Abstract
Mitochondrial metabolism has traditionally been thought of as a source of cellular energy in the form of ATP. The recent renaissance in the study of cellular metabolism, particularly in the cancer field, has highlighted the fact that mitochondria are also critical biosynthetic and signaling hubs, making these organelles key governors of cellular outcomes.1-5 Using the epidermis as a model system, our recent study looked into the role that mitochondrial metabolism and ROS production play in cellular differentiation in vivo.6 We showed that conditional deletion of the mitochondrial transcription factor, TFAM within the basal cells of the epidermis results in loss of mitochondrial ROS production and impairs epidermal differentiation and hair growth. We demonstrated that mitochondrial ROS generation is required for the propagation of Notch and β-catenin signals which promote epidermal differentiation and hair follicle development respectively. This study bolsters accumulating evidence that oxidative mitochondrial metabolism plays a causal role in cellular differentiation programs. It also provides insights into the role that mitochondrial oxidative signaling plays in a cell type-dependent manner.
Keywords: mitochondrial metabolism, ATP, reactive oxygen species, TFAM, cellular differentiation
Mitochondrial ROS Regulate Signal Transduction
Mitochondria produce reactive oxygen species (ROS) during oxidative metabolism through the single-electron reduction of molecular oxygen (O2).7 The resulting superoxide anion (O2•−), is converted to hydrogen peroxide (H2O2) by cellular superoxide dismutases (SODs). Similar to phosphorylation, ubiquitination, and acetylation, oxidation of protein cysteine residues represents a form of reversible, posttranslational protein modification which can regulate signaling. Oxidation of cysteine thiol groups (–SH) by H2O2 results in formation of sulphenic acid (–SOH) which quickly forms disulfide (–SS–) or sulfenyl amide (–SN–) bonds, changing protein function.8,9 The cellular thioredoxin and glutathione reductase systems restore these oxidatively modified residues back to their reduced forms in a manner analogous to phosphatases, deubiquitinating enzymes, or histone deacetylases.
Mitochondrial ROS (mROS) generation is required for the propagation of numerous cellular signaling pathways including those regulating tumorgenesis,10-12 immune responses,13-15 and cellular adaptation to stresses such as hypoxia.16-19 In most cases it is unknown exactly what oxidative events are required for the effects of ROS on these pathways and it is likely that multiple oxidative targets potentiate transduction through a given pathway.
Increasing evidence suggests that mitochondria and mROS generation play key roles in cellular differentiation programs. Cellular mitochondrial content and oxidative capacity are increased when mouse embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), or mesenchymal stem cells are induced to differentiate.20-24 Furthermore, increased cellular ROS levels and oxidation products correlate with the differentiation of ESCs and iPSCs as well as mesenchymal, neural, and epithelial stem cells.23,25-30 Hematopoietic stem cells (HSCs) contain significantly lower levels of ROS than do the more-differentiated common myeloid progenitor cells. Aberrantly increased ROS levels promote HSC differentiation while inhibition of ROS production prevents differentiation.31-34 Thus increased mitochondrial and oxidant content appears to correlate with stem cell differentiation while low mitochondrial mass and oxidant content correlates with stem cell maintenance. In our recent report, we used the mammalian epidermis as a model system to test the hypothesis that mitochondrial metabolism and ROS production promote signaling through pathways required for differentiation of mammalian stem cell populations in vivo.6
Mitochondrial ROS Promote Epidermal Differentiation and Hair Growth
The epidermis is a self-renewing stratified squamous epithelium and is thus regulated by stem cell populations. To maintain epidermal homeostasis, cells within the proliferative basal layer withdraw from the cell cycle and differentiate as they process outward through the suprabasal layers, compensating for cellular loss via desquamanation from the outermost epidermal layer.35 The epidermis also elaborates appendages such as hair follicles (HFs) which are themselves regulated by stem cell populations.36 Multiple transcriptional networks are associated with differentiation within the epidermis including Notch, p63, C/EBP, and AP2 (interfollicular epidermis) and β-catenin (HF).37 It is incompletely understood how these various factors are regulated to promote epidermal homeostasis and what role cellular metabolism might play in this regulation.
