Abstract
There has been a sharp rise in allergic asthma and asthma-related deaths in the developed world; in contrast to many childhood illnesses that have been reduced or eliminated. The hygiene hypothesis proposes that excessively sanitary conditions early in life result in autoimmune and allergic phenomena because of a failure of the immune system to receive proper microbial stimulation during development. We demonstrate that antibodies generated against conserved bacterial polysaccharides are reactive with and dampen the immune response against chitin and Aspergillus fumigatus. A reduction in antigen uptake, cell influx, cell-activation, and cytokine production occurred in the presence of anti-polysaccharide antibodies, resulting in a striking decrease in the severity of allergic airway disease in mice. Overall, our results suggest that antigen exposure during the neonatal period, derived from environmental sources, self-antigens, or vaccination, have dramatic effects on the adult antibody response and modify the development of allergic airway disease.
Introduction
The incidence of asthma, an increasingly significant public health issue with a clear association with immune allergies, is more prevalent in Western-style societies. The hygiene hypothesis attributes this increase to reduced stimulation of the immune system by microorganisms, due in part to the increased sanitary conditions early in life (1). Perinatal and early childhood periods are considered critical for development of a normal Th1/Th2 balance of effector CD4 T cells and it is thought that the absence of appropriate microbial exposure during this period leads to a shift from a Th1 to a Th2 CD4 T cell cytokine profile. This shift is accompanied by increased allergic phenomena, including production of allergen-specific IgE antibodies that exacerbate asthma pathology. However, the similar rise in autoimmune diseases during this period cannot be explained through the Th1:Th2 paradigm (2). In addition, identification of specific infectious agents or assessment of the underlying immunological mechanisms responsible for these increases have yielded conflicting results (3). We propose an adjunct hypothesis that antibodies may contribute to the mechanism of protection proposed by the hygiene hypothesis.
Allergens involved in asthma and other allergic diseases are a highly diverse group of molecules; it is becoming increasingly clear that their ability to induce allergies resides in their presentation as “cargo” associated with innate immune-activating components (4, 5). One such immune-activating molecule that has attracted recent attention is chitin, a naturally occurring β-1,4-linked N-acetyl-glucosamine (GlcNAc) homopolymer. As the second most abundant biopolymer on earth, chitin is ubiquitously associated with a multitude of organisms implicated in human allergies including: fungi, molds, crustaceans, insects, and parasites. Furthermore, purified chitin particles exert size-dependent effects on innate and adaptive immunity, leading to the proposal that chitin and chitinases play a role in pulmonary inflammatory and allergic responses (6, 7). However, the physical nature of purified commercially available chitin used by most investigators bears little resemblance to organism-associated chitin. In its natural unpurified state, chitin is covalently linked to proteins and other glucans, as well as other organic and inorganic molecules, particularly in fungi (8). Therefore, chitin’s role in asthma and allergic diseases is best studied in the context of its naturally occurring state within the environment.
Aspergillus fumigatus, a ubiquitous pathogenic fungus and the other chitin containing organisms mentioned above have been shown to be linked to allergic airway diseases dependent on a variety of geographical and environmental associations (9, 10). A. fumigatus expresses an array of highly conserved cell wall-associated polysaccharides during its lifecycle, including chitin (7-15%), α-1,3 (35-46%) and β-1,3 glucans (20-35%) (11). There is a variety of innate receptors for these fungal cell wall polysaccharides including the mannose receptor (CD206) (12) and TLR2 (13) for chitin, dectin-1 (14, 15) and CD36 (16) for β-glucans, to name a few [extensively reviewed in (17)]. Interactions of these cell wall structures and innate receptors are involved in a wide range of inflammatory and allergic responses induced by these organisms. Interestingly, fungi share similar polysaccharide epitopes with commensal and pathogenic bacteria. For example, Enterobacter cloacae (18) (19) Streptococcus pyogenes (Group A Streptococci, GAS), and Streptococcus agalactiae (Group 1b Streptococcus, GBS1b) (20) express α-1,3 glucans, GlcNAc, and sialyllacto-N-tetraose respectively, all of which induce polysaccharide-specific antibodies following immunization/infection. We have taken all these findings together and developed an adjunct hypothesis to the prevailing idea that infections early in life may modify the Th1:Th2 balance and prevent the development of allergies/asthma. We propose that natural antibodies generated against conserved bacterial polysaccharides alter the interactions between allergen-bearing microorganisms and innate receptors in the lung microenvironment and dampen susceptibility to asthma and other allergy-associated diseases.
Throughout infancy, childhood and adolescence, the immune system is in a constant state of development and maturation and these processes are susceptible to extrinsic influences from the environment. The discovery of genes associated with asthma is in its infancy but it is unlikely that a single mechanism will be found responsible for induction of this complex disease. With allergic asthma often developing early in childhood, we propose that the highly plastic clonal B cell repertoire is altered during a critical time in B cell development by early exposure to environmental antigens. Such alterations in B cell clonal frequencies and the BCR repertoire produce long-lasting effects on adult natural antibody levels and thus, antibody-mediated protection or susceptibility to allergen-induced airway disease. In the present study, we demonstrate that antibodies generated against conserved bacterial polysaccharides are reactive with and dampen inflammatory and allergic responses to A. fumigatus after intratracheal (i.t.) challenge. Utilizing a chronic A. fumigatus i.t. sensitization protocol, we show that passive transfer of germline gene-encoded antibodies to adults or bacterial immunization of neonates inhibits the influx and activation of innate and adaptive immune cells in both the lung tissue and alveolar spaces. The inhibition of cellular influx was associated with reduced production of asthma-associated cytokines and chemokines. Furthermore, the presence of these bacteria- and fungi-reactive antibodies reduced the uptake and activation of chitin and A. fumigatus by macrophages and dendritic cells. The current studies reveal that neonatal antigen exposure induces alterations in the B cell clonal frequency and/or repertoire, which has long-lasting effects on the adult antibody repertoire and thus, provides a level of resistance to the development of A. fumigatus-induced allergic airway disease (AAD) in mice.
Experimental Procedures
Animals
C57BL/6 mice were purchased from the Jackson Laboratory and maintained under specific pathogen-free conditions using approved animal protocols from the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Preparation of fungus
Aspergillus fumigatus isolate 13073 (ATCC, Manassas, Virginia, United States) was maintained on potato dextrose agar for 5 days at 37 °C. Conidia were harvested by washing the culture flask with 50 ml of sterile PBS. Conidia were filtered through a sterile 40μm nylon membrane to remove hyphal fragments, enumerated on a hemocytometer and stored at 4°C in distilled water until use. A. fumigatus was germinated in DMEM+10% FCS at 37°C for various periods of time prior to staining and analysis by flow cytometry.
CD4 T cell cytokine staining
For determination of in vivo IL-4 production, total lung lymphocytes were isolated by collagenase digestion 24 hours after final A. fumigatus challenge. 106 lymphocytes were cultured in a 96-well plate in RPMI+ 10% FCS and Brefeldin A (BD Biosciences) for 6 hours in the presence or absence of 5 μg/ml plate-bound anti-CD3 and -CD28 (Ebiosciences). Cells were then stained with anti-CD4, fixed in Cytofix/Cytoperm (BD Biosciences) and stained for IL-4 or isotype control in Permwash (BD Biosciences).
ELISpot Analysis
The total numbers of GlcNAc-specific antibody-secreting cells (ASCs) in the spleen and bone marrow were determined as previously described in (21), using EIA/RIA plates (Costar, Corning, NY) coated with PGN-GAC overnight at 4°C at a concentration of 2 μg/ml. 10 - 15 × 106 bone marrow or splenic cells from individual C57Bl/6 mice neonatally immunized with GAS on day 3 or day 14 of life and sacrificed at 8 weeks of age were added to the first well and then diluted 5-fold in RPMI with 5% FCS to generate quantifiable spots.
Histology
Lungs were fixed in 4% PFA, dehydrated by sequentially increasing concentrations of ethanol, incubated in xylene and embedded in paraffin. Four μm sections of lung were cut, rehydrated, and stained with hematoxylin and eosin or Periodic Acid-Schiff (PAS) and Alcian blue 8GX (Sigma-Aldrich). Sections were then dehydrated, incubated in xylene, and mounted in Permount (Fisher Scientific). Tissue sections were viewed as previously described (22).
Immunofluorescence
A. fumigatus conidia were bound to poly-L-lysine coated glass slides and were incubated for 0,4, 8 and 11 hours in RPMI 1640+ 2%FCS. The slides were stained with SMB19, GAC39 and 1-21 monoclonal antibodies and viewed with a Leica/Leitz DMRB fluorescence microscope equipped with appropriate filter cubes and phase contrast.
