Abstract

Iteration of Pd-catalyzed reaction of alkynyl- and oligoynylzincs with (E)-ICH=CHCl followed by metalation-termination with electrophiles(E) has provided a linear route to conjugated tri- and tetraynes, while Pd-catalyzed monoalkynylation of 1,1-dibromoenynes accompanied by dehydrobromination has provided a convergent route to conjugated tri-, tetra-, and pentaynes. Both display unprecedent high efficiency and selectivity.
A large number of naturally occurring conjugated diynes and oligoynes exhibiting antibacterial, antifungal, anti-inflammatory, anti-angiogenic, antimicrobial, cytotoxic, larvicidal, and other biological activities are known.1 Additionally, a growing number of non-natural oligo- and polyynes of optical, electrical, and other materials chemical as well as structural chemical interest have been prepared and investigated.2 The parent polyynes of high degrees of polymerization represent the hypothetical sp carbon allotrope “carbyne” of considerable current interest.2b,3
Although various Cu-mediated4 and Pd-catalyzed5,6 methods for the synthesis of unsymmetrically substituted conjugated diynes via alkynyl-alkynyl coupling are known, the available data indicate that none appears to be widely and predictably satisfactory, the major side-reaction being competitive formation of the two homodimers derivable from the two starting alkynes presumably via alkynyl ligand scrambling. For example, we recently reported a highly cross-selective reaction of BrZnC≡CCO2Me with nHexC≡CI producing nHexC≡CC≡CCO2Me in 86% yield7 but still failed to develop it into a predictably general and satisfactory route to conjugated diynes. An ingenious application of the Fritsch-Buttenberg-Wiechell rearrangement8 by Tykwinski et al.1b,9 provides an alternative convergent route to conjugated oligoynes. However, application of these basically convergent protocols to the synthesis of tetra- and higher conjugated oligoynes requires one or two shorter oligoynyl intermediates. In this context, a recent modification of the Cadiot-Chodkiewicz reaction permitting its iterative use by Kim et al.10 is noteworthy, but each homologation cycle requires two steps, namely halogenation of alkynylsilanes and alkynyl-alkynyl coupling. It is clearly desirable to develop protocols requiring just one step for each homologation or even less, i.e., homologation by two or more ethynyl units in one step.
We report herein a highly efficient and strictly “pair-selective”11 linear iterative protocol for the synthesis of conjugated tri- and tetraynes (Schemes 1 and 2). Also reported are some results in the development of a complementary convergent protocol for the synthesis of tri- and higher oligoynes (Schemes 3 and 4) through application of strictly “pair-selective” Pd-catalyzed alkynyl-alkenyl coupling.
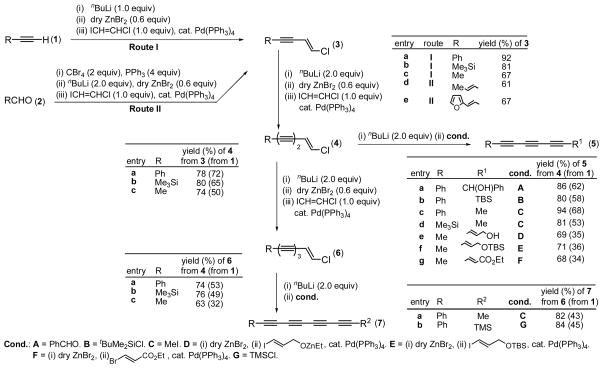
Scheme 1.
Scheme 2.

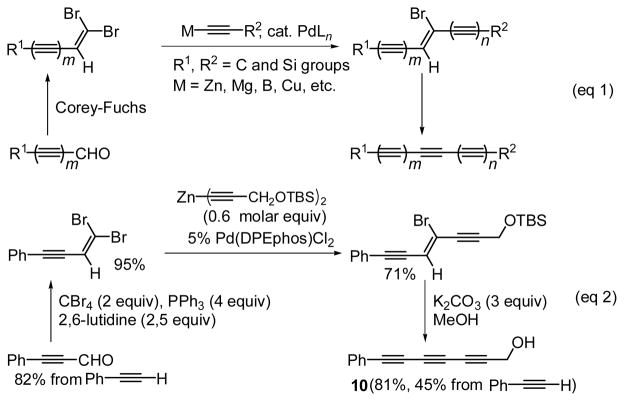
Scheme 3.