To test the hypothesis that mitochondria play an active role in the regulation of epidermal homeostasis, we conditionally deleted TFAM (transcription factor A, mitochondrial) in basal, undifferentiated epidermal keratinocytes. TFAM is required for the replication and transcription of the mitochondrial genome, and cells lacking TFAM are unable to conduct oxidative phosphorylation or produce mROS.38 Mice conditionally lacking TFAM in epidermis (TFAM cKO mice) lacked hair and developed an epidermal barrier defect which contributed to their perinatal mortality. Histologically, the skin of these mice displayed signs of impaired keratinocyte differentiation.
Similar to in vivo, primary keratinocytes derived from TFAM cKO mice displayed impaired differentiation in vitro. Differentiation of wild-type keratinocytes was inhibited by antioxidant treatment, and differentiation marker expression in TFAM cKO cells could be partly restored by treatment with exogenously applied H2O2, clearly demonstrating that oxidative signaling promotes keratinocyte differentiation. We went on to demonstrate that mROS generation is required for activation of the Notch and β-catenin transcriptional programs that promote epidermal differentiation and hair growth respectively. Together, our results show that mROS act as pro-differentiation signals and are key upstream regulators of stem cell fate decisions.
While the role for mROS in signal transduction is becoming increasingly accepted, the identities of the exact targets of oxidation which promote signaling remain unknown in most situations. Our work identified nucleoredoxin (NXN) as a putative target of mROS-mediated Wnt-β-catenin signaling. NXN is a member of the thioredoxin family which has previously been demonstrated to regulate β-catenin-dependent transcription in a redox-sensitive manner.39 Our results show that NXN becomes oxidized when wild-type keratinocytes are treated with the β-catenin activator Wnt-3a. This did not occur in TFAM cKO keratinocytes, suggesting that mROS-mediated NXN oxidation plays a causal role in the transduction of signals between Wnt receptors and β-catenin. Further studies will be required to determine how mROS regulate Notch transcriptional activity during keratinocyte differentiation.
Future Directions: Metabolism as the Gatekeeper to Cellular Differentiation
Our study demonstrates that mitochondrial metabolism and ROS generation promote keratinocyte differentiation. This raises the interesting possibility that aberrant keratinocyte metabolism may be associated with, and presents targets for, the treatment of epidermal disease. While the genetic causes of these diseases are increasingly studied, little is known about how disease-causing mutations might affect cellular metabolism. More generally, the interactions between cellular differentiation programs and cellular metabolism will be increasingly studied in years to come. The development of regenerative therapies depends on the ability to maximize the renewal capacity of stem cells, and subsequently, to promote complete differentiation into desired cell types. Cellular metabolism represents an intriguing “rheostat” which may be used to modulate these ends. A further understanding of the mechanisms by which differentiation programs both regulate, and are regulated by metabolism will prove useful in this regard.
It is unknown how stem and differentiated cells maintain their respective metabolic phenotypes. Proper regulation is likely dependent on cell type-specific factors as well as environmental influences such as hypoxia. In keratinocytes, elevation of extracellular calcium is the most common method for inducing differentiation in vitro. As an epidermal calcium gradient exists in vivo, it is hypothesized that calcium concentrations regulate epidermal differentiation in vivo as well.40 Calcium uptake into mitochondria is known to stimulate the activity of TCA cycle enzymes (pyruvate-, isocitrate-, and oxoglutarate-dehydrogenases), promoting mitochondrial respiration.41 We found that inhibition of mitochondrial calcium uptake prevented keratinocyte differentiation in vitro, suggesting that mitochondrial calcium uptake might promote the mitochondrial metabolism and ROS production required for epidermal differentiation.
