Abstract
Differential gene expression is facilitated by transcriptional regulatory mechanisms and chromatin modifications through DNA–protein interactions. One of the widely used assays to study this is chromatin immunoprecipitation (ChIP) assay, which enables analysis of association of regulatory molecules to specific promoters and histone modifications in vivo. This is of immense value as ChIP assays can provide glimpse of the regulatory mechanisms involved in gene expression in vivo. This article outlines the general strategies and protocols to study ChIP assays in differential recruitment of transcriptional factors (TFs) and also global analysis of transcription factor recruitment is discussed. Further, the applications of ChIP assays for discovering novel genes that are dependent on specific transcription factors were addressed.
Keywords: ChIP, dapk1, IFN- γ, C/EBP- β, Transcription factor activity, Regulation of gene expression, DNA–protein interactions, Transcription factor recruitment
1. Introduction
A myriad of biological processes like gene transcription, DNA replication and recombination, DNA repair, chromosome segregation, chromosomal stability, cell cycle progression, epigenetic silencing, and regulation of gene expression are mediated through protein–DNA interactions. Thus, it is of principal importance to understand the significance of these interactions in driving a biological response.
Techniques commonly used to characterize protein–DNA interactions include electrophoretic mobility shift assay (EMSA) (1), DNAse footprinting (2) and chromatin immunoprecipitation (ChIP) assays. Though, EMSA is a rapid and sensitive method to detect protein–DNA interactions, despite its advantages it has limitations like the samples are not in chemical equilibrium during the electrophoresis step and also many complexes are significantly more stable in the gel than at free solution. ChIP assays minimize these limitations and provide an efficient tool to determine these protein–DNA interactions occurring in vivo, by immunoprecipitating chromatin with specific antibodies. These assays provide unbiased observations into the chromatin changes occurring in response to extracellular signals or during differentiation and development (3, 4) . Although transcription factor recruitment to the promoter is very dynamic, ChIP assays provide an efficient means to study these events.
The ChIP assay has been adopted as a powerful method for the analysis of proteins interacting within a native chromatin environment and is versatile enough for adaptation for a variety of purposes (5, 6) . This assay has been utilized in yeast (7), drosophila (8), tetrahymena (9), Caenorhabditis elegans (10), various mammalian cell lines, and even on whole mouse embryos (11, 12) for the analysis of low abundance transcription factor binding. In addition to focused study of a single or group of genes, some groups used it for systematic promoter cloning (13, 14) and identification of gene targets using promoter microarrays (7, 15) . Also, ChIP has been used to determine the allele-specific transcription factor binding patterns (16) and to measure long-range enhancer binding of specific proteins (17) . Additionally, in combination with other techniques, ChIP assays are used to uncover an extraordinarily rich and dynamic chromatin environment (13, 18, 19) . Finally, ChIP assay systems can provide useful end-point measurement for delineation of components within the signal transduction pathways (20) .
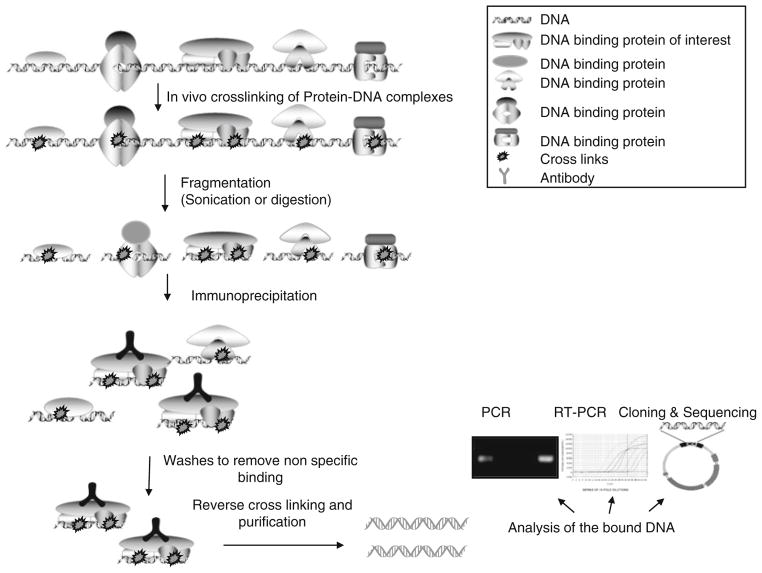
ChIP assay is a multistep process (Fig. 1) and each step needs to be standardized for obtaining optimum results. In this technique, intact cells are treated with formaldehyde to covalently link protein to DNA (X-ChIP), the nucleoprotein complexes are then sheared either mechanically or by enzymatic digestion. The resultant soluble cross-linked DNA–protein complexes enriched by immunoprecipitation (21) . The retrieved complexes are then analyzed by PCR amplification with gene-specific primers to detect and quantify specific DNA targets. The main drawback of ChIP is its inherent variability. This may arise due to variabilities in the cross-linking, immunoprecipitation, and protein–DNA washing efficiencies. Stringent control over experimental conditions may reduce these variabilities. Other limitations include the dependence on the availability of a highly specific antibody and the need for relatively high level as well as broad expression DNA-binding protein of interest. Endogenous ChIP, i.e., without overexpressing these factors, poses additional challenges, including the difficulty of handling large population if the protein of interest is expressed at a low level.
Fig. 1.
Schematic representation of the steps involved in chromatin immunoprecipitation (ChIP) assay.
