemisphere, suggesting that there are neural systems in AR children that are not present in TD children—a possible compensatory mechanism. The case for normalization can also be made for the N2 component since TD and AR children differed from the IR children, but the TD children did not differ from AR children.
Support for Hypothesis 2
There was some support for our second hypothesis that children in both TD and AR groups would show hemisphere differences with the left hemisphere showing more activation than the right hemisphere. In this study, we found no statistically significant hemisphere differences for the AR group; the hemisphere differences that were found for the TD group (in the P300 response) go in the opposite direction of those hypothesized (LH < RH). This finding differs from previous studies of intervention response (using fMRI and MEG). However, this comparison is difficult given that the outcome measure in previously reported MEG studies of RTI were based on the number of dipoles activated in each hemisphere, which may not directly map onto the surface ERP waveforms. Comparisons with previous fMRI work is also difficult, as fMRI does not offer the temporal resolution offered by ERPs, and is a hemodynamic measure, which is secondary to the actual neural activity. The lack of a hemisphere effect in early peaks has been acknowledged in previous ERP findings. Taylor and Keenan (1990) found no Group × Hemisphere effects for any component. Ackerman, et al. (1994) reported no differences to visual evoked potentials using word stimuli in early peaks between children with dyslexia, borderline slow-learners, and children with ADD. Similarly, Bergmann, Hutzler, Klimesch, and Wimmer (2005) identified hemisphere differences in the N220, which showed larger amplitudes in the left hemisphere for word presentations for children with dyslexia. However, many other ERP studies report data that would have fallen in line with our hypotheses (e.g., Lovrich et al., 2003; Molfese et al., 2006).
Given the mixed literature, it is unclear why the AR children whose reading performance was similar to TD children who did not also show a hemisphere difference. It is possible that the AR children continued to show large activation in the right hemisphere, even after increasing activation in the left hemisphere, which differentiated them from both the TD children (in right hemisphere) and IR children (in left hemisphere). It is also possible that given our small sample size and complexity of the design (Group × Condition × Hemisphere), that this analysis in particular was underpowered.
Support for Hypothesis 3
Consistent with our third hypothesis, our results show that P3 was larger to non-rhyming than rhyming stimuli, consistent with Ackerman et al. (1994) and Bonte and Blomert (2004) who also showed ERP waveform sensitivity to differences in rhyming and non-rhyming stimuli. These results are found for all groups. However, a hypothesized Group × Condition interaction was found for the TD group alone who showed a larger left hemisphere effect. We attribute the ability of all groups to differentiate the stimuli to the relative ease of the words used in these tasks.
Combining data from the three peaks in the Exploratory Analyses added information about the consistency of these data for discriminating groups. While the classification accuracy was high in the three group and two group models, there are some explainable misclassifications. The misclassifications of the AR and TD children may reflect differences in brain processing that are still present in some AR children despite participation in reading interventions that successfully resulted in them reaching grade level performance and present in TD children while interfering with their reading performance. The ERP responses to phonological tasks such as those used here reflect sensitivity to stimuli meaningfully related to reading performance. As such, ERPs appear to contribute additional information on mechanisms underlying children’s reading skills that complement that provided by performance scores alone. Further research using ERPs to differentiate within and between performance based reading groups is needed.
Limitations
This study has some limitations. First, ERP testing was conducted at only one time period such that children were tested at the end of second grade. This was after the AR and IR children had participated in ongoing reading intervention. Because of this limitation, it is not possible to make conclusions as to whether or how the brain changes in response to participation in the reading intervention. In this case, it is possible to extrapolate to previous studies to show that as with Simos et al. (2002, 2005), Shaywitz et al. (2004), and Meyler et al. (2008), children who responded adequately to the intervention showed differences from TD children, even after the reading intervention. The findings from these studies appear to align with the results of our discriminant function analyses.
The second limitation of the study is the small sample size. While 29 participants were originally recruited, a total of five participants were unable to be included in the data analyses due to reading ability, data quality, or equipment malfunction. Although our sample was comparable to previous published studies, future studies of intervention response and neuroimaging should seek to recruit larger samples.
Finally, accuracy and reaction time data were obtained from participants button presses for each condition. These data were lost due to hardware failure. However, each participant was also asked to read the words in each pair aloud. Thus, while the participants were accurate in reading the words, which were mainly pre-primary level words with a few first and second grade words, there are no data specifically on children’s identification of the words as rhyming or non-rhyming. Future research should collect both types of performance data.
Acknowledgments
Supported in part by a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, 1 P50 HD052117, Texas Center for Learning Disabilities. The views expressed are not necessarily the views of the NICHD.