A recent study suggests that mitochondrial uncoupling proteins may play a role in regulating differentiation-dependent metabolism.42 Uncoupling protein 2 (UCP2) is highly expressed in iPSCs, with reduced expression in differentiated cells. Ectopic UCP2 expression inhibited oxidative metabolism of pyruvate, reduced cellular ROS content, and prevented differentiation suggesting that this protein could act as a metabolic switch that controls stem cell differentiation. How UCP2 expression is repressed during differentiation remains to be determined and it will also be of interest to determine if UCP2 regulates the metabolism of less-pluripotent, tissue-specific stem cell populations.
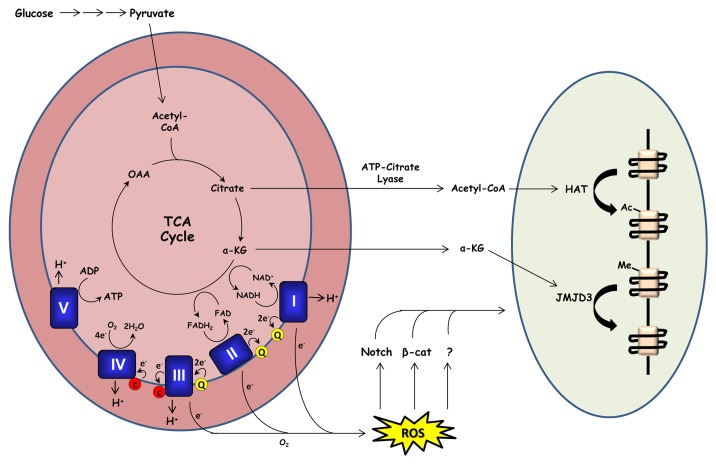
Beyond promotion of oxidant-dependent signaling, the mechanisms by which mitochondrial metabolism promotes cellular differentiation remain poorly understood. Some differentiated lineages may require the efficient ATP production provided by oxidative phosphorylation; however, another intriguing possibility involves the nutrient-dependent regulation of chromatin (Fig. 1). Acetyl-CoA, a key fuel for oxidative metabolism, is also a substrate used by histone acetyltransferases to acetylate histones and other cellular proteins. Global increases in histone acetylation are associated with differentiation of ESCs, 3T3-L1 preadipocytes, and keratinocytes.43-45 Treatment of primary human keratinocytes with histone deacetylase inhibitors is sufficient to induce cell cycle arrest and expression of several differentiation markers.46,47 Although differentiation programs likely regulate histone acetylation in a gene and time-dependent manner; these reports suggest that cellular differentiation may depend on availability of acetyl-CoA to carry out acetylation reactions. In mammalian cells, most cytosolic acetyl-CoA is produced through the cleavage of TCA-cycle-derived citrate by the enzyme ATP-citrate lyase (ACL). Inhibition of ACL expression prevents global increases in histone acetylation and differentiation of 3T3-L1 cells.48
Figure 1. Mitochondrial metabolism promotes keratinocyte differentiation. During oxidative metabolism, electrons are transferred from TCA cycle substrates to complexes I and II of the electron transport chain via NADH and FADH2 respectively. These electrons are transferred to complex III (via coenzyme Q) and subsequently to complex IV (via cytochrome c) where they are used to reduce molecular oxygen to water. Complexes I, II, and III of the respiratory chain contain sites in which electrons can prematurely react with oxygen, producing ROS. Our recent report demonstrates that mitochondrial ROS production is required for activation of Notch and β-catenin transcriptional programs which promote epidermal differentiation and hair development respectively. Mitochondrial metabolism may also promote keratinocyte differentiation by providing substrates for histone acetyltransferases (HATs) and histone demethylases (such as JMJD3).