Scheme 4.
Since 1984, we have reported a series of “pair-selective” syntheses of conjugated diynes via Pd- catalyzed alkynyl-alkenyl coupling reactions12–15 with (E)-1-iodo-2-chloroethylene,12,13 (E)-1-iodo-2-bromoethylene,13 and vinylidene dichloride.14 However, their applicability to the synthesis of higher oligoynes has not been investigated. In the initial search for a highly favorable ethynyl synthon, conversion of PhC≡CH to PhC≡CC≡CMe was performed with (E)-ICH=CHCl,12,16 (E)-ICH=CHBr,13,17 Cl2C=CH2 ($ 6.6/mol, Aldrich), and ClCH=CCl2 ($ 1.2/mol, Aldrich) as ethynyl synthons. The yields of PhC≡CC≡CMe were 84, 76, 72, and 55%, respectively. Despite the lower product yields, the significantly lower costs of Cl2C=CH2 and ClCH=CCl2 might appear to favor their use in cases where the starting alkynes are relatively inexpensive.
However, their cross-coupling reactions require severalfold excesses of them, which complicates their iterative use for the synthesis of oligoynes. In view of the higher product yield and cleaner reaction profile, (E)-ICH=CHCl was chosen in this study. As an ethynyl synthon, (E)-ICH=CHBr does not generally offer any notable advantage over (E)-ICH=CHCl. Some representative results of the syntheses of tri- and tetraynes from the corresponding monoynes (1) or aldehydes (2) in three and four steps, respectively, are summarized in Scheme 1. Specifically, several triynes (5) were prepared in 34–62% overall yields from 1, and a couple of tetraynes (7) were prepared in 43 and 45% overall yield.
Many of the requisite starting terminal alkynes are commercially available. In cases where appropriately structured aldehydes are economically available, as in the case of (E)-crotonaldehyde, 2-furylaldehyde, and (E)-3-(2-furyl)acrolein, the corresponding alkynylzinc reagents can be readily generated via Corey-Fuchs18 and other carbonyl alkynylation reactions and used in situ for the preparation of (3) in one pot (Route II in Scheme 1). An efficient synthesis of a dienetriyne derivative (8) from (E)-crotonaldehyde, (E)-ICH=CHCl, and (E)-ICH=CHCH2OTBS in 42% overall yield shown in Scheme 2 is exemplary. In this case, the dienemonoyne (3d) and dienediyne (9) intermediates were obtained as crudely worked-up materials without purification and used in the subsequent steps. For their full identification, however, they were chromatographically purified and isolated. This additional operations, however, led to an overall yield of 35% for the synthesis of 8. Omission of purification of intermediates was also applied to the synthesis of 1-phenyl-1,3,5-heptatrieyne (5c). Even though the overall yield of 67% from PhC≡CH is only comparable to that shown in Entry c (68%), the overall process is simpler and more efficient.19 In all of the other cases shown in Scheme 1, however, all indicated intermediates were isolated and purified primarily for their full identification.