Our group exploited ChIP methodology for fine mapping of transcription factor-binding regions to regulatory elements of a cell death regulatory gene, DAPK1, under control of IFN- γ. The region of DNA containing the full potential for specific transcription factor can be mutated to identify the elements present. Loss or enhancement of transcription factor association with active elements within the DNA can be quantified. Mutations can be generated in specific regulatory elements within the DNA regions which prevent transcription factor binding and the mechanism of gene regulation can be defined. Gain or loss of function experiments for these transcription factors can also be quantified using a ChIP assay. For example, transcription factors can be overexpressed or/its expression can be knocked down by RNAi or/knocked out to determine the role of individual factors in the regulation of specific element (22–24) . Also, ChIP assay can be used to isolate specific transcription factor gene targets thus identifying both consensus and nonconsensus-binding sites (14) . Here, we outline in detail general strategies and an optimized protocols for ChIP (for adherent and suspension cells as the chromatin source) along with troubleshooting guides.
2. Materials
2.1. Cell Culture
Mouse embryonic fibroblasts (MEFs) were grown in Dulbecco’s modified Eagle’s medium (GIBCO) supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic agent.
Mouse IFN- γ(PBL) eliquoted into small volumes and stored at —20°C till use.
2.2. Stock Solutions and Reagents
Phosphate-buffered saline (PBS): 10× PBS stock is prepared by adding 1.37 M NaCl, 27 mM KCl, 100 mM Na 2 HPO4, 18 mM KH2 PO4 (pH should be adjusted to 7.4 with HCl if necessary) and is autoclaved before storing at room temperature. Working solution is prepared by diluting one part with nine parts water.
Phenylmethylsulphonylfluoride (PMSF): 1 mM PMSF is prepared by dissolving in isopropanol.
Protease inhibitor cocktail: Diluted in water to make 100× stock and frozen in aliquots. Caution: Toxic!!! Should be handled using gloves.
RIPA lysis buffer: 20 mM Tris pH 7.4, 1% NP-40, 75 mM NaCl, 1 mM EDTA, 25 mM βglycerophosphate, 10% glycerol. Lysis buffer is supplemented with 1 mM PMSF, 1 mM Na3 VO4, and 1× protease inhibitor cocktail just before use.
ChIP dilution buffer: 0.01% SDS, 1.1%Triton X-100, 1.2 mM EDTA, 16.7 mM Tris–HCl, pH 8.1, 167 mM NaCl. ChIP dilution buffer is supplemented with 1 mM PMSF, 1 mM Na3 VO4, and 1× protease inhibitor cocktail just before using.
C/EBP βantibody (Santa Cruz Biotech) or other specific antibody.
Rabbit IgG (Sigma) serves as a negative control. In case the antibody used for ChIP is a monoclonal, it is recommended to use an isotype control antibody.
Protein G magnetic beads (Active Motif).
Proteinase K diluted to 20 mg/ml in water and frozen (—20 °C) in aliquots of 20 ml.
Low salt immune complex wash buffer: 0.1% SDS, 1%Triton X-100, 2 mM EDTA, 20 mM Tris–HCl, pH 8.1, 150 mM NaCl. This buffer is stored at 4°C till use and vortexed briefly before use.
High salt immune complex wash buffer: 0.1% SDS, 1%Triton X-100, 2 mM EDTA, 20 mM Tris–HCl, pH 8.1, 500 mM NaCl. This buffer is stored at 4 °C till use and vortexed briefly before use.
LiCl Immune complex wash buffer: 0.25 M LiCl, 1% IGEPALCA630, 1% deoxycholic acid, 1 mM EDTA, 10 mM Tris, pH 8.1. This buffer is stored at 4 °C till use.
TE Buffer: 10 mM Tris–HCl, 1 mM EDTA, pH 8.0. This buffer is stored at 4 °C till use.
Elution buffer: Prepared just before eluting by combining 1% SDS, 0.1 M NaHCO3 at room temperature.
PCR purification kit (QIAGEN) or other similar kits.
Oligonucleotide primers: User specific. The lyophilized primers are dissolved in 10 mM Tris–Cl (pH 8.0) to prepare a 100 μM stock.
IDTaq™ DNA polymerase Kit (ID Labs) or other similar reagents.
SYBR Green Jump start Taq Ready Mix (Sigma) or other similar reagents.
2.3. Equipment
Humidified 37°C incubator.
Table top centrifuge.
Rotating wheel/platform maintained at 4°C and 20°C.
Shaking incubator.
Branson’s sonicator fitted with microtip (or equivalent).
Teflon cell scrapers (Fisher).
Timer.
Variable volume (5–1,000 μl) pipettes and tips.
Magnetic stand (Ambion).
3. Methods
In order to establish the ChIP protocol using an antibody against a protein of interest, three critical steps have to be optimized: (1) fixation time, (2) amount of input material, and (3) amount of antibody used. For the optimization step, it is helpful if there is a known target gene for the DNA-binding protein of interest. This serves as a positive control.
Optimization of chemical cross-linking time (fixation time). One of the critical steps in ChIP is to capture the DNA–protein interactions within a cellular context, which is achieved using a chemical cross-linking agent, such as formaldehyde (5, 25) . The extent of cross-linking is the most important parameter. An excess of cross-linking can result in the reduced antigen availability in chromatin for the antibody. The relative sensitivity of the antigen epitopes to formaldehyde also should be taken into consideration. This step may be problematic, as the extent of cross-linking may vary from sample to sample. The cross-linking time needs to be empirically determined when setting up ChIP for the first time. Because the handling of materials varies significantly among different users, we suggest each user to optimize the cross-linking time. Since the extent of crosslinking affects IP by a given antibody, the length of cross-linking must be empirically optimized for each antibody initially.