Flow cytometry
Flow cytometry was performed as described previously (23). FITC-labeled anti-B220, anti-IgE, PE-labeled anti-Ly6G, anti-IL-4, anti-SiglecF, anti-CD44, Pacific Blue-labeled anti-CD4, anti-IFN-γ, anti-B220; APC-labeled anti-CD117, and anti-CD8 antibodies were purchased from BD Pharmingen; goat anti-mouse IgM was purchased from SBA (Birmingham, AL). FcR blocker Ab93, HGAC39 and HAC78, 1-21, and SMB19 monoclonal antibodies were generated and/or maintained in our laboratory. All FACS analyses were performed on a FACS Calibur (BD Biosciences) or LSR II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Immunization
3- and 14-day-old mice were immunized i.p. or subcutaneously with 5×107 heat-killed, pepsin treated Streptococcus pyogenes (Strain J174A). Sera were collected at 8 weeks (day 0) before re-immunization with 1×108 S. pyogenes and again on day 7 after re-immunization and stored at −80°C until use.
ELISA
GlcNAc-specific IgM and total IgE levels were determined by ELISA as previously described (24) using plates coated with rat anti-mouse IgE (SBA, Birmingham, AL) or GAC-PGN (Lee Labs) and detected with alkaline phosphatase-conjugated goat anti-mouse IgE (SBA, Birmingham, AL) or goat anti-mouse IgM (SBA, Birmingham, AL). Standard curves were prepared using known quantities of mouse IgE (Southern Biotechnology Associates) or GAC78, a GlcNAc-specific IgM purified in our laboratory. p-Nitrophenyl phosphate (Sigma-Aldrich) was added, and color development was determined on a SPECTROstar Omega reader (BMG Labtech) at 405nm.
A. fumigatus-specific ELISAs
A .fumigatus-specific antibodies in the BAL and serum were measured by coating Costar 96-well plates (Fisher Scientific) with a 1:100 dilution of A. fumigatus allergenic extract (Hollister Stier, Spokane, WA). Antibodies were detected with alkaline phosphatase-conjugated goat anti-mouse Ig (SBA, Birmingham, AL) and addition of p-Nitrophenyl phosphate (Sigma-Aldrich). Optical density was measured using a SPECTROstar Omega reader (BMG Labtech) at 405nm. Sera and BAL from naïve and PBS-treated mice were used as controls.
Reverse-Transcriptase PCR
RT-PCR was performed as previously described (21). Briefly, total RNA was isolated using TRIzol according to the manufacturer’s instructions (Invitrogen). Total RNA was quantified by NanoDrop analysis (Thermo Scientific) and cDNA was generated using Omniscript RT-PCR kit (Qiagen).
In vivo i.t. challenge, lung and BAL analysis
Mice were anesthetized using 3-5% isoflurane and 100 μg of purified chitin or 5×105 live A. fumigatus conidia were administered i.t. in a volume of 50 μl and in the presence or absence of polysaccharide-binding antibodies. Mice were immobilized on a slanted/vertical board using a suture string looped around their upper incisors, with taped tail to help support body weight, and the tongue was extended from oral cavity using blunt end forceps. Liquid (50 μl) was pipetted in the oral cavity and the nares were manually plugged using fingers to force the mouse to inhale the liquid. For an allergic airway disease model, A. fumigatus conidia were administered Mondays and Fridays for 8 consecutive weeks. After a week off this regimen mice were challenged i.t. with 5×105 conidia. One to 3 days after challenge mice were sacrificed and total BAL and lung cells were analyzed by flow cytometry, ELISA, and cytokine analysis. BAL cells were enumerated using a hemocytometer and trypan blue dye exclusion. Cells were isolated from the lung using collagenase digestion as described previously (25).
Chitin preparations
Chitin particles from crab shells (Sigma) were prepared using a protocol published previously (12). Chitin was labeled with 488 and 647 Alexa Fluorochromes according to manufacture’s instructions (Molecular Probes/Invitrogen Life Technologies). Chitin contained <0.1 U/ml of endotoxin as determined by Etoxate (Sigma), a Limulus lysate assay with a detection level of 0.1 U/ml.
Anti-A. fumigatus antibodies-
All anti-α-1,3 glucan hybridomas were generated in our laboratory (18, 19). Hybridomas producing SMB-19 (26), HGAC78 (27), and HGAC39 (28) have been previously described. IgG3 antibody was purified from the supernatant by absorption to protein G-Sepharose affinity columns from Amersham Biosciences and IgM antibodies were purified on Sepharose-6B columns coupled with RS3.1 anti-IgMa Mab. Purified antibodies were labeled with Alexa fluorochromes according to instructions (Molecular Probes/Invitrogen Life Technologies). Antibodies were determined to have <0.1 U/ml of endotoxin activity using Etoxate (Sigma). The “Antibody Combo” contains HGAC78, SMB-19 and an anti-α-1,3 glucan IgM.
BAL Cytokine Analysis
The first milliliter of fluid extracted from the BAL of sacrificed mice was used to measure the levels of 23 cytokine and chemokine proteins using the Bio-Plex Multiplex Suspension Cytokine Array (Bio-Rad Laboratories) according to the manufacturer’s instructions. The data were analyzed using Bio-Plex Manager software (Bio-Rad Laboratories).
In vitro cell cultures
Bone marrow derived macrophages (BMDM) were generated as described previously (29). A459 epithelial cells or BMDMs were seeded at 500,000 cell/well in 6 well plates in DMEM+10% FCS, and 100μM 2-ME. The cells were incubated with either unlabeled or Alexa 647-labeled chitin, or Alexa 488-labeled A. fumigatus in the presence or absence of 10μg/ml A. fumigatus-binding antibodies or isotype controls for 2-24 hours at 37°C. In A. fumigatus, B. anthracis dual uptake studies, 106 Alexa 647-labeled B. anthracis spores (Sterne strain) were added to cultures containing 5×105 Alexa 488-labeled A. fumigatus conidia. Uptake of spores and conidia were analyzed by flow cytometry or rt-PCR.
Statistics
Statistical comparisons were performed using Prism 4.0 software (GraphPad). Data with three or more groups were analyzed by a one-way ANOVA test followed by post hoc analysis, while data with two groups were analyzed by a two-tailed unpaired t test to determine whether overall statistically significant differences existed. Statistically significant results were described by a p value of *<0.05, ** <0.01, ***<0.001.
Results
Antibodies generated against conserved bacterial polysaccharides bind chitin and A. fumigatus
Monoclonal antibodies (mAbs) mostly of IgM, IgG3 and IgA isotypes were isolated from mice immunized with E. cloacae (18, 19), S. pyogenes (30), and S. agalactiae (26). Since immunization of mice with these bacteria generates large amounts of serum antibodies against their respective cell wall polysaccharides (PS), we investigated whether antibodies generated by bacterial immunization bound fungi. Two different represenative IgM and IgG3 anti-GlcNAc mAbs generated from GAS immunized mice bound purified chitin particles, <10μm in size (Figure 1A). In addition, live resting or 18 hr germinated A. fumigatus conidia were stained with anti-sialyllacto-N-tetraose (SMB19), anti-GlcNAc (GAC39), and anti-α-1,3 glucan (1-21) but not by an isotype control anti-human myelomonocytic antibody (MMA), (Figure 1B). Furthermore, the expression of these antigens increased with time of germination and became maximal during the hyphal stage (Figure 1C). These studies show that multiple mAbs generated against commensal and pathogenic bacteria also react with ubiquitous cell wall components of A. fumigatus and other fungi (data not shown).
Figure 1. Monoclonal antibodies generated against conserved bacterial polysaccharides bind chitin and A. fumigatus.
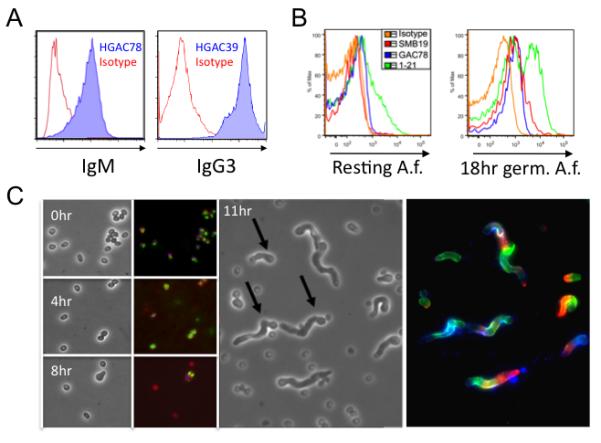
(A) Purified chitin particles (<10μm) were incubated with anti-GlcNAc IgM (HGAC78, blue), IgG3 (HGAC39, blue), or isotype control (Isotype, red) antibodies and analyzed by flow cytometry. (B) Live resting or 18 hr germinated A. fumigatus conidia were incubated with an IgM isotype control (Isotype, orange), anti-sialyllacto-N-tetraose (SMB19-IgM, red), anti-GlcNAc (GAC39-IgG3,blue), or anti-α-1,3 glucan (1-21-IgM, green) antibodies and analyzed by flow cytometry. (C) A. fumigatus conidia bound poly-L-lysine coated glass slides were incubated for 0, 4, 8, and 11 hrs in RPMI1640 + 2% FCS. Columns 1 and 3 show a field under phase contrast with germinated conidia (arrow), while columns 2 and 4 shows staining with SMB19 (red), GAC39 (blue), and 1-21 (green).