Another histone modification potentially regulated by cellular metabolism is methylation. Histone methylation status is regulated by histone methyltransferases and demethylases with various methylation “codes” associated with either transcriptional activation or repression. One particular methyl modification—trimethylation of histone 3 on lysine 27 (H3K27me3)—is associated with transcriptional repression of epidermal differentiation markers.49 Genes such as keratin 1 and involucrin display high levels of H3K27me3 in undifferentiated keratinocytes, and lose this mark as cells differentiate. Demethylation of H3K27me3 requires the demethylase JMJD3 and cells lacking JMJD3 expression are unable to differentiate in culture.49 JMJD3 is a member of the Jumanji-C domain containing family of demethylases which use oxygen, Fe(II), and the TCA cycle intermediate α-ketoglutarate as cofactors and are thus potentially sensitive to cellular metabolic state.50,51
Many other nutrient-sensitive mechanisms are likely to regulate stem cell maintenance and differentiation in both the epidermis and in other compartments. Cancer researchers are currently striving to identify cancer-specific metabolic signatures which can be therapeutically targeted. Metabolic study of cellular differentiation is an emerging area which, in concert with genetic approaches, has the potential to lead to strategies for targeted control of cell fate decisions and therapies for diseases associated with aging.
Footnotes
Previously published online: www.landesbioscience.com/journals/cellularlogistics/article/25456
References
- 1.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 4.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–13. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6:ra8. doi: 10.1126/scisignal.2003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandes N, Schmitt S, Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 14.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–36. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–20. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–14. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–8. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–9. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facucho-Oliveira JM, Alderson J, Spikings EC, Egginton S, St John JC. Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci. 2007;120:4025–34. doi: 10.1242/jcs.016972. [DOI] [PubMed] [Google Scholar]
- 21.Chung S, Arrell DK, Faustino RS, Terzic A, Dzeja PP. Glycolytic network restructuring integral to the energetics of embryonic stem cell cardiac differentiation. J Mol Cell Cardiol. 2010;48:725–34. doi: 10.1016/j.yjmcc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S60–7. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–33. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 24.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–8. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 25.Yanes O, Clark J, Wong DM, Patti GJ, Sánchez-Ruiz A, Benton HP, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–7. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, et al. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells. 2010;28:661–73. doi: 10.1002/stem.307. [DOI] [PubMed] [Google Scholar]
- 27.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011;14:537–44. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–7. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–50. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 31.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 33.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–41. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–8. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs E. Finding one’s niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–3. doi: 10.1242/jcs02793. [DOI] [PubMed] [Google Scholar]
- 37.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–6. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 39.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–8. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 40.Dotto GP. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit Rev Oral Biol Med. 1999;10:442–57. doi: 10.1177/10454411990100040201. [DOI] [PubMed] [Google Scholar]
- 41.Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011;30:4860–73. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCool KW, Xu X, Singer DB, Murdoch FE, Fritsch MK. The role of histone acetylation in regulating early gene expression patterns during early embryonic stem cell differentiation. J Biol Chem. 2007;282:6696–706. doi: 10.1074/jbc.M609519200. [DOI] [PubMed] [Google Scholar]
- 44.Yoo EJ, Chung JJ, Choe SS, Kim KH, Kim JB. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J Biol Chem. 2006;281:6608–15. doi: 10.1074/jbc.M508982200. [DOI] [PubMed] [Google Scholar]
- 45.Frye M, Fisher AG, Watt FM. Epidermal stem cells are defined by global histone modifications that are altered by Myc-induced differentiation. PLoS One. 2007;2:e763. doi: 10.1371/journal.pone.0000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders N, Dicker A, Popa C, Jones S, Dahler A. Histone deacetylase inhibitors as potential anti-skin cancer agents. Cancer Res. 1999;59:399–404. [PubMed] [Google Scholar]
- 47.Elder JT, Zhao X. Evidence for local control of gene expression in the epidermal differentiation complex. Exp Dermatol. 2002;11:406–12. doi: 10.1034/j.1600-0625.2002.110503.x. [DOI] [PubMed] [Google Scholar]
- 48.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–80. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 2008;22:1865–70. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaelin WG, Jr., McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metab. 2012;16:9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]