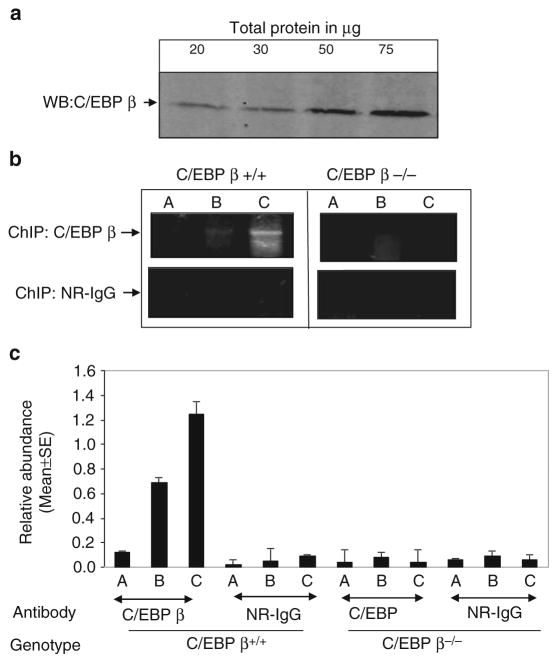
Optimization of the amount of input material . As DNA-binding proteins are expressed over a wide range in various tissues, the amount of protein of interest available for IP varies widely. For example, for highly expressed proteins, the amount of total protein required for immunoprecipitation will be significantly lower than that of a low abundance protein. Therefore, ChIP must be optimized for the amount of input material (total protein) required for IP using each antibody against the protein of interest. In addition, before beginning to do any ChIP experiment, it is important to determine the expression level or pattern of the protein of interest using western blotting or in situ (Fig. 2). Also in some cases less amount of protein may interact with DNA, although the protein may be widely expressing. In such cases, more ChIP-ed DNA may be required for the detection.
Optimization of amount of the antibody used for ChIP . The cross-linked DNA–protein complex is immunoprecipitated using a specific antibody against the protein of interest. The quality and specificity of the antibody contributes immensely to the outcome of the ChIP procedure (26) . As antibody preparations differ considerably in specificity and titer, the amount of antibody used to immunoprecipitate the cross-linked protein–DNA complex needs to be empirically determined. Different antibodies should be used to optimize the ChIP assay because certain antibodies may efficiently immunoprecipitate the native protein but may not be effective in immunoprecipitating proteins cross-linked to chromatin. Also the specificity of antibody can be checked by Western blot to ensure that it detects the correct size product. An antibody that detects multiple nonspecific bands is not recommended for ChIP. We optimized an antibody against the C/EBP β(Santa Cruz Biotechnology Catalog # sc-150) a rabbit polyclonal antibody for our assays. Before performing ChIP, we ensured its specificity by a Western blot analysis.
Fig. 2.
(a) Western blot analysis to determine the expression levels of the C/EBP βprotein in wildtype MEFs. The indicated amounts of the total protein was resolved on 10% SDS-PAGE and transferred to PVDF membrane. The membrane was blocked with 5% nonfat dry milk powder and probed with 1:1,000 dilution of the Ab (in 5% milk) for 3 h. Following three washes (10 min each) with 1× TBST, the membrane was incubated in 1:5,000 diluted anti-rabbit secondary Ab (GE) for 1 h. The membrane was washed three times with 1× TBST (10 min each) and Super Signal West Pico detection system (Pierce Biotechnology) was used to visualize the signal (1 min exposure time). The bands highlight the expression level of the protein in MEFS cells. (b) Optimization of the input amount for chromatin immunoprecipitation (ChIP). An aliquot of (a) 0.5 × 107, (b) 1 × 107, and (c) 2 × 107 million MEFS cells were cross-linked and sonicated. Cell lysate was immunoprecipitated with 5 μg of C/EBP βor NR-IgG antibody. After reverse cross-linking DNA is purified and 5 μl of it is subjected to PCR with specific primers for 30 cycles. The binding of C/EBP βor NR-IgG to the dapk1 promoter was used to evaluate the efficiency of ChIP. (c) Real-time PCR analysis of dapk1 promoter fragments recovered in ChIP assays performed with the indicated antibodies using chromatin prepared from wild type and C/EBP β−/− MEFs. Each bar represents the mean abundance of dapk1 promoter fragments ± SE of six separate reactions from two independent experiments.
Experimental controls and repeats
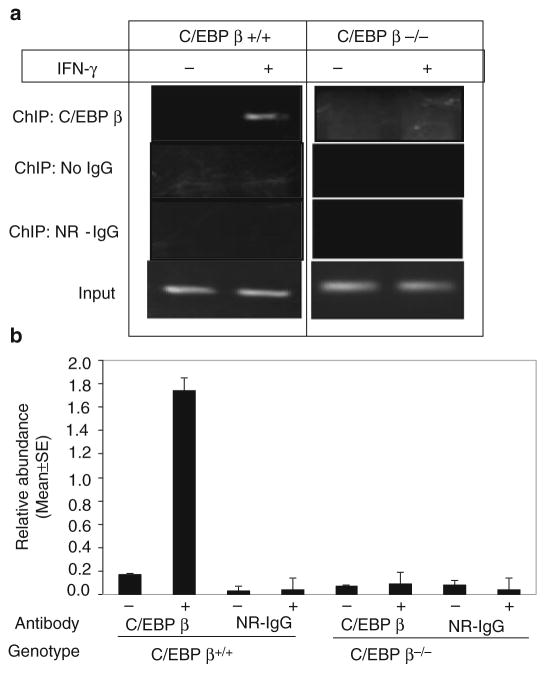
As with any experiment, proper controls are absolutely necessary for correct interpretation of data obtained from ChIP experiments. Positive controls are difficult to obtain, as the number of experimentally defined direct protein–DNA interactions currently available is limited. At least one gene that is known to interact with the transcription factor of interest must be used as a positive control, when available. However, good negative controls lead to proper interpretation of the results. The knock out cell lines of the protein of interest provides specific advantage in ChIP, thus enabling comparison of the degree of target DNA enrichment in biological samples expressing or lacking the protein of interest (Fig. 3). However, such cell lines should be verified for complete lack of the protein of interest, as a partially functional protein (such as a truncated product) may still bind DNA and lead to misinterpretation of the results. Alternatively, RNA i may be used to knock down the gene, although care should be taken to judge the efficacy of the RNA i . ChIP is a long protocol involving multiple steps. As such, it is important to have multiple technical as well as biological repeats. Technical repeats (triplicates within each experiment to assess the variability due to sample handling) can be generated by performing three separate immunoprecipitation reactions and detections after the cells have been sonicated. The specificity of ChIP reactions should further be ascertained by pre-incubating with the antibody with recombinant antigen or peptide. The following protocol is based on our studies on transcription factor C/EBP- β and its transcriptional co-activator Med1 on gene regulation.