Anti-GlcNAc antibodies dampen chitin-induced cellular recruitment and activation in the lung
As a proof of principle, we first examined whether bacteria-induced chitin-reactive antibodies could alter the immune response to defined chitin particles in the lung. Purified chitin particles have been shown to induce the recruitment of asthma/allergy-associated cell types (6). Administration of IgM anti-GlcNAc antibody, via either the i.t. or i.v. route, inhibited chitin-induced recruitment of neutrophils into the bronchial alveolar lavage fluid (BAL) by > 50% when compared to the isotype control antibody (Figure 2B, gating scheme is shown in Figures 2A and S1). To investigate potential mechanisms through which the anti-GlcNAc antibody inhibits cellular recruitment, cytokine levels in BAL were measured 24 hours after chitin administration. The production of the proinflammatory cytokines KC, Rantes, MIP-1α, and TNFα,, was inhibited by ~50% after anti-GlcNAc administration (Figure 2C). These cytokines and chemokines are produced by various cell types in the lung including: vascular endothelium, epithelial cells, macrophages, dendritic cells, and T lymphocytes. Cells and mRNA were obtained from lung tissue digests and, similar to our findings in the BAL, the presence of anti-GlcNAc antibody partially inhibited the recruitment of all cell types into the lung tissue including neutrophils and CD4 T cells (Figure 2D). Additionally, reduced mRNA transcript levels of CCL17 and CCL22 were observed in the lungs in the presence of anti-GlcNAc antibodies (Figure 2E). Taken together, these data show that the presence of anti-GlcNAc antibody greatly reduces chitin-induced inflammation and influx of cells into the lung tissue and alveolar spaces, thus dampening the overall immune response to chitin.
Figure 2. Anti-GlcNAc antibody dampens chitin-induced cellular recruitment and activation in the lung.
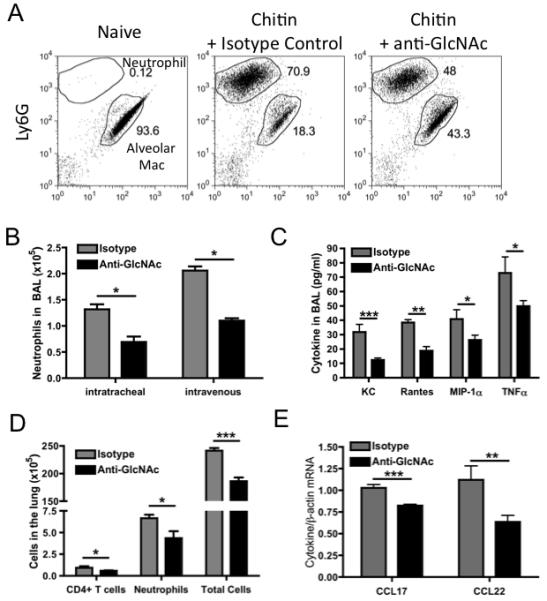
C57BL/6 mice, treated i.v. with 5μg IgM isotype control or anti-GlcNAc antibodies (i.v. and i.t. in B), were challenged i.t. with 100μg of chitin particles. BAL was collected 24 hours after chitin challenge and the total numbers of neutrophils (Ly6G+) and alveolar macrophages (determined by their autofluorescence) (gating scheme shown in A) were enumerated by flow cytometry (B). Cytokine expression was measured by a Bio-plex cytokine array (C). Cells collected from collagenase-treated lungs and total cell numbers, neutrophils, and CD4+ T cells were calculated (D). Total RNA was collected from lung tissue-derived cells and analyzed by RT-PCR for CCL17 and CCL22 expression (E). Values represent the mean ± SEM from 3 independent experiments with 3-5 mice per group. Data were analyzed by a two-tailed unpaired t test. * = p<0.05, ** = p<0.01, *** = p<0.001 See also Figure S1
Anti-GlcNAc antibodies decrease the uptake of chitin particles in vitro and in vivo
While the data in the previous figures clearly demonstrate that anti-GlcNAc antibody binds chitin and dampens the immune response to lung-administered chitin, the mechanism by which this occurs is unclear. To determine whether anti-GlcNAc antibodies were affecting the binding or cellular uptake of chitin, Alexa 647-labeled chitin was used to quantify the number of chitin positive cells in an uptake assay (gating scheme shown in Figure 3A). Bone marrow derived macrophages (BMDM) and A549 epithelial cells were cultured with Alexa 647-labeled chitin, in the presence or absence of an IgM isotype control or anti-GlcNAc antibody, and cellular binding and uptake of A. fumigatus was measured by flow cytometry. The anti-GlcNAc antibody decreased the uptake of chitin particles by both BMDM (Figure 3B) and A549 cells (Figure 3C). We next treated C57BL/6 mice intratracheally with an IgM isotype control or anti-GlcNAc antibody and subsequently challenged i.t. with Alexa 647-labeled chitin particles. BAL was collected at 0, 1, 2, and 3 hours. Similar to our the findings in vitro, alveolar macrophages (Figure 3D) and dendritic cells (Figure 3E) bound or internalized less chitin in the presence of anti-GlcNAc antibody, as shown by flow cytometry. Taken together, the data show that anti-GlcNAc antibody decreases the uptake of chitin particles both in vitro and in vivo, suggesting that it interferes with the cellular recognition mechanisims for chitin.
Figure 3. Anti-GlcNAc antibody decreases the uptake of chitin particles in vitro and in vivo.
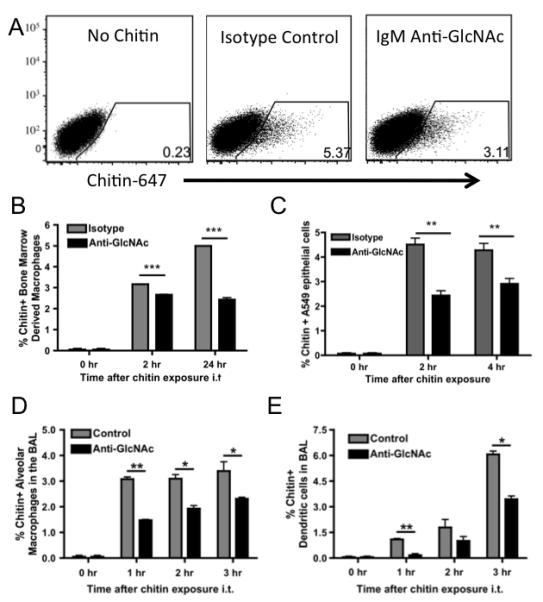
(A) Representative gating scheme for determining chitin positive cells. (B) Bone marrow derived macrophages (BMDM) and (C) A549 epithelial cells were cultured with 100μg of Alexa 647-labeled chitin, in the presence or absence of an IgM isotype control or anti-GlcNAc antibody, and chitin uptake was determined by flow cytometry. C57BL/6 mice, treated i.t. with an IgM isotype control or anti-GlcNAc antibodies, were challenged i.t. with 100μg of Alexa 647-labeled chitin particles and BAL was collected at 0, 1, 2, and 3 hours. Chitin positive (D) alveolar macrophages and (E) dendritic cells were detected by flow cytometry. Data represent the mean ± SEM from 3 independent experiments with 6 wells or 3-5 mice per group. Data were analyzed by a two-tailed unpaired t test. * = p<0.05, ** = p<0.01, *** = p<0.001
Anti-A. fumigatus antibodies dampen the allergic airway response induced by A. fumigatus
To further determine the effects of A. fumigatus-binding antibodies, we adapted a low dose airway sensitization model (31) using live A. fumigatus conidia, to more closely mimic the physiological process of allergen sensitization in humans. Eight-week-old mice were sensitized by i.t. instillation of a non-inflammatory, low dose A. fumigatus for 8 weeks. This dose was previously established to have no effect, as a single dose, on cell infiltration or recruitment into the lung (data not shown). Mice were then rested for one week and rechallenged i.t. with A. fumigatus (protocol depicted in Figure 4A). This regimen of chronic A. fumigatus sensitization and rechallenge reproducibly induces a strong allergic response, with increases in total BAL cells (>10-fold), eosinophils (>12-fold), neutrophils (>5-fold), mast cells (>10-fold) and total serum IgE (>4-fold) (data not shown). Using this model system, we sought to determine if anti-GlcNAc and other A. fumigatus-binding anti-polysaccharide mAbs would dampen the induction of an allergic airway response to A. fumigatus. Mice were treated with anti-GlcNAc, anti-α-1,3 glucan and anti-sialyllacto-N-tetraose antibodies singly or in combination i.v. 2 hours prior to the final i.t. challenge with A. fumigatus. The mice were sacrificed three days later and the BAL and total lung-associated leukocytes were analyzed by flow cytometry (gating scheme shown in Figure S1). Passive administration of anti-GlcNAc or the antibody combination decreased the total number of neutrophils and eosinophils present in the BAL by at least 50% (Figure 4B). We found that these antibodies also reduced the total number of neutrophils (Figure 4C), eosinophils (Figure 4C), CD8 T cells (Figure 4E), and the CD44 expression on CD4 T cells (as shown by MFI, Figure 4D) within the lung tissue by approximately 50%. Individual antibodies of both IgM and IgG3 isotypes exhibited a hierarchy of ability to protect with anti-GlcNAc and anti-sialyllacto-N-tetraose antibodies inhibiting neutrophil influx equally, while the anti-α1,3 glucan antibody was less effective (data not shown). These data clearly demonstrate the ability of these anti-PS antibodies to alter the allergic response to A. fumigatus in the lung.