APPENDIX. RHYMING TASK STIMULI
| Rhyming | Non-Rhyming | ||
|---|---|---|---|
| BIG | DIG | CALL | COLD |
| CAT | BAT | SEE | BAT |
| BOLD | COLD | RAN | DIG |
| CAN | TAN | FIND | TAKE |
| BIG | DIG | MAKE | KIND |
| TAR | CAR | LET | CAR |
| KIT | BIT | RUN | BIT |
| RUN | FUN | KIT | FUN |
| LET | SET | TAR | SET |
| MAKE | TAKE | BIG | TAN |
| FIND | KIND | CAN | DIG |
| RAN | CAN | BOLD | BALL |
| SEE | BEE | CAT | BEE |
| CALL | BALL | BIG | CAN |
Contributor Information
Peter J. Molfese, Haskins Laboratories, New Haven, Connecticut
Jack M. Fletcher, Department of Psychology, University of Houston, Houston, Texas
Carolyn A. Denton, Department of Pediatrics, University of Texas Health Science Center at Houston, Houston, Texas
References
- Ackerman PT, Dykman RA, Oglesby DM. Visual event-related potentials of dyslexic children to rhyming and nonrhyming stimuli. Journal of Clinical and Experimental Neuropsychology. 1994;16(1):138–154. doi: 10.1080/01688639408402624. [DOI] [PubMed] [Google Scholar]
- Al Otaiba S, Fuchs D. Characteristics of children who are unresponsive to early literacy intervention: A review of the literature. Remedial and Special Education. 2002;23:300–315. [Google Scholar]
- Aylward EH, Richards TL, Berniger VW, Nagy WE, Field KM, Grimme AC, Cramer SC. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61:212–219. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- Bergmann J, Hutzler F, Klimesch W, Wimmer H. How is dysfluent reading reflected in the ERP? Journal of Neurolinguistics. 2005;18:153–165. [Google Scholar]
- Bonte ML, Blomert L. Developmental dyslexia: ERP correlates of anomalous phonological processing during spoken word recognition. Cognitive Brain Research. 2004;21:360–376. doi: 10.1016/j.cogbrainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Compton DL, Fuchs D, Fuchs LS, Bryant JD. Selecting at-risk readers in first grade for early intervention: A two-year longitudinal study of decision rules and procedures. Journal of Educational Psychology. 2006;98:394–409. [Google Scholar]
- Davis N, Barquero L, Compton DL, Fuchs LS, Fuchs D, Gore JC, Anderson AW. Functional correlates of children’s responsiveness to intervention. Developmental Neuropsychology. 2011;36(3):288–301. doi: 10.1080/87565641.2010.549875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainer KB, Klorman R, Salzman LF, Hess DW, Davidson PW, Michael RL. Learning-disordered children’s evoked potentials during sustained attention. Journal of Abnormal Child Psychology. 1981;9(1):79–94. doi: 10.1007/BF00917859. [DOI] [PubMed] [Google Scholar]
- Denton CA, Cirino PT, Barth AE, Romain M, Vaughn S, Wexler J, Francis DJ, Fletcher JM. An experimental study of scheduling and duration of “Tier 2” first grade reading intervention. Journal of Research on Educational Effectiveness. 2011;4:208–230. doi: 10.1080/19345747.2010.530127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton CA, Tolar TD, Barth AE, Cirino PT, Romain M, Fletcher JM, Vaughn S. Effects of intensive reading intervention for students previously unresponsive to supplemental intervention. (under review) [Google Scholar]
- Dien J. Issues in the application of the average reference: Reviews, critiques, and recommendations. Behavioral Research Methods, Instruments, & Computers. 1998;30(1):34–43. [Google Scholar]
- Farris EA, Odegard TN, Miller HL, Ring J, Allen G, Black J. Functional connectivity between the left and right inferior frontal lobes in a small sample of children with and without reading difficulties. Neuroscase. 2011;17(5):425–439. doi: 10.1080/13554794.2010.532141. [DOI] [PubMed] [Google Scholar]
- Fletcher JF, Lyon GR, Fuchs LS, Barnes MA. Learning disabilities: From identification to intervention. New York, NY: Guilford Press; 2007. [Google Scholar]
- Fletcher JF, Steubing KK, Barth AE, Denton CA, Cirino PT, Francis DJ, Vaughn S. Cognitive correlates of inadequate response to reading intervention. School Psychology Review. 2011;40:3–22. [PMC free article] [PubMed] [Google Scholar]
- Frost SJ, Landi N, Mencl WE, Sandak R, Fulbright RK, Tejada ET, Pugh KR. Phonological awareness predicts activation patterns for print and speech. Annals of Dyslexia. 2009;59(1):78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Fuchs LS. Introduction to response to intervention: What, why, and how valid is it? Reading Research Quarterly. 2006;41:92–99. [Google Scholar]
- Holcomb PJ, Ackerman PT, Dykman RA. Cognitive event-related brain potentials in children with attention and reading deficits. Psychophysiology. 1985;22(6):656–667. doi: 10.1111/j.1469-8986.1985.tb01663.x. [DOI] [PubMed] [Google Scholar]
- Justice LM. Evidence-based practice, response to intervention, and the prevention of reading difficulties. Language, Speech, and Hearing Services in Schools. 2006;37:284–297. doi: 10.1044/0161-1461(2006/033). [DOI] [PubMed] [Google Scholar]