Fig. 3.
(a) PCR analysis to compare the target dapk1 promoter fragment enrichment for C/EBP β in C/EBP β+/+ and C/EBP β−/− MEFs with or without IFN- γ treatment (16 h, 500 U/ ml). (b) Real-time PCR analysis of dapk1 promoter fragments recovered in ChIP assays performed with the indicated antibodies with or without IFN- γ treatment (16 h, 500 U/ml) using chromatin prepared from wild type and C/EBP β− /− MEFs. Each bar represents the mean abundance of dapk1 promoter fragments ± SE of six separate reactions from two independent experiments.
3.1. In Situ Cross- Linking and Harvesting
MEFs (2 × 107) were grown in confluence and stimulated or treated with mouse IFN γor appropriate inducing agent for conditions, where transcriptional activation of the gene of interest has been demonstrated (see Note 1).
Proteins were cross-linked to DNA by adding 1% formaldehyde (270 μl of 37% (W/V) formaldehyde) directly to cells growing in 10 ml of growth medium on plate and incubating for 15 min at 37°C (see Note 2) (27) . For cross-linking suspended cells, see Subheading 3.7, step 1 .
The cross-linking reaction is quenched by adding glycine to a final concentration of 0.125 M (141 ml of 1 M glycine per 1 ml of medium) and incubating for 5 min at 37°C (see Note 3).
The medium in the plates is aspirated as completely as possible.
The cells were scraped and collected by centrifugation (2,000 × g for 5 min at 4°C), then washed twice with cold 1× PBS containing protease inhibitors (1 mM PMSF, and 1× protease inhibitor cocktail).
At this stage, cell pellets can be lysed or stored at–80°C up to 1 year.
3.2. Lysis (Must Be Performed on Ice or at 4°C)
The cell pellet is lysed in 200 μl of RIPA buffer containing protease inhibitors (and phosphatase inhibitors if needed). The pellet should be suspended by pipetting up and down several times in a microcentrifuge tube (there will be a lot of insoluble material) (see Note 4).
The lysate (resuspended cell pellet) is incubated on ice or at 4°C for 15 min and followed with sonication.
3.3. Chromatin Fragmentation
The lysate is sonicated to shear the chromatin to 300 and 1,000 base pairs in length keeping the samples ice cold.
Our experience with most of the cell lines indicate that chromatin is sheared to the appropriate length with six cycles of 20 s pulses at 30% of maximum power with intermittent cooling on ice for 30 s using Branson’s sonicator equipped with a 2 mm tip (see Note 5). However, sonication settings should be determined empirically for each cell type and antibody. (Note: Sonication generates heat. Excessive heat denatures the antigen, which significantly diminishes the detection of chromatin fragments by some antibodies).
Digestion with micrococcal nuclease can be done as an alternative, to cleave the chromatin into nucleosomes. This method does not involve cross-linking step and is referred to as “native chromatin immunoprecipitation.” Native ChIP cannot be adopted for these studies as nucleosomes are dynamic and without cross-linking there is chance of their rearrangement during the enzymatic digestion (see Note 6).
The sonicated lysates were cleared by centrifuging at 5,700 μg in microcentrifuge for 10 min at 4°C. The supernatants were transferred to new tubes and the pellet was discarded.
An aliquot of sheared chromatin (equivalent to 0.2 million cells) is transferred to a new microcentrifuge tube and DNA is recovered to determine shearing efficiency.
If conditions for sonication are optimized, the lysates can be directly aliquoted for immunoprecipitation or can be stored at —80°C for months.
3.4. Immunoprecipitation
-
Following immunoprecipitation reactions were set up with the equal quantities of the lysates:
No antibody.
Nonreactive antibody–rabbit IgG (fraction from the same species in which the specific antibodies were produced).
-
Specific antibody–C/EBP βantibody.
A portion of the supernatant (20% of the sample) is labeled as input and stored to further quantitate the amount of DNA present. If multiple antibodies are to be used with the same DNA preparation, a single mock IP is sufficient as a control for all the antibodies used (see Note 7) .
The above samples were diluted ten times with ChIP dilution buffer and 5 μg of specific/mock antibody per reaction was added. The mixture was rotated overnight at 4°C by securing the caps tightly.
Preclearing ChIP lysates is optional when protein G magnetic beads were being used (for preclearing protocol see Subheading 3.7, step 2) (see Note 8) . We highly recommend the use of protein-G-magnetic beads to avoid false positive ChIPs. The chances of sample loss was minimized when Protein-G magnetic beads was used for capturing the ChIP products.
The samples were directly incubated with 30 μl of protein G magnetic beads (50% slurry) for 1 h at room temperature with rotation to collect the antibody/protein–DNA complex.
The samples with magnetic beads were collected by incubation on “Magnetic stand” for 1 min and the supernatant that contains unbound, nonspecific DNA was carefully aspirated.
The samples with beads were washed once with each of the low salt immune complex wash buffer, high salt immune complex wash buffer, LiCl immune complex wash buffer, and thrice with TE buffer, respectively. A wash consists of resuspending the beads with the specified buffer, rotating for 5 min on a rotating platform at room temperature with 1 ml of the specified buffers, pelleting the beads by incubating for 1 min on magnetic stand, and aspirating the supernatant (see Notes 9 and 10).