Figure 4. Anti-A. fumigatus antibodies dampen the allergic airway disease induced by A. fumigatus.
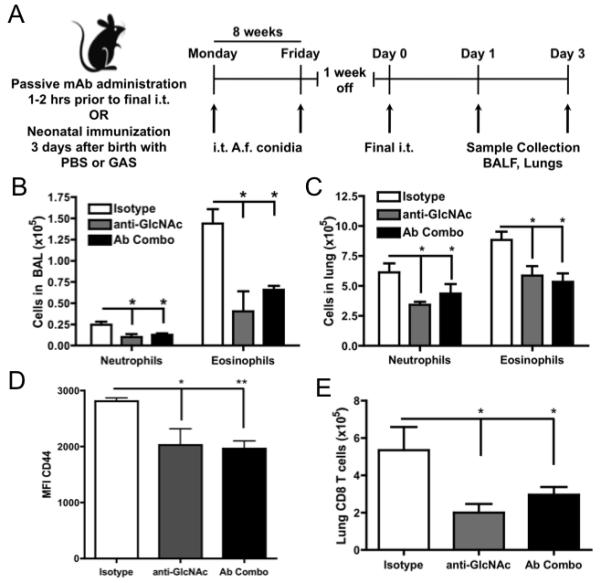
(A) C57BL/6 mice were subjected to 5×105 live A. fumigatus conidia, starting at 8 weeks of age, administered i.t. twice a week for 8 weeks. After one week without challenge, allergic airway disease was elicited using the same dose of A. fumigatus conidia, in the presence or absence of an isotype control, anti-GlcNAc antibody, or a combination of anti-GlcNAC, -sialyllactose-N-tetraose, -α-1,3 glucan), and lungs were analyzed 3 days later. (B) BAL and (C) cellular lung digests were collected and analyzed for neutrophil and eosinophil cell infiltration using flow cytometry. The level of (D) CD44 expression by CD4+ T cells and (E) total CD8+ T cells was measured by flow cytometry. Data represent the mean ± SEM from 3 independent experiments with 3-5 mice per group. Data were analyzed by a two-tailed unpaired t test. * = p<0.05, ** = p<0.01, *** = p<0.001 See also Figures S1.
Anti-A. fumigatus antibodies decrease the uptake of A. fumigatus in vitro and in vivo
The previous figures clearly show that anti-PS antibodies bind to and dampen the immune response against A. fumigatus conidia, and our studies with purified chitin and anti-GlcNAc antibodies suggest that the antibodies can block interactions between chitin and its cellular receptors, we examined if the A. fumigatus-binding anti-PS antibodies could inhibit uptake and/or binding of A. fumigatus conidia to cells. BMDM and A549 epithelial cells were cultured with Alexa 488-labeled A. fumigatus conidia, in the presence of either anti-PS antibodies or an IgM isotype control. Binding or cellular uptake was then measured using flow cytometry (gating scheme shown in Figure 5A). BMDM and A549 bound or internalized less A. fumigatus in the presence of anti-PS antibodies at 2 and 4 hrs (Figure 5B and 5C). To determine whether this finding was specific to our antibodies binding A. fumigatus or a cell-intrinsic inhibition initiated by antigen-antibody complexes, we measured cell uptake of simultaneously administered Alexa 488-labeled A. fumigatus conidia and Alexa 647-labeled Bacillus anthracis spores in the presence of anti-PS or isotype control antibodies in BMDMs and A549 cells. Anti-PS antibodies bound and decreased the uptake of A. fumigatus while the uptake of B. anthracis was unchanged (Figure S2A and S2B). To measure A. fumigatus uptake in vivo, C57BL/6 mice were treated with either an IgM isotype control or an anti-PS antibody combination, challenged i.t. with Alexa 488-labeled A. fumigatus conidia, and BAL analyzed at 0, 2, and 4 hours. The presence of anti-PS antibodies decreased the percentage of A. fumigatus positive alveolar macrophages (Figure 5D) and dendritic cells in the BAL (Figure 5E). These results show collectively that treatment with anti-PS antibodies decreased the binding or internalization of A. fumigatus by APCs in vitro and in vivo, similar to our findings with chitin.
Figure 5. Anti-A. fumigatus antibodies decrease the uptake of A. fumigatus in vitro and in vivo.
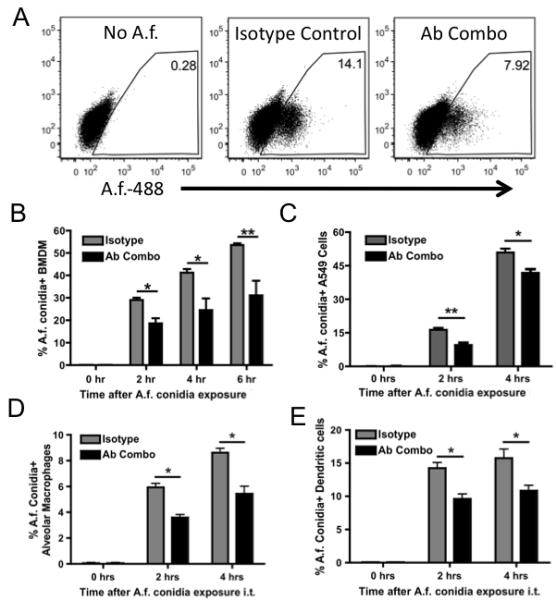
(A) Representative gating scheme for determining A. fumigatus positive cells. (B) Bone marrow derived macrophages (BMDM) and (C) A549 epithelial cells were cultured with 1×105 of Alexa 488-labeled A. fumigatus conidia, in the presence or absence of an IgM isotype control or an antibody combination (Ab combo) of anti-GlcNAC, -sialyllactose-N-tetraose, -α-1,3 glucan, and A. fumigatus uptake was measured by flow cytometry. C57BL/6 mice, treated i.t. with an IgM isotype control or antibody combo were challenged i.t. with 5×105 of Alexa 488-labeled A. fumigatus conidia and BAL was collected at 0, 2, and 4 hours. (D) A. fumigatus-positive alveolar macrophages and (E) dendritic cells were determined using flow cytometry. Data represent the mean + SEM from 3 independent experiments with 6 wells or 3-5 mice per group. Data were analyzed by a two-tailed unpaired t test. * = p<0.05, ** = p<0.01, *** = p<0.001
Neonatal GAS immunization primes the adult antibody response and dampens the immune response to chitin
We have hypothesized that the lack of microbial stimulation early in life and its effect on B cells and antibody production is partially responsible for the increased development of AAD. The previous experiments show that passive administration of purified monoclonal antibodies, by i.v. or i.t. routes, reduces the allergic inflammatory response in previously sensitized adult mice. To examine how neonatal antigen exposure can generate antibody repertoires that dampen the development of AAD, we immunized C57BL/6 mice neonatally with GAS at 3 or 14 days after birth. Serum levels of GlcNAc-specific IgM in these mice at 8 weeks of age were ~9 and 50 μg/ml respectively compared with 5 μg/ml in PBS treated mice, showing the initial neonatal immunization had a lasting effect. Upon rechallenge with GAS, these day 3 and day 14 neonatally primed animals produced a 4- and 10-fold increase, respectively in GlcNAc-specific IgM when compared to the PBS-treated animals (Figure 6A). Additionally, the levels in anti-GlcNAC IgM in the serum of day 3 immunized mice increased 310-fold over pre-immune serum levels, compared to a 100-fold increase in PBS-treated mice. ELISpot analysis demonstrated an increase in GlcNAc-specific IgM splenic ASCs in the GAS-primed mice when compared to PBS-treated mice (Figure 6B). Further analysis showed increased levels of GlcNAc-specific IgM in the BAL (Figure 6C), which was associated with a decreased influx of neutrophils in response to chitin (Figure 6D). To confirm that the priming of the anti-GlcNAc antibody response was not a phenomenon associated only with i.p. neonatal GAS immunization, we subcutaneously immunized mice with GAS at three days of age and rechallenged with GAS as adults. These subcutaneously immunized mice exhibited an increase in serum levels of anti-GlcNAc IgM similar to mice receiving GAS i.p as neonates (Figure S3A). These data show that neonatal priming with GAS induces long-lasting effects on serum levels of anti-GlcNAc antibody and, resulted in a subsequent dampening of the immune response to chitin and A. fumigatus in the lung.