During the development of the above-described linear iterative protocol, it occurred to us that 1,1-dibromo-1-alkenes should be used not as mere precursors to 1-alkynes (Routes II in Schemes 1 and 2) but for devising a convergent protocol shown in Scheme 3 for the synthesis of triynes and higher oligoynes by combining two shorter monoynyl and/or oligoynyl intermediates, the latter of which are now efficiently and selectively preparable, as discussed above. The critical step involves the Pd-catalyzed trans-selective monoalkynylation of 1,1-dibromo- and 1,1-dichloro-1-alkenes developed recently by us.20
For the proof of principle, 7-phenyl-2,4,6-heptatriyn-1-ol (10) was prepared in 45% overall yield in 4 steps from PhC≡CH and HC≡CCH2OTBS, as shown in equation 2 of Scheme 3.21,22 Application of the convergent protocol shown in Scheme 3 to the synthesis of conjugated tetraynes, however, was complicated by competitive formation of the expected monobromotriynes and the eventual target tetraynes. Specifically, the reaction of 4-phenyl-1,1-dibromo-1-buten-3-yne with 1.1 equiv of an enediynylzinc derivative 11, generated in situ by treating (E,E)-1-chloro-1,5-heptadien-3-yne, in the presence of 5 mol% of Pd(DPEphos)Cl2 at 23°C gave 12 and 13 in <10 and 32% yields, respectively, with 49% of the starting dibromide remaining unreacted. Only traces, if any, of the stereoisomer of 12 and the dialkynylated product were present. Although the precise mechanism of formation of 13 is unclear, 11 must have been partially consumed as a mere base to neutralize HBr. As expected, use of two equiv of 11 produced 13 in 66% yield along with only traces of 12 and the starting dibromide. Although this reaction is clean, it is not synthetically attractive, as it requires 1.0 molar equiv (or a two-fold excess) of the alkynylzinc reagent. Our recent development of the Pd-catalyzed alkynylation with free terminal alkynes in the presence of NEt3 and ZnBr2,23 however, provided a handy solution. Thus, a combination of 11 (0.55 molar equiv) and NEt3 (1.0 equiv) in place of a two-fold excess (1.0 molar equiv) of 11 cleanly produced 13 in 66% yield (eq 1 of Scheme 4). This procedure was successfully applied to the synthesis of 14 in 68% yield from 4-(p-tolyl)-1,1-dibromo-1-buten-3-yne and bis(4-phenyl-1,3-butadiynyl)zinc (eq 2 of Scheme 4) as well as three conjugated pentaynes 15a–15c (eq 3 of Scheme 4).
Although further condition optimization is needed to improve the modest yields of 43–55%, these examples appear to represent the first set of “pair-selective” syntheses of unsymmerically substituted conjugated pentaynes.
Supplementary Material
Acknowledgments
We thank the National Science Foundation (CHE-0309613), the National Institutes of Health (GM 36792), and Purdue University for support of this research.
Footnotes
Warning! Upon concentration and exposure to air, 1-trimethylsilyl-1,3,5,7-nonatetrayne spontaneously ignited in a manner of fireworks. Although the exact nature of the phenomenon remains unclear, it is advisable to exercise appropriate precautions in handling conjugated oligoynes.3
Supporting Information Available: Experimental details of representative reactions as well as spectral and analytical data of isolated products including 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews, see: Bohlmann F, Burkhardt H, Zdero C. Naturally Occurring Acetylenes. Academic Press; New York: 1973. p. 547.Shi Shun ALK, Tykwinski RR. Angew Chem Int Ed. 2006;45:1043. doi: 10.1002/anie.200502071.
- 2.See, for example, Ye F, Orita A, Yaruva J, Hamada T, Otera J. Chem Lett. 2004;33:528.Gibtner T, Hampel F, Grisselbrecht JP, Hirsch A. Chem Eur J. 2002;8:408. doi: 10.1002/1521-3765(20020118)8:2<408::AID-CHEM408>3.0.CO;2-L.
- 3.For a recent paper reporting on an explosion of conjugated polyynes, see: Baughman RH. Science. 2006;312:1009. doi: 10.1126/science.1125999.
- 4.(a) Cadiot P, Chodkiewicz W. In: Chemistry of Acetylenes. Viehe HG, editor. Marcel Dekker; New York: 1969. pp. 597–647. [Google Scholar]; (b) Brandsma L, Vasilevski SF, Verkruijsse HD. Application of Transition Metal Catalysis in Organic Synthesis. Springer-Verlag; Berlin: 1999. pp. 49–105. [Google Scholar]
- 5.For a recent review summarizing the reported results of the Pd-catalyzed alkynyl-alkynyl coupling reactions, see: Negishi E, Anastasia L. Chem Rev. 2003;103:1979. doi: 10.1021/cr020377i.
- 6.For papers reporting the use of Sonogashira alkynyl-aklynyl coupling, see: Wityak J, Chan JB. Synth Commun. 1991;21:977.Alzeer J, Vasella A. Helv Chim Acta. 1995;78:177.Amatore C, Blart E, Genêt JP, Jutand A, Lemaire-Audoire S, Savignac M. J Org Chem. 1995;60:6829.Alami M, Ferri F. Tetrahedron Lett. 1996;37:2763.