3.5. Reverse Crosslinking and Recovery of DNA
The protein–DNA complex from the antibody was eluted by adding 250 μl of freshly prepared elution buffer to the above beads. The beads with elution buffer were briefly vortexed and incubated at room temperature for 15 min with rotation. The magnetic beads were pelleted as described above and the supernatant fraction is transferred to new tube and one more elution is repeated. The two supernatants (eluates) were pooled (total volume 500 μl). If the recovered DNA has to be proceeded with cloning see Subheading 3.7, step 3 .
The pooled eluates and the previously stored input were now reverse cross-linked by incubating at 65°C for 4 h/overnight after adding 20 μl of 5 M NaCl (see Note 11).
To the reverse-cross-linked samples 10 μl of 0.5 M EDTA, 20 μl of 1 M Tris–HCl, pH 6.5, and 2 μl of 10 mg/ml proteinase K were added and incubated for 1 h at 45°C. The samples should be handled carefully as some DNA may be lost in subsequent purification steps.
DNA in the reverse-cross-linked samples was recovered by column chromatography using Qiagen Qiaquick PCR purification kit. The input and the recovered DNA were recovered with 250 and 50 μl of 10 mM Tris–HCl, pH 8.5, respectively. At this stage, samples are ready for PCR analyses.
If heteromeric interaction between different transcription factors binding to a native promoter has to be analyzed, see Note 12.
At this step, the samples can be directly amplified to detect the bound DNA or stored for 1 month at —20°C.
3.6. Analysis of the Bound DNA
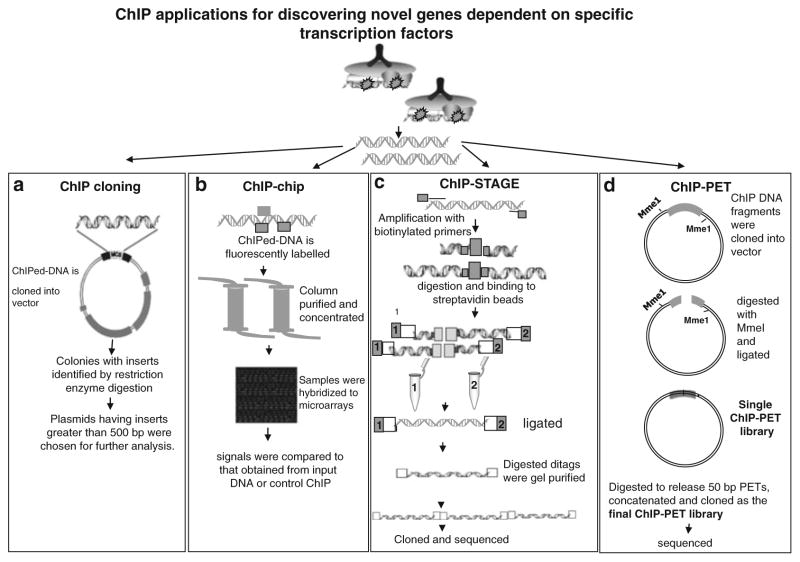
General strategies of some commonly used techniques for analyzing transcription factor activity were discussed here, including ChIP applications for identifying specific transcription factors (Fig. 4). The detailed protocols for these analyses were out of the realm of this chapter.
Fig. 4.
ChIP applications for discovering novel genes dependent on specific transcription factors (a) ChIP cloning: ChIPed DNA was ligated and cloned into vector. Colonies having inserts were identified by restriction enzyme digestion using enzymes in the polylinker. Plasmids with inserts greater than 500 bp were studied further. (b) ChIP-chip: The enriched DNA sample is fluorescently labeled, column purified and concentrated. The concentrated samples were hybridized to microarray and signal was compared to that obtained from input DNA or control ChIP. (c) ChIP-STAGE: High-throughput sequencing of defined length concatamerized ditags were involved in the STAGE technique. The ChIP DNA was amplified randomly by using biotinylated primers. Amplified DNA fragments were digested and isolated by binding to streptavidin beads. They were ligated separately to one of the two linkers. DNA with linkers were digested and ligated to create 21 bp ditags. Ditags were gel-purified concatamerized, cloned, and sequenced. (d) ChIP-PET: ChIP DNA was cloned into vector, which contains two MmeI recognition sites. The ligations were transformed and digested with MmeI and ligated with T4 DNA polymerase. The resulting vector containing a signature tag from each terminal of the ChIP DNA insert was self-ligated and then transformed to form the “single-PET” library. The plasmids from this library were digested to release 50 bp PETs, which were concatenated into long fragments (1–2 kb) and cloned as the final ChIP-PET library for sequencing.
PCR: The recovered DNA is amplified by PCR using specific primers to the sequence of interest to detect the bound DNA. At least three nonoverlapping primer pairs of about 150 to 200 bp apart for each region of interest should be designed (see Note 13). This also helps for an identification of “real positive” reactions. For PCR, the input (starting material), no antibody, nonreactive antibody, and specific antibody ChIPed DNA is included. The amount of sample used per reaction must be determined empirically. In general, 5 μl of ChIPed DNA and 1 μl of the input DNA would be sufficient for PCR. PCR conditions vary according to the primers used; generally, 30–35 cycles of amplification will produce in visible bands on agarose gels. The PCR products were resolved on an agarose gel and visualized with ethidium bromide. For analysis, see Note 12.
Real-time-PCR (RT-PCR): For RT-PCR, we used SYBR Green Master Mix with 15 μl reactions in 96-well plates on STRATAGENE (default three-step method, 40 cycles) machine. The relative occupancy of the immunoprecipitated factor at a locus is estimated using the following equation 2^(Ct MOCK–Ct SPECIFIC), where Ct MOCK and Ct SPECIFIC are mean threshold cycles of PCR done in triplicate on DNA samples from mock and specific immunoprecipitations (28) (see Note 14).