Figure 6. Neonatal GAS immunization primes the adult antibody response and dampens the immune response to chitin.
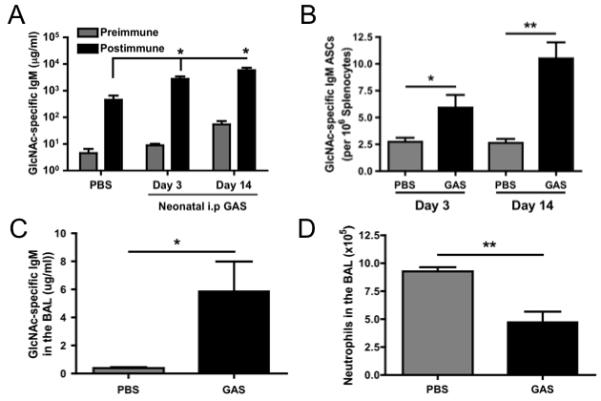
Neonatal mice were injected i.p. with PBS or GAS at 3 or 14 days of age. At 8 weeks of age, all groups were immunized i.v. with GAS and sera were collected before (preimmune) and 7 days (postimmune) after re-immunization. (A) The level of serum GlcNAc-specific IgM was determined by ELISA. (B) ELISPOT analysis was performed 7 days after the neonatal immunizations, Day 10 and Day 21 respectively, for GlcNAc-specific IgM ASCs. The post-immune mice were challenged i.t. with 100μg chitin (<10μm) 7 days following re-immunization at 8 weeks and the BAL level of GlcNAc-specific IgM (C) was determined by ELISA and total neutrophil influx (D) was determined by flow cytometry 24 hours following i.t. challenge. Data represent the mean + SEM from 3 independent experiments with 3-5 mice per group. Data were analyzed by a two-tailed unpaired t test. * = p<0.05, ** = p<0.01
Neonatal GAS immunization dampens the adult AAD induced by A. fumigatus
We next used a similar approach to determine if neonatal immunization with GAS affected the sensitization or provocation of AAD induced by chronic A. fumigatus exposure to the lungs. Mice were treated neonatally with PBS or GAS, as described above, and then subjected to the chronic A. fumigatus sensitization protocol described in Figure 4A (gating scheme for FACS analysis is shown in Figure S1). Neonatal GAS immunization resulted in the recruitment of fewer total cells, neutrophils, eosinophils, CD4+ T cells, mast cells, and IgE+ B cells in the BAL (Figures 7A and 7B). Mice receiving subcutaneous GAS immunization as neonates and subjected to the chronic A. fumigatus sensitization protocol described in Figure 4A also exhibited a decrease in the influx of immune cells into the BAL, similar to mice neonatally immunized i.p with GAS (Figure S3B-E). In contrast to the dampening of cellular influx to the BAL induced by A. fumigatus sensitization following neonatal GAS treatment, immunization of adult mice with GAS prior to an 8 week A. fumigatus sensitization protocol had no effect on the prevention of the allergic response (Supplementary Figure 4).
Figure 7. Neonatal GAS immunization dampens the adult allergic airway disease induced by A. fumigatus.
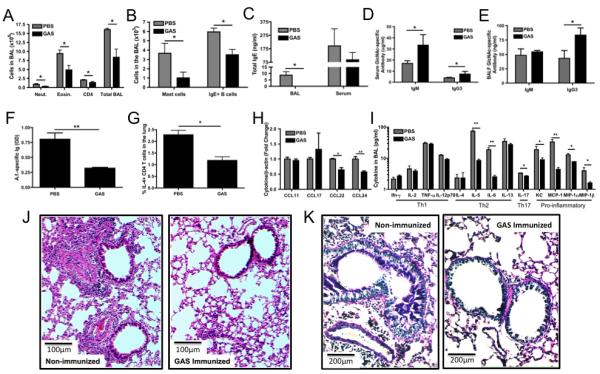
Neonatal C57BL/6 mice were immunized once i.p. with PBS or GAS at 3 days of age and grown up to 6-8 weeks before starting the chronic sensitization model outlined in Figure 4A. (A, B) BAL was collected 3 days following elicitation of the response and analyzed by flow cytometry for (A) neutrophils (neut.), eosinophils (eosin.), CD4+ T cells (CD4), total BAL cells and (B) mast cells and IgE+ B cells. Sera and BAL was analyzed for (C) total IgE, (D, E) GlcNAc-specific IgM and IgG3, and (F) A. fumigatus-specific total Ig against purified A. fumigatus allergic extract using ELISA. (G) Total lung leukocytes were stimulated with anti-CD3/CD28 in the presence of Brefeldin A and the percentage of CD4+IL-4+ cells was determined by flow cytometry. (H) Total RNA was collected from lung-derived cells and analyzed by RT-PCR for CCL11, 17, 22 and 24 expression. (I) BAL was analyzed for cytokine expression using the Bio-plex cytokine assay. Data were analyzed by an unpaired t test. * = p<0.05 See also Figures S1 and S2. Paraffin embedded lung sections from PBS and GAS immunized neonatal mice were stained with (J) hematoxylin and eosin (H&E) and (K) Alcian Blue-Periodic acid Schiff (AB-PAS). Data represent the mean ± SEM from 3 independent experiments with 3-5 mice per group.
Isolates from the digested lung tissue showed similar reductions in these cell types (data not shown). BAL and serum levels of total IgE (Figure 7C) were found to be lower in the mice immunized neonatally with GAS. While GlcNAc-specific IgG3 was higher in the BAL after the final challenge with A. fumigatus (Figure 7D, E), the decrease in GlcNAc specific IgM in neonatally immunized GAS immunized mice may be due to the depletion of IgM by A. fumigatus conidia, since GlcNAc-specific IgM prior to sensitization with A. fumigatus was higher in the BAL of mice immunized neonatally with GAS than in mice given PBS neonatally. The levels of A. fumigatus-specific total Ig in the BAL reactive with soluble A. fumigatus allergic extract containing allergens from A. fumigatus were lower in mice receiving neonatal GAS (Figure 7F). Total lung leukocytes were isolated 24 hours after final A. fumigatus administration and stimulated in vitro with anti-CD3/CD28 in the presence of Brefeldin A. The percentage of CD4+IL-4+ cells detected by flow cytometry was lower in GAS immunized mice compared to PBS treated mice (Figure 7G). Total RNA was collected from the lung tissue-derived cells and analyzed by RT-PCR for CCL11, 17, 22 and 24 expression (Figure 7H) while the BAL (Figure 7I) and sera (Figure S2D) were analyzed for cytokine expression using the Bio-Plex cytokine assay. Th1 cytokine levels were essentially unchanged, in contrast to the decreased expression of Th2, Th17, and other pro-inflammatory cytokines, showing that neonatal GAS exposure does not initiate a general immune shift from a Th2 to a Th1 response. To evaluate tissue histology and airway mucus production, paraffin embedded lung sections from mice neonatally immunized with GAS or treated with PBS after the 8-week A. fumigatus sensitization, and 24 hours after final A. fumigatus administration were stained with hematoxylin and eosin (H&E) (Figure 7J) and Alcian Blue-Periodic acid Schiff (AB-PAS) (Figure 7K). Mice sensitized and challenged, but not neonatally immunized with GAS, showed marked peribronchovascular inflammation, thickening of the basement membrane and bronchial epithelium, and increased mucus secretion. Alternatively, neonatally GAS-immunized mouse lungs showed minimal signs of remodeling. Our data show that the presence of anti-GlcNAc antibodies in the lung, induced by neonatal immunization with GAS, is associated with a striking decrease at 18 weeks in the development and severity of the AAD induced by chronic A. fumigatus exposure. As summarized in Figure 8A, we propose a model by which anti-GlcNAc and similar antibodies bind A. fumigatus conidia in the lung, preventing them from binding to receptors on resident lung cells as well as immune cells present on the lung. Blockade of these receptor:ligand interactions decreases or diverts uptake of the conidia to other pathways, resulting in a dampening of cytokine and chemokine production as well as decreasing the presentation to and priming of CD4 T cells. In this way, antibodies can modulate the development of allergic airway disease during its development.
