Abstract
Acetyl CoA carboxylase of Escherichia coli has been resolved into three functionally dissimilar proteins: (1) biotin-carboxyl carrier protein (BCCP); (2) a biotin carboxylase component that catalyzes the Mn-ATP-dependent carboxylation of BCCP to form CO2--BCCP; and (3) a transcarboxylase component that catalyzes the transfer of the carboxyl group from CO2--BCCP to acetyl CoA to form malonyl CoA.
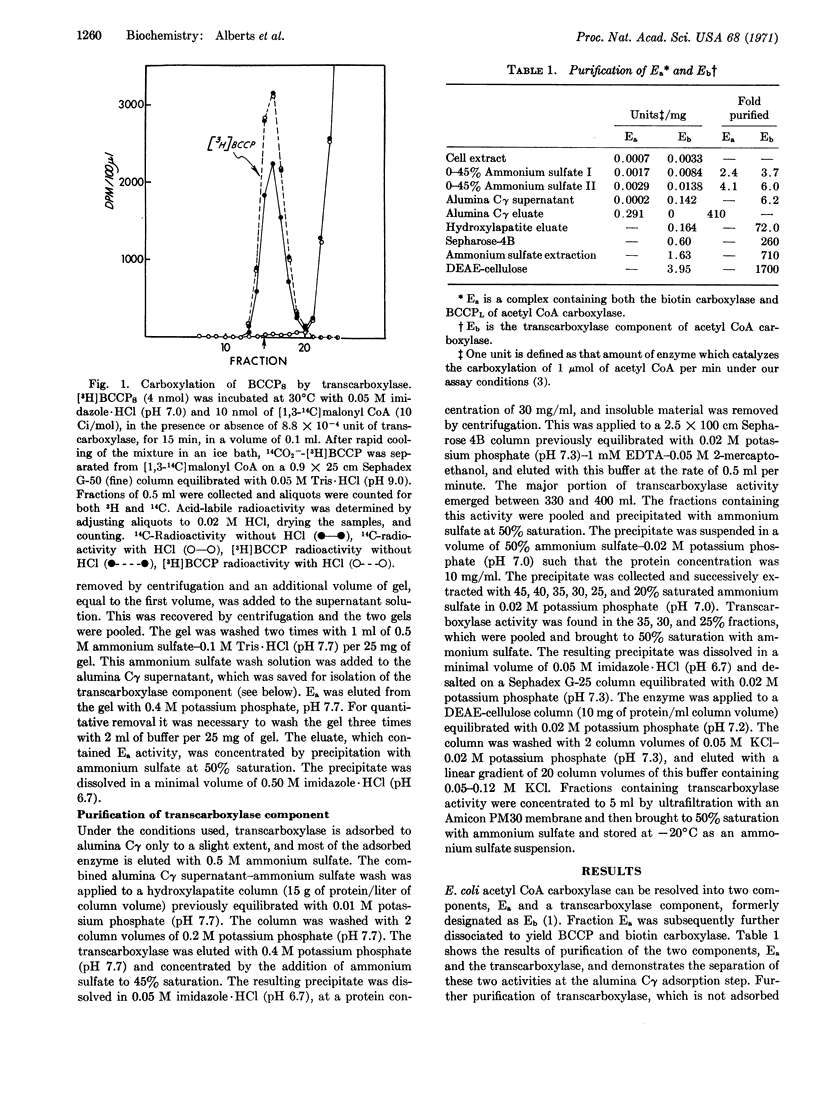