ChIP cloning: For analysis of the bound DNA by cloning, immunoprecipitated DNA was ligated and cloned into the vector. Colonies having inserts were identified by restriction enzyme digestion using enzymes in the polylinker or by PCR amplification with specific primers. Plasmids having inserts were studied further (14, 29) .The putative sites identified can be checked with ChIP assay by synthesizing primers. In spite of some drawbacks with this technique (see Note 15), the main advantage of this protocol lies in that it allows the isolation of known and/or unknown individual target genes.
ChIP-chip: Combination of microarray analysis with the ChIP technique led to the improvements in the identification of transcription factor–DNA interactions. After immunoprecipitation, the enriched DNA sample is fluorescently labeled. The control and enriched samples are hybridized to various microarrays and signal was compared to that obtained from input DNA or control ChIP. The two most commonly used microarray formats for the analysis of ChIP-chip samples are PCR product arrays and oligonucleotide arrays. Ideally, to achieve a comprehensive and unbiased glimpse of protein DNA interactions, the DNA microarrays used in ChIP-chip assays should contain elements that represent the entire genome. The in vivo nature of ChIP-chip allows observations into changes in protein–DNA interaction during cellular processes and in response to stimuli (30–33) .
ChIP-STAGE (for sequence tag analysis of genomic enrichment): Advances in DNA sequencing led to the application of high-throughput DNA sequencing methods to the analysis of protein–DNA interactions. By increasing the number of fragments sequenced, the amount of DNA sampled was increased further enhancing the identification of the enriched DNA fragments. This approach was used to study the regulatory networks of the transcription factors (34, 35) .
ChIP-PET (For paired-end ditag): In the ChIP-PET method, chromatin bound by DNA-binding proteins is enriched as in ChIP-chip. The enriched DNA sequences are cloned randomly into a plasmid vector, where the insert is flanked by type II restriction enzyme MmeI sites. These plasmids are digested with the restriction enzyme, which cleaves 18 bp into the cloned genomic DNA fragment. The single-stranded plasmid ends are blunt-ended and ligated to form short ditag signature sequences within the plasmid, combining 18 bp from the 5 ′ end and 18 bp from the 3 ′ end of the ChIP DNA fragment. Together, the two halves of the ditag signature sequence represent the full length of the ChIP fragment originally present. The ditags derived from all enriched ChIP DNA fragments are then isolated, concatenated, and cloned into a second plasmid, creating the ChIP-PET library. This library is then extensively sequenced, and the sequences resulting from the enriched ChIP DNA fragments were mapped back to the genome and thus the interacting sites were determined (36, 37) .
3.7. Support Protocols for ChIP Assay
-
In vivo cross-linking and harvesting for suspension cells:
In the fume hood, formaldehyde (37% stock) was added directly to tissue culture media to a final concentration of 1%.
After adding formaldehyde, the cells were incubated in fume hood on a stir plate for 10′ at RT.
The cross-linking reaction was stopped by adding powdered glycine directly to flask to a final concentration of 0.125 M continuing to rock/spin for 5′ at RT.
The cells were collected by centrifugation (2,000 × g for 5 min at 4°C), and washing twice with cold 1× PBS containing protease inhibitors (1 mM PMSF, 1× protease inhibitor cocktail).
The ChIP assay is continued as described in Subheading 3.1 from step 6 onwards.
-
Preclearing ChIP lysates:
Two micrograms of irrelevant antibody of the same species and isotype as the specific antibody, or normal serum (rabbit is preferred by some researchers) (38) is added to the ChIP lysates.
The lysates were incubated for 1 h on ice.
Twenty microliters of protein G magnetic bead slurry was added to the lysates.
The samples were incubated for 30 min at 4°C with gentle agitation.
The samples with magnetic beads were incubated for 1 min on magnetic stand and the precleared supernatant is carefully collected. (f) The ChIP assay is continued as described in Subheading 3.4 from step 14 onwards.
-
ChIP DNA recovery for cloning:
The protein–DNA complex from the antibody was eluted by adding 30 μl of freshly prepared elution buffer to the above beads.
The beads with elution buffer were briefly vortexed and incubated at room temperature for 15 min with rotation.
The magnetic beads were pelleted as discussed earlier and the supernatant fraction is transferred to new tube.
The eluate was then diluted with ChIP dilution buffer to 300 μl and proceeded once more with immunoprecipitation as described in Subheading 3.4 .
Table 1.
Troubleshooting guide for frequent problems encountered with ChIP assays
| Problem | Possible reason | Solution |
|---|---|---|
| Lower specific IP/ mock IP signal ratio | Antibody is not binding to chromatin due to:
|
|
| Lower PCR signal |
|
|
| No PCR signal |
|
|
Acknowledgments
This work is supported by NIH grants CA78282 and CA105005 to D.V.K.
Footnotes
The number of cells to be started with has to be determined exponentially and has to be optimized as discussed above. Cell cultures should be healthy and not density arrested prior to cross-linking. In general, we use 2 × 107 cells per antibody per ChIP (fewer cells, as low as 2 × 106 cells, can be used but may result in lower signal to noise ratio).
The cross-linking time and formaldehyde concentration can affect both the efficiency of chromatin shearing and the efficiency of precipitating a specific antigen. Shorter cross-linking times (<10 min), lower formaldehyde concentrations or both may improve shearing efficiency; however, for some proteins, especially those that do not directly bind DNA, this might reduce the efficiency of cross-linking and thus the yield of precipitated chromatin. Another point of concern is the loss of antigen epitopes due to denaturation. Formaldehyde is a moderately denaturating agent for proteins and is known to interfere with the secondary and, in particular, tertiary structures, resulting in the unfolding of the protein of interest. The general sensitivity of proteins to formaldehyde in vivo has to be determined empirically.