Figure 8. Model for prevention from allergic airway disease by anti-PS antibodies.
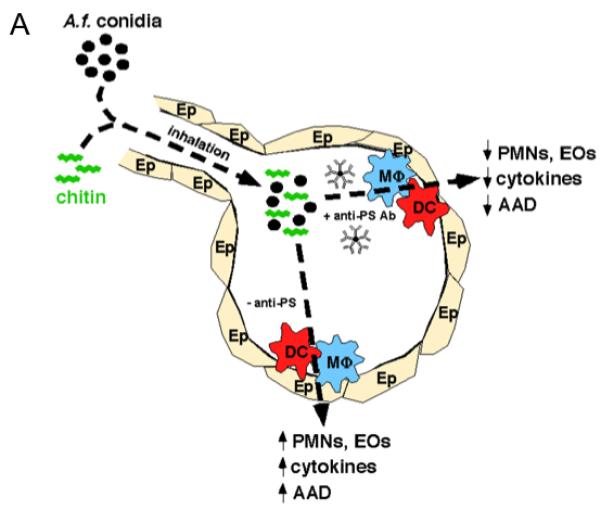
(A) This schematic shows our proposed model for the protection against A. fumigatus-induced allergic airway disease.
Discussion
There is increasing evidence that primary sensitization to environmental antigens occurs early in life, and that the perinatal and early childhood period is considered critical for the development of an appropriate Th1:Th2 balance. However, there is little information regarding how microbial exposure may influence the developing immune system. We found that a single immunization of mice, during the neonatal period, with GAS vaccine induces anti-GlcNAc antibodies that are sustained well into adulthood and protect against the local inflammatory responses induced by chitin and A. fumigatus in the lung. In addition, neonatally induced B cells secrete antibodies that decrease the allergic response to A. fumigatus. Our results suggest that antigen exposure during the neonatal period, whether derived from environmental sources, self-antigens, or vaccination, has dramatic effects on the adult antibody response and attenuates the development of AAD.
A highly significant negative correlation between GAS infections (Scarlet Fever) and asthma was found by annual, seasonal (by month) and geographic (by state) comparisons (32). Another study found that a strong inverse relationship of allergic asthma, but not atopy, was associated with concentrations of peptidoglycan-associated muramic acid in mattress dust (33). The cell wall of Gram-positive bacteria, such as GAS contain large amounts of peptidoglycan, a biopolymer consisting of alternating units of GlcNAc and N-acetylmuramic acid. Our data are in agreement with these studies as we found that neonatal immunization with GAS dampened the development and severity of AAD induced by A. fumigatus in mice. Multiple studies suggested that the timing between infection and allergen exposure is critical as to whether protection or disease ensues in humans (34). In our previous publications, we have shown that neonatal exposure to bacteria expressing phosphorylcholine and α-1,3 glucans result in permanent and distinct alterations in the heavy and light chain composition of B cells with these specificities in the adult repertoire (35). Similar changes occur in the repertoire of B cell clones involved in the anti-GlcNAc response to Group A Streptococci after neonatal GAS versus adult immunization. (Dizon and Kearney, manuscript submitted for publication). Furthermore, the timing of neonatal GAS immunization, day 3 vs. day 14, resulted in a significant difference in the baseline levels and recall responses of anti-GlcNAc antibodies. In agreement with these findings, our current results show that the timing of antigen or microbial exposure is critical in influencing the development of antibody-producing B cell clones that aid in the prevention of AAD. In contrast to the protective effect of neonatal immunization with GAS on A. fumigatus sensitization, immunization of adult mice with GAS prior to an 8-week A. fumigatus sensitization protocol had no effect on the prevention of the allergic response (Supplementary Figure 4), even though the anti-GlcNAc antibodies at the time of initiation of the sensitization protocol are similar to those described in Figure 6A, PBS group.
Numerous studies have shown that antibody responses to both T-dependent antigens and purified polysaccharides in general are poor in the neonatal period, findings partially attributed to a deficit in bone marrow stromal support of antibody secreting cells (36). However, certain B cell clones are generated only during fetal and neonatal life and remain as permanent members of the adult repertoire (37). We observed that neonatal immunization has long-lasting effects on the adult B cell antibody repertoire. These data show that systemic neonatal exposure to GAS produces a long-lived adult IgM response to GlcNAc in mice, which plays a protective role against lung sensitization to chitin and A. fumigatus. Young infants and children rarely get significant GAS infection until age 3, but it is a very frequent cause of illness in school age children. These observations correlate with experiments showing that normal GAS-uninfected children <4 yrs old in the USA have undetectable levels of specific anti-GlcNAc antibodies perhaps because the carrier rate of S. pyogenes in healthy populations is only 5%. These very low levels of GlcNAc-inhibitable, anti-Group A reactive antibody gradually increases as the children get older (38). It will be of interest to determine through retrospective analyses possible correlations between infant levels of anti-GlcNAc antibody and subsequent development of AAD. Our model has concentrated on GAS to generate anti-GlcNAc antibodies in our mouse model, however GlcNAc-containing antigens are expressed by many other organisms including Staphylococcus aureus, Escherichia coli (39), Listeria monocytogenes, (40) fungi, and helminthes. We have shown that these organisms generate GlcNAc-specific antibodies in mice raising the likelihood that infections or carriage of these and other similar microorganisms may also be associated with protection against AAD.
If such protective antibodies are produced by deliberate immunization with bacteria, why are similar antibodies not produced to the same A. fumigatus epitopes during the sensitization period? We and others have found that deliberate immunization of adult mice with chitin or A. fumigatus induces very little antibody reactive with fungal cell wall polysaccharide components ((11) and data not shown), nor in humans infected with A. fumigatus are humoral responses elicited (41, 42). These findings contrast with the comparatively high and long-lived anti-PS responses produced following neonatal immunization and re-challenge with the bacterial vaccines. This may be due to the way the A. fumigatus polymeric cell wall antigens are presented, or a lack of crucial co-stimulatory molecules similar to those associated with bacteria or immuno-inhibitory molecules produced by A. fumigatus (43, 44). In our model, mice developed antibodies against soluble allergic antigens found in the A. fumigatus allergic extract used in clinical settings. GAS-immunized mice had significantly lower antibody responses to the allergic extract than PBS-treated mice. These results suggest that the anti-GlcNAc antibodies are clearing or shielding the A. fumigatus from the immune system resulting in lower antibody responses to soluble protein antigens.
The mechanisms by which antibodies regulate biology and host defense are diverse and can include neutralization, ADCC, opsonization, and antigen presentation [(reviewed in (45)], in addition to other mechanisms that are only beginning to be appreciated (46). IgG antibodies have been studied in great detail, while aspects of the induction and role of natural IgM antibodies have been studied less extensively. One of the reasons that the functional significance of IgM is often ignored is the assumption that insufficient concentrations exist at the site of antigen exposure. B1 cells in the peritoneal and pleural cavities and MZ B cells in the spleen give rise primarily to IgM isotype secreting cells (47, 48) and recent reports show that IgM plays underappreciated protective roles in a variety of microbial infections (49-52). Although the alveolar epithelia are associated with mostly plasma-derived monomeric IgA and IgG, there is low but detectable IgM (53-55). Furthermore, a recent study suggests that IgM promotes macrophage-mediated clearance of antigen in a size dependent manner (56), specifically promoting clearance of small sized (less the 3μM) particles, which are smaller than swollen conidia and our chitin particles (4-10μM). Our results are in agreement with these findings as we observe that IgM fails to promote the uptake of chitin and A. fumigatus by macrophages, DC, and epithelial cells, actually inhibiting uptake by 25-50%. Natural IgM antibodies have been shown to play a role in mediating immunity against Pneumocystis murina (57) and our data indicate that anti-GlcNAc IgM antibodies dampen the binding or internalization of chitin and A. fumigatus. Taken together, the decreased uptake of antigen and subsequent sensitization to A. fumigatus in our chronic AAD model suggests that anti-PS IgM alters the recognition, activation, and immune response against A. fumigatus. As we show, IgG3 is consistently observed in lung fluids and sera of GAS immunized mice. Mice immunized with natural bacterial (not conjugated) carbohydrate polymers consistently produce largely IgM and to a lesser extent, IgG3 isotype and some IgA in serum. These types of T-independent antibody responses do not normally induce the other IgG isotypes that have the potential to bind via complexes to trigger well-characterized IgG Fc gamma receptors. In contrast IgG3 antibodies do not appear to bind known IgG Fc receptors (58). Although it has not yet been shown conclusively, IgG3 complexes, in contrast to other IgG isotypes, (59) may also give different signals to antigen presenting cells and in this way dampen their cytokine responses and antigen-presenting capabilities, similar to those proposed for IgM and the human IgG3 equivalent (IgG2), as discussed for other model systems (60).