- Lovrich D, Cheng JC, Velting DM. ERP correlates of form and rhyme letter tasks in impaired reading children: A critical evaluation. Child Neuropsychology. 2003;9(3):159–174. doi: 10.1076/chin.9.3.159.16458. [DOI] [PubMed] [Google Scholar]
- Mathes PG, Denton CA, Fletcher JM, Anthony JL, Francis DJ, Schatschneider C. The effects of theoretically different instruction and student characteristics on the skills of struggling readers. Reading Research Quarterly. 2005;40(2):148–182. [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JDE, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: A longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese DL, Key AF, Kelly S, Cunningham N, Terrell S, Ferguson M, Bonebright T. Below-average, average, and above-average readers engage in different and similar brain regions while reading. Journal of Learning Disabilities. 2006;39(4):352–363. doi: 10.1177/00222194060390040801. [DOI] [PubMed] [Google Scholar]
- Nelson JR, Benner GJ, Gonzalez J. Learner characteristics that influence the treatment effectiveness of early literacy interventions: A meta-analytic review. Learning Disabilities Research and Practice. 2003;18:255–267. [Google Scholar]
- Odegard TN, Ring J, Smith S, Biggan J, Black J. Differentiating the neural response to intervention in children with developmental dyslexia. Annals of Dyslexia. 2008;58:1–14. doi: 10.1007/s11881-008-0014-5. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Bolger DJ. The brain might read that way. Scientific Studies of Reading. 2004;8(3):293–304. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184– 187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pugh K, Frost S, Sandak R, Landi N, Rueckl J, Constable R, Mencl E. Effects of stimulus difficulty and repetition on printed word identification: An fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. Journal of Cognitive Neuroscience. 2008;20:1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K, Mencl E, Jenner A, Ren Lee J, Katz L, Frost S, Shaywitz B. Neuroimaging studies of reading development and reading disability. Learning Disabilities Research & Practice. 2001;16:240–249. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychology. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Denton C, Sarkari S, Billingsley-Marshall R, Papanicolaou AC. Magnetic source imaging studies of dyslexia interventions. Developmental Neuropsychology. 2006;30(1):591–611. doi: 10.1207/s15326942dn3001_4. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley-Marshall R, Denton CA, Papanicolaou AC. Intensive instruction affects brain magnetic activity associated with oral word reading in children with persistent reading disabilities. Journal of Learning Disabilities. 2007;40(1):37–48. doi: 10.1177/00222194070400010301. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Francis DJ, Castillo EM, Papanicolaou AC. Early development of neurophysiological processes involved in normal reading and reading disability: A magnetic source imaging study. Neuropsychology. 2005;19(6):787–798. doi: 10.1037/0894-4105.19.6.787. [DOI] [PubMed] [Google Scholar]
- Symann-Louett N, Gascon GG, Matsumiya Y, Lombroso C. Wave form difference in visual evoked responses between normal and reading disabled children. Neurology. 1977;27:156–159. doi: 10.1212/wnl.27.2.156. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Keenan NK. Event-related potentials to visual and language stimuli in normal and dyslexic children. Psychophysiology. 1990;27(3):318–327. doi: 10.1111/j.1469-8986.1990.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JD. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Alexander AW, Wagner RK, Rashotte CA, Voeller KKS, Conway T. Intensive remedial instruction for children with severe reading disabilities: Immediate and long-term outcomes from two instructional approaches. Journal of Learning Disabilities. 2001;34(1):33–58. doi: 10.1177/002221940103400104. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Scanlon DM, Small S, Fanuele DP. Response to intervention as a vehicle for distinguishing between reading disabled and non-reading disabled children: Evidence for teh role of kindergarten and first grade intervention. Journal of Learning Disabilities. 2006;39(2):157–169. doi: 10.1177/00222194060390020401. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Scanlon DM, Zhang H, Schatschneider C. Using response to kindergarten and first grade intervention to identify children at-risk for long term reading difficulties. Reading and Writing. 2008;21:437–480. [Google Scholar]
- Woodcock RM, Johnson MB. Woodcock-Johnson Psychoeducational Battery–Revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]