Also, cross-linking for longer periods than 30 min may cause cells to form aggregates that cannot be sonicated efficiently. Quenching with glycine is more critical while working with suspended cells when compared to adherent cells.
We employ RIPA buffer to resuspend the cell pellet as it produces less foam during sonication over conventional SDS lysis buffer. The cell material will be very viscous at this stage owing to the release of DNA, and thus will be hard to pipette. The viscosity of the material will decrease after sonication.
Sonication conditions must be determined empirically for each cell or tissue type, and sonicator model; optimal average DNA fragment sizes are 0.3–1 kb. Heating of the sample during sonication, is supposed to denature the proteins so the sample should be held on ice. Foaming should be avoided as it decreases sonication efficiency. Dipping the probe all the way to the bottom avoids foaming. Also 1.5 ml-microcentrifuge tube with conical bottom should be used for sonication as it increases sonication efficiency when compared to 2 ml round bottom ones. Larger volumes for sonication can decrease sonication efficiency. So it is desirable to maintain lower volumes. In our hands, 300–500 μl worked well.
The protocol for cell lysis and nuclei preparation in native ChIP assays is described elsewhere (39–41) .
The amount of antibody added should be in excess of the factor being precipitated, and thus it should be determined empirically for each factor and/or antibody. The incubation time vary from 1 to 12 h and should be determined empirically with each antibody.
Preclearing ChIP lysates is a good method to reduce nonspecific backgrounds and eliminate false positives during the assay. The end result will be a lowering of background and an improved signal-to-noise ratio. While it may not be necessary to preclear in all cases, it should be carried out if nonspecific background bands are observed in PCR.
Efficient washing is critical to reduce background. The beads should be washed each time with at least 1 ml of the buffer. Transferring the beads to a new tube after the first wash helps to reduce nonspecific bands. The supernatant should be aspirated at each time by placing the tubes on magnetic stand. Care should be taken not to lose beads during the wash steps. If more noise is encountered in negative controls in PCR, then the stringency and/or the number of washings has to be increased.
For eliminating false positives during the assay in addition to washings, if two separate (with distinct specificities) antibodies are available for a specific transcription factor, one could ChIP the chromatin with the first antibody. The first round products are re-ChIPed with a second antibody.
Reverse cross-linking step is critical as there is chance of sample leakage. The tube caps should be punched and protected by wrapping with parafilm. At this step, the sample can be stored at —20°C and the protocol can be continued next day.
To detect the heteromeric interactions between two different transcription factors binding to an element at the native promoter, those two ChIP reactions are first precipitated with an antibody against factor 1. The immunoprecipitated products from this step are then subjected to ChIP with an antibody against factor 2. Immunoprecipitation reactions with individual factor-specific antibodies and negative control antibodies should be performed to demonstrate the specificity of these reactions. If ChIP reactions with individual antibodies and 2-step ChIP yield similar level of PCR products (as measured by real time PCR), it suggests that two different transcription factors bind to the same promoter elements as heteromer.
ChIP PCR primers should be initially tested on genomic DNA for amplification. To achieve high resolution, PCR product size should not be too big usually the amplicon size should be in between 150 and 250 bp. The relative occupancy of a factor at a locus is estimated as the ratio of the intensity of the specific IP band to that of the mock IP band in gel electrophoresis.
For real-time-PCR, it is critical that each primer pair yields only one amplicon, which can be determined by analyzing the amplicon dissociation plot, which should yield a single peak. The PCR may then be loaded on a 1% agarose gel to visualize whether a single amplicon is being amplified. Also, Ct values >35 are disregarded and more concentrated DNA should be used in a reanalysis, if necessary. The input sample should ideally be in the 20–30 Ct range and should be adjusted (i.e., diluted) accordingly.
In ChIP cloning to decrease the amount of nonspecific DNA, the ChIPed DNA sample may be re-immunoprecipitated, which usually results in a lower amount of precipitated DNA . Also, the size of DNA fragments is critical for ChIP cloning. To reduce the number of nonspecific clones, the chromatin should be sonicated to 1–2 kb fragments and only clones containing DNA fragments of 500 bp or larger should be analyzed further .
One should be extremely careful and meticulous throughout this assay. The PCR amplification is highly sensitive. All possible precautions should be taken to prevent contamination from other DNA sources. Amplification in a dedicated space, use of fresh gloves, sterile filter tips, properly calibrated pipettes and clean tubes are highly recommended for this assay.
The success of ChIP can be determined from several perspectives and is an art rather than a technique. Sample handling should be appropriate and uniform. For trouble-shooting, see Table 1 .