- 7.Negishi E, Qian M, Zeng F, Anastasia L, Babinski D. Org Lett. 2003;5:1597. doi: 10.1021/ol030010f. [DOI] [PubMed] [Google Scholar]
- 8.(a) Fritsch P. Liebigs Ann Chem. 1894;279:319. [Google Scholar]; (b) Buttenberg WP. Liebigs Ann Chem. 1894;279:327. [Google Scholar]; (c) Wiechell H. Liebigs Ann Chem. 1894;279:332. [Google Scholar]
- 9.Eisler S, Tykwinski RR. J Am Chem Soc. 2000;122:10737.Shi Shun ALK, Tykwinski RR. J Org Chem. 2003;68:6810. doi: 10.1021/jo034734g.Luu T, Shi W, Lowary TL, Tykwinski RR. Synthesis. 2005:3167.For an application of the Tykwinski protocol to the synthesis of (−)-ichthyothereol, see: Mukai C, Miyakoshi N, Hanaoka M. J Org Chem. 2001;66:5875. doi: 10.1021/jo0104532.
- 10.Kim S, Kim S, Lee T, Ko H, Kim D. Org Lett. 2004;6:3601. doi: 10.1021/ol0484963. [DOI] [PubMed] [Google Scholar]
- 11.In place of “pair-selective”, a Greek-derived term “couploselective” may be used along with “stereoselective”, “regioselective”, and so on.
- 12.Negishi E, Okukado N, Lovich SF, Luo FT. J Org Chem. 1984;49:2629.See also: Kende AS, Smith CA. J Org Chem. 1988;53:2655.
- 13.Negishi E, Hata M, Xu C. Org Lett. 2000;1:3687. doi: 10.1021/ol000270m. [DOI] [PubMed] [Google Scholar]
- 14.Qian M, Negishi E. Org Proc Res Dev. 2003;1:412. [Google Scholar]
- 15.For a recent review, see: Negishi E, Hu Q, Huang Z, Qian M, Wang G. Aldrichimica Acta. 2005;38:71. See also Ref. 5.
- 16.Van de Walle H, Henne A. Bull Cl Sci, Acad R Belg. 1925;11:360.Chem Abstr. 1926;20:1050.(b) For a detailed procedure, see Ref. 12.
- 17.(a) Negishi E, Alimardanov A, Xu C. Org Lett. 2000;2:65. doi: 10.1021/ol990336h. [DOI] [PubMed] [Google Scholar]; (b) Negishi E, Zeng X. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, editor. John Wiley & Sons; Inc; New York: 2002. [Google Scholar]; (c) Bartlome A, Stampfli U, Neuschwander M. Chimia. 1991;45:346. [Google Scholar]
- 18.Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;36:3769. [Google Scholar]
- 19.For the synthesis of oligoenynes via the Pd-catalyzed aklynylation through the use of ICH=CHBr involving formation of two or more C–C bonds without isolation-purification, see: Negishi E, Alimardanov A, Xu C. Org Lett. 2000;2:65. doi: 10.1021/ol990336h.Ghasemi H, Antunes LM, Organ MG. Org Lett. 2004;6:2913. doi: 10.1021/ol0489853.
- 20.(a) Shi J, Zeng X, Negishi E. Org Lett. 2003;5:1825. doi: 10.1021/ol030017x. [DOI] [PubMed] [Google Scholar]; (b) Negishi E, Shi J, Zeng X. Tetrahedron. 2005;61:9886. [Google Scholar]
- 21.For related syntheses of conjugated diynes, see: Bryant-Friedrich A, Neidlein R. Synthesis. 1995:1506.Shen W, Thomas SA. Org Lett. 2000;2:2857. doi: 10.1021/ol006282p.
- 22.A recent synthesis of 10 from PhC≡CH and HOCH2C≡CCH2OH was achieved in 17% overall yield over 7 steps.9c
- 23.Anastasia L, Negishi E. Org Lett. 2002;3:3111. doi: 10.1021/ol010145q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.