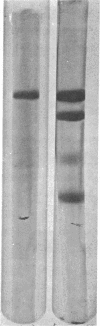
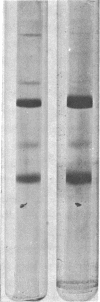
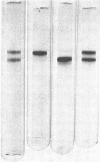
Abstract
Two new forms of carboxypeptidase B have been isolated from spontaneously activated bovine pancreatic juice. The fully active enzymes contain an internal split at residues 92-93 and 95-96, respectively. Sequenator analysis of the amino terminal segments of the two chains of the enzyme has extended the sequence information by 51 amino acid residues. Comparison of 125 residues strengthens the hypothesis that carboxypeptidases A and B are homologous both in amino acid sequence and in three-dimensional conformation and implicates Asp-255 as the anionic site of substrate binding of the B enzyme.
Keywords: amino acid sequence, active site, proteolytic cleavage
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A., Ericsson L. H., Walsh K. A., Neurath H. The amino acid sequence of bovine carboxypeptidase A. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1389–1394. doi: 10.1073/pnas.63.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw R. A., Neurath H., Walsh K. A. Considerations of the concept of structural homology as applied to bovine carboxypeptidases A and B. Proc Natl Acad Sci U S A. 1969 Jun;63(2):406–411. doi: 10.1073/pnas.63.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H., Lai C. Y. Primary structure of bovine carboxypeptidase B. 3. The carboxyl-terminal sequence. Arch Biochem Biophys. 1968 Feb;123(2):353–360. doi: 10.1016/0003-9861(68)90145-8. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H. Primary structure of bovine carboxypeptidase B. II. Tryptic peptides from the reduced, aminoethylated protein. Arch Biochem Biophys. 1968 Feb;123(2):343–352. doi: 10.1016/0003-9861(68)90144-6. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H. Primary structure of bovine carboxypeptidase B. IV. Amino acid sequence of a disulfide-containing loop. Arch Biochem Biophys. 1968 Feb;123(2):361–367. doi: 10.1016/0003-9861(68)90146-x. [DOI] [PubMed] [Google Scholar]
- KELLER P. J., COHEN E., NEURATH H. The proteins of bovine pancreatic juice. J Biol Chem. 1958 Aug;233(2):344–349. [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Quiocho F. A., Reeke G. N., Jr The structure of carboxypeptidase A. IX. The x-ray diffraction results in the light of the chemical sequence. Proc Natl Acad Sci U S A. 1969 Sep;64(1):28–35. doi: 10.1073/pnas.64.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Neurath H., Walsh K. A., Winter W. P. Evolution of structure and function of proteases. Science. 1967 Dec 29;158(3809):1638–1644. doi: 10.1126/science.158.3809.1638. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr Isolation and sequence of peptides at the active center of bovine carboxypeptidase B. J Biol Chem. 1969 Oct 10;244(19):5246–5253. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- WALSH K. A., NEURATH H. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:884–889. doi: 10.1073/pnas.52.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERSBERGER E., COX D. J., NEURATH H. Bovine pancreatic procarboxypeptidase B. I. Isolation, properties, and activation. Biochemistry. 1962 Nov;1:1069–1078. doi: 10.1021/bi00912a017. [DOI] [PubMed] [Google Scholar]
- WINTERSBERGER E., COX D. J., NEURATH H. Bovine pancreatic procarboxypeptidase B. I. Isolation, properties, and activation. Biochemistry. 1962 Nov;1:1069–1078. doi: 10.1021/bi00912a017. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wintersberger E. Isolation and structure of an active-center peptide of bovine carboxypeptidase B containing the zinc-binding sulfhydryl group. Biochemistry. 1965 Aug;4(8):1533–1536. doi: 10.1021/bi00884a011. [DOI] [PubMed] [Google Scholar]