References
- 1.Fried MG. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis. 1989;10:366–76. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- 2.Christy RJ, et al. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3- L1 preadipocytes. Proc Natl Acad Sci USA. 1991;88:2593–7. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillai S, Dasgupta P, Chellappan SP. Chromatin immunoprecipitation assays: analyzing transcription factor binding and histone modifications in vivo. Methods Mol Biol. 2009;523:323–39. doi: 10.1007/978-1-59745-190-1_22. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y, Chen H, Costa M. Chromatin immunoprecipitation assays. Methods Mol Biol. 2004;287:9–19. doi: 10.1385/1-59259-828-5:009. [DOI] [PubMed] [Google Scholar]
- 5.Nowak DE, Tian B, Brasier AR. Two-step cross-linking method for identification of NF-kappaB gene network by chromatin immunoprecipitation. Biotechniques. 2005;39:715–25. doi: 10.2144/000112014. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta P, Chellappan SP. Chromatin immunoprecipitation assays: molecular analysis of chromatin modification and gene regulation. Methods Mol Biol. 2007;383:135–52. doi: 10.1007/978-1-59745-335-6_9. [DOI] [PubMed] [Google Scholar]
- 7.Kurdistani SK, Grunstein M. In vivo protein-protein and protein-DNA cross-linking for genomewide binding microarray. Methods. 2003;31:90–5. doi: 10.1016/s1046-2023(03)00092-6. [DOI] [PubMed] [Google Scholar]
- 8.Breiling A, et al. General transcription factors bind promoters repressed by Polycomb group proteins. Nature. 2001;412:651–5. doi: 10.1038/35088090. [DOI] [PubMed] [Google Scholar]
- 9.Dedon PC, et al. A simplified formaldehyde fixation and immunoprecipitation technique for studying protein-DNA interactions. Anal Biochem. 1991;197:83–90. doi: 10.1016/0003-2697(91)90359-2. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay A, et al. Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat Prot. 2008;3:698–709. doi: 10.1038/nprot.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan MQ, et al. Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cell Biol. 2004;24:9248–61. doi: 10.1128/MCB.24.20.9248-9261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botquin V, et al. New POU dimer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by Oct-4 and Sox-2. Genes Dev. 1998;12:2073–90. doi: 10.1101/gad.12.13.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinmann AS, Farnham PJ. Identification of unknown target genes of human transcription factors using chromatin immunoprecipitation. Methods. 2002;26:37–47. doi: 10.1016/S1046-2023(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 14.Weinmann AS, et al. Use of chromatin immunoprecipitation to clone novel E2F target promoters. Mol Cell Biol. 2001;21:6820–32. doi: 10.1128/MCB.21.20.6820-6832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martone R, et al. Distribution of NF-kappaB-binding sites across human chromosome 22. Proc Natl Acad Sci USA. 2003;100:12247–52. doi: 10.1073/pnas.2135255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heckman CA, Boxer LM. Allele-specific analysis of transcription factors binding to promoter regions. Methods. 2002;26:19–26. doi: 10.1016/S1046-2023(02)00004-X. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KD, Bresnick EH. Dissecting long-range transcriptional mechanisms by chromatin immunoprecipitation. Methods. 2002;26:27–36. doi: 10.1016/S1046-2023(02)00005-1. [DOI] [PubMed] [Google Scholar]
- 18.Cosma MP, Tanaka T, Nasmyth K. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 19.Metivier R, et al. Transcriptional complexes engaged by apo-estrogen receptor-alpha isoforms have divergent outcomes. EMBO J. 2004;23:3653–66. doi: 10.1038/sj.emboj.7600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flanagin S, et al. Microplate-based chromatin immunoprecipitation method, Matrix ChIP: a platform to study signaling of complex genomic events. Nucleic Acids Res. 2008;36:e17. doi: 10.1093/nar/gkn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahl JA, Collas P. Q2ChIP, a quick and quantitative chromatin immunoprecipitation assay, unravels epigenetic dynamics of developmentally regulated genes in human carcinoma cells. Stem Cells. 2007;25:1037–46. doi: 10.1634/stemcells.2006-0430. [DOI] [PubMed] [Google Scholar]
- 22.Gade P, et al. Critical role for transcription factor C/EBP-beta in regulating the expression of death-associated protein kinase 1. Mol Cell Biol. 2008;28:2528–48. doi: 10.1128/MCB.00784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, et al. The Med1 subunit of transcriptional mediator plays a central role in regulating CCAAT/enhancer-binding protein-beta-driven transcription in response to interferon-gamma. J Biol Chem. 2008;283:13077–86. doi: 10.1074/jbc.M800604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gade P, et al. Down-regulation of the transcriptional mediator subunit Med1 contributes to the loss of expression of metastasis-associated dapk1 in human cancers and cancer cells. Int J Cancer. 2009;125:1566–74. doi: 10.1002/ijc.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlando V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem Sci. 2000;25:99–104. doi: 10.1016/s0968-0004(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen J, et al. Assessment of sera for chromatin-immunoprecipitation. Biotechniques. 2008;44:66–68. doi: 10.2144/000112681. [DOI] [PubMed] [Google Scholar]
- 27.Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods. 2002;26:48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JD, et al. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34:e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hug BA, et al. A chromatin immunoprecipitation screen reveals protein kinase Cbeta as a direct RUNX1 target gene. J Biol Chem. 2004;279:825–30. doi: 10.1074/jbc.M309524200. [DOI] [PubMed] [Google Scholar]
- 30.Hartman SE, et al. Global changes in STAT target selection and transcription regulation upon interferon treatments. Genes Dev. 2005;19:2953–68. doi: 10.1101/gad.1371305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odom DT, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–81. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Euskirchen G, et al. CREB binds to multiple loci on human chromosome 22. Mol Cell Biol. 2004;24:3804–14. doi: 10.1128/MCB.24.9.3804-3814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cawley S, et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004;116:499–509. doi: 10.1016/s0092-8674(04)00127-8. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, et al. Mapping DNA-protein interactions in large genomes by sequence tag analysis of genomic enrichment. Nat Methods. 2005;2:47–53. doi: 10.1038/nmeth726. [DOI] [PubMed] [Google Scholar]
- 35.Impey S, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–54. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 36.Wei CL, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 38.Bonifacino JS, Dell’Angelica EC. Immunoprecipitation. Curr Protoc Cell Biol. 2001;Chapter 7(Unit 7):2. doi: 10.1002/0471143030.cb0702s00. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill LP, Turner BM. Immunoprecipitation of native chromatin: NChIP. Methods. 2003;31:76–82. doi: 10.1016/s1046-2023(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 40.Thorne AW, Myers FA, Hebbes TR. Native chromatin immunoprecipitation. Methods Mol Biol. 2004;287:21–44. doi: 10.1385/1-59259-828-5:021. [DOI] [PubMed] [Google Scholar]
- 41.Hebbes TR, et al. Core histone hyper-acetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 1994;13:1823–30. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]