Sensitization in many mouse asthma/allergy studies is achieved by using alum adjuvant-based models with highly purified allergens or surrogate sensitizing antigens administered systemically (61). However, since sensitization by allergen-bearing organisms involved in human asthma occurs via airways or skin after chronic exposure, sensitization through these portals constitutes a more relevant mouse model. Moreover, alum-based models have proven ineffective in evaluating interventional procedures to control the development of allergic disease (62, 63), which in many cases occurs over a period of time by incremental exposure to sensitizing allergens. It is clear that the immune responses in the lung to particulate conidia versus purified or recombinant A. fumigatus allergens are significantly different (64-66). A recent review chronicles the drawbacks of these models and emphasizes the need for better models that more closely mimic the development and progression of human allergic airway disease (5). Our use of a chronic low dose model for induction of AAD, using live A. fumigatus conidia without artificial adjuvants, has revealed that increased natural antibodies through neonatal vaccination elicits significant protection against pulmonary challenge with A. fumigatus.
In mice, carbohydrates and carbohydrate-modified proteins induce multiple types of innate immune responses by ligating a variety of selectins, toll-like receptors, CR3 or MAC1, mannose receptor, and dectin-1. For example, β-glucans expressed on A. fumigatus promote strong inflammatory cytokine production (67-69) and it has been proposed that α-1,3 glucans shield dectin-1 from binding to β-glucans in Histoplasma capsulatum (70). In a similar fashion, anti-PS antibodies binding A. fumigatus may shield a variety of innate receptor:ligand interactions and lower the levels of asthma-associated cytokines and chemokines produced. Our current results support this hypothesis and show that levels of IL-5, IL-6, MCP-1, MIP1α/β, IL-17, CCL17, CCL22, and CCL24, all of which play roles in AAD (71), are decreased in the presence of anti-PS antibodies. Such receptors expressed on macrophages and DCs are also involved in the clearance or intracellular fate of microorganisms (72) and antigen processing and presentation pathways (41, 73). However, there are conflicting data within the literature suggesting that chitin is associated with both enhancement (6, 10) and inhibition (74, 75) of AAD. We consider that naturally occurring chitin particles and other conserved cell wall components act as immunogenic carriers, not as allergens themselves, with the potential to bring protein allergens into cells as “cargo”. These carbohydrates may play a role in regulating the presentation of antigens/allergens to T cells (76-78). Our data support the idea that antibodies to these conserved structures play a role in diverting their allergenic “cargo” into pathways less conducive to T cell sensitization. Taken together, the results from our model show that natural antibodies from B cells generated and primed during neonatal bacterial exposure play a role in altering innate receptor:ligand interactions, subsequent inflammatory responses, and antigen processing and presentation.
The development of asthmatic allergic disease is a long and complex process, related to the development of a specific phenotype, environmental factors and genetic susceptibility. As a result, it is highly unlikely that there will be a single mechanistic explanation for the induction, maintenance or especially prevention of such diseases. Nevertheless, our data clearly show that the timing of antigen exposure during the neonatal period has long-lasting effects on the baseline natural antibody levels and recall responses as an adult. We show that the antibody responses induced by conserved bacterial PS antigens have dampening effects on the development and severity of A. fumigatus-induced AAD by down-regulating both the influx of allergen-associated cell types, and multiple cytokines and chemokines associated with AAD. The ability to drastically alter the magnitude and specificity of an antibody response, through the timing of neonatal exposure, is an important observation and may lead to vaccination or probiotic protocols during early life that will dampen the development of airway disease. Exposure of neonates to microbial stimulation may shift the Th1:Th2 cytokine balance, but it may also involve changes in the magnitude and repertoire of the responding antibodies, a property that may be exploited for therapeutic intervention.
Supplementary Material
Acknowledgements
We gratefully acknowledge Dr. Nicolas Berbari, Lisa Jia, and Di Lau (University of Alabama at Birmingham) for invaluable technical assistance and Dr. Tamer Mahmoud, Dr. Robert Flynn and J. Stewart New (University of Alabama at Birmingham) for critical reading of the manuscript.
Footnotes
This work was supported by research funds from the National Institutes of Health (NIH) Grant AI14782. J.F.K. is a recipient of a Senior Investigator Award from the American Asthma Foundation. N.W.K. is a recipient of an F32 NRSA Postdoctoral Fellowship Grant AI078662.
References
- 1.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey CD, Celedon JC. The hygiene hypothesis and asthma. Curr Opin Pulm Med. 2005;11:14–20. doi: 10.1097/01.mcp.0000145791.13714.ae. [DOI] [PubMed] [Google Scholar]
- 4.Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010;3:104–110. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 8.Muzzarelli RA. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar Drugs. 2010;8:292–312. doi: 10.3390/md8020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah A, Panjabi C. Allergic bronchopulmonary aspergillosis: a review of a disease with a worldwide distribution. J Asthma. 2002;39:273–289. doi: 10.1081/jas-120002284. [DOI] [PubMed] [Google Scholar]
- 10.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal Chitin from Asthma-Associated Home Environments Induces Eosinophilic Lung Infiltration. J Immunol. 2011 doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastebois A, Clavaud C, Aimanianda V, Latge JP. Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiol. 2009;4:583–595. doi: 10.2217/fmb.09.29. [DOI] [PubMed] [Google Scholar]
- 12.Shibata Y, Metzger WJ, Myrvik QN. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J Immunol. 1997;159:2462–2467. [PubMed] [Google Scholar]
- 13.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17 A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 15.Willment JA, Gordon S, Brown GD. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–43823. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 16.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. The Journal of experimental medicine. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney JF, McCarthy MT, Stohrer R, Benjamin WH, Jr., Briles DE. Induction of germ-line anti-alpha 1-3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol. 1985;135:3468–3472. [PubMed] [Google Scholar]
- 19.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard DG, Egan ML, Gray BM, Dillon HC., Jr. Immunochemical characterization of the polysaccharide antigens of group B streptococci. Rev Infect Dis. 1988;10(Suppl 2):S367–371. doi: 10.1093/cid/10.supplement_2.s367. [DOI] [PubMed] [Google Scholar]
- 21.Kin NW, Y Chen,, Stefanov EK, Gallo RL, Kearney JF. Cathelin-related Antimicrobial Peptide Differentially Regulates T and B cell Function(1) European journal of immunology. 2011 doi: 10.1002/eji.201141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver AM, Grimaldi JC, Howard MC, Kearney JF. Independently ligating CD38 and Fc gammaRIIB relays a dominant negative signal to B cells. Hybridoma. 1999;18:113–119. doi: 10.1089/hyb.1999.18.113. [DOI] [PubMed] [Google Scholar]
- 23.Kin NW, Crawford DM, Liu J, Behrens TW, Kearney JF. DNA microarray gene expression profile of marginal zone versus follicular B cells and idiotype positive marginal zone B cells before and after immunization with Streptococcus pneumoniae. J Immunol. 2008;180:6663–6674. doi: 10.4049/jimmunol.180.10.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kin NW, Sanders VM. CD86 Regulates IgG1 Production via a CD19-Dependent Mechanism. J Immunol. 2007;179:1516–1523. doi: 10.4049/jimmunol.179.3.1516. [DOI] [PubMed] [Google Scholar]
- 25.Swiecki MK, Lisanby MW, Shu F, Turnbough CL, Jr., Kearney JF. Monoclonal antibodies for Bacillus anthracis spore detection and functional analyses of spore germination and outgrowth. J Immunol. 2006;176:6076–6084. doi: 10.4049/jimmunol.176.10.6076. [DOI] [PubMed] [Google Scholar]
- 26.Pritchard DG, Gray BM, Egan ML. Murine monoclonal antibodies to type Ib polysaccharide of group B streptococci bind to human milk oligosaccharides. Infect Immun. 1992;60:1598–1602. doi: 10.1128/iai.60.4.1598-1602.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shikhman AR, Cunningham MW. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J Immunol. 1994;152:4375–4387. [PubMed] [Google Scholar]
- 28.Greenspan NS, Davie JM. Serologic and topographic characterization of idiotopes on murine monoclonal anti-streptococcal group A carbohydrate antibodies. J Immunol. 1985;134:1065–1072. [PubMed] [Google Scholar]
- 29.Oliva CR, Swiecki MK, Griguer CE, Lisanby MW, Bullard DC, Turnbough CL, Jr., Kearney JF. The integrin Mac-1 (CR3) mediates internalization and directs Bacillus anthracis spores into professional phagocytes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1261–1266. doi: 10.1073/pnas.0709321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahm MH, Clevinger BL, Davie JM. Monoclonal antibodies to streptococcal group A carbohydrate. I. A dominant idiotypic determinant is located on Vk. J Immunol. 1982;129:1513–1518. [PubMed] [Google Scholar]
- 31.Denis O, van den Brule S, Heymans J, Havaux X, Rochard C, Huaux F, Huygen K. Chronic intranasal administration of mould spores or extracts to unsensitized mice leads to lung allergic inflammation, hyper-reactivity and remodelling. Immunology. 2007;122:268–278. doi: 10.1111/j.1365-2567.2007.02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vargas MH. Ecological association between scarlet fever and asthma. Respir Med. 2006;100:363–366. doi: 10.1016/j.rmed.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 33.van Strien RT, Engel R, Holst O, Bufe A, Eder W, Waser M, Braun-Fahrlander C, Riedler J, Nowak D, von Mutius E, Team AS. Microbial exposure of rural school children, as assessed by levels of N-acetyl-muramic acid in mattress dust, and its association with respiratory health. J Allergy Clin Immunol. 2004;113:860–867. doi: 10.1016/j.jaci.2004.01.783. [DOI] [PubMed] [Google Scholar]
- 34.Holt PG, Sly PD. Prevention of allergic respiratory disease in infants: current aspects and future perspectives. Curr Opin Allergy Clin Immunol. 2007;7:547–555. doi: 10.1097/ACI.0b013e3282f14a17. [DOI] [PubMed] [Google Scholar]
- 35.Vakil M, Briles DE, Kearney JF. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Dev Immunol. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pihlgren M, Friedli M, Tougne C, Rochat AF, Lambert PH, Siegrist CA. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J Immunol. 2006;176:165–172. doi: 10.4049/jimmunol.176.1.165. [DOI] [PubMed] [Google Scholar]
- 37.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 38.Shackelford PG, Nelson SJ, Palma AT, Nahm MH. Human antibodies to group A streptococcal carbohydrate. Ontogeny, subclass restriction, and clonal diversity. J Immunol. 1988;140:3200–3205. [PubMed] [Google Scholar]
- 39.Cerca N, Maira-Litran T, Jefferson KK, Grout M, Goldmann DA, Pier GB. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7528–7533. doi: 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirm M, Kalmokoff M, Aubry A, Thibault P, Sandoz M, Logan SM. Flagellin from Listeria monocytogenes is glycosylated with beta-O-linked N-acetylglucosamine. J Bacteriol. 2004;186:6721–6727. doi: 10.1128/JB.186.20.6721-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-alpha and IFN-gamma expression potentials. Cellular immunology. 2003;224:114–121. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bozza S, Gaziano R, Lipford GB, Montagnoli C, Bacci A, Di Francesco P, Kurup VP, Wagner H, Romani L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002;4:1281–1290. doi: 10.1016/s1286-4579(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 43.Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, van Kooyk Y, Bozza S, Moretti S, Schwarz F, Trichot C, Aebi M, Delepierre M, Elbim C, Romani L, Latge JP. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7:e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, Sarfati J, D’Angelo C, Perruccio K, Bonifazi P, Zagarella S, Moretti S, Bistoni F, Latge JP, Romani L. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183:2407–2414. doi: 10.4049/jimmunol.0900961. [DOI] [PubMed] [Google Scholar]
- 45.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10:101–111. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 46.Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nat Immunol. 2011;13:21–28. doi: 10.1038/ni.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 48.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 49.Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dadachova E, Bryan RA, Huang X, Ortiz G, Moadel T, Casadevall A. Comparative evaluation of capsular polysaccharide-specific IgM and IgG antibodies and F(ab’)2 and Fab fragments as delivery vehicles for radioimmunotherapy of fungal infection. Clin Cancer Res. 2007;13:5629s–5635s. doi: 10.1158/1078-0432.CCR-07-0870. [DOI] [PubMed] [Google Scholar]
- 51.Dadachova E, Revskaya E, Sesay MA, Damania H, Boucher R, Sellers RS, Howell RC, Burns L, Thornton GB, Natarajan A, Mirick GR, DeNardo SJ, DeNardo GL, Casadevall A. Pre-clinical evaluation and efficacy studies of a melanin-binding IgM antibody labeled with 188Re against experimental human metastatic melanoma in nude mice. Cancer Biol Ther. 2008;7:1116–1127. doi: 10.4161/cbt.7.7.6197. [DOI] [PubMed] [Google Scholar]
- 52.Taborda CP, Casadevall A. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J Immunol. 2001;166:2100–2107. doi: 10.4049/jimmunol.166.3.2100. [DOI] [PubMed] [Google Scholar]
- 53.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. The Journal of experimental medicine. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renegar KB, Small PA, Jr., Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 55.Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. The Journal of experimental medicine. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litvack ML, Post M, Palaniyar N. IgM promotes the clearance of small particles and apoptotic microparticles by macrophages. PLoS One. 2011;6:e17223. doi: 10.1371/journal.pone.0017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. The Journal of experimental medicine. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 59.Vukovic P, Hogarth PM, Barnes N, Kaslow DC, Good MF. Immunoglobulin G3 antibodies specific for the 19-kilodalton carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein 1 transfer protection to mice deficient in Fc-gammaRI receptors. Infect Immun. 2000;68:3019–3022. doi: 10.1128/iai.68.5.3019-3022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gronwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kung TT, Jones H, Adams GK, 3rd, Umland SP, Kreutner W, Egan RW, Chapman RW, Watnick AS. Characterization of a murine model of allergic pulmonary inflammation. Int Arch Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 62.Conrad ML, Yildirim AO, Sonar SS, Kilic A, Sudowe S, Lunow M, Teich R, Renz H, Garn H. Comparison of adjuvant and adjuvant-free murine experimental asthma models. Clin Exp Allergy. 2009;39:1246–1254. doi: 10.1111/j.1365-2222.2009.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epstein MM. Do mouse models of allergic asthma mimic clinical disease? Int Arch Allergy Immunol. 2004;133:84–100. doi: 10.1159/000076131. [DOI] [PubMed] [Google Scholar]
- 64.Kurup VP, Choi H, Murali PS, Resnick A, Fink JN, Coffman RL. Role of particulate antigens of Aspergillus in murine eosinophilia. Int Arch Allergy Immunol. 1997;112:270–278. doi: 10.1159/000237465. [DOI] [PubMed] [Google Scholar]
- 65.Kurup VP, Resnick A, Kalbfleish J, Fink JN. Antibody isotype responses in Aspergillus-induced diseases. J Lab Clin Med. 1990;115:298–303. [PubMed] [Google Scholar]
- 66.Murali PS, Pathial K, Saff RH, Splaingard ML, Atluru D, Kurup VP, Fink JN. Immune responses to Aspergillus fumigatus and Pseudomonas aeruginosa antigens in cystic fibrosis and allergic bronchopulmonary aspergillosis. Chest. 1994;106:513–519. doi: 10.1378/chest.106.2.513. [DOI] [PubMed] [Google Scholar]
- 67.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattila PE, Metz AE, Rapaka RR, Bauer LD, Steele C. Dectin-1 Fc targeting of aspergillus fumigatus beta-glucans augments innate defense against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52:1171–1172. doi: 10.1128/AAC.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1366–1370. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. The Journal of clinical investigation. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168:2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 73.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 74.Chen CL, Wang YM, Liu CF, Wang JY. The effect of water-soluble chitosan on macrophage activation and the attenuation of mite allergen-induced airway inflammation. Biomaterials. 2008;29:2173–2182. doi: 10.1016/j.biomaterials.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Strong P, Clark H, Reid K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin Exp Allergy. 2002;32:1794–1800. doi: 10.1046/j.1365-2222.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 76.Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, Pamer EG. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25:665–675. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Syme RM, Spurrell JC, Amankwah EK, Green FH, Mody CH. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcgamma receptor II for presentation to T lymphocytes. Infect Immun. 2002;70:5972–5981. doi: 10.1128/IAI.70.11.5972-5981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Syme RM, Spurrell JC, Ma LL, Green FH, Mody CH. Phagocytosis and protein processing are required for presentation of Cryptococcus neoformans mitogen to T lymphocytes. Infect Immun. 2000;68:6147–6153. doi: 10.1128/iai.68.11.6147-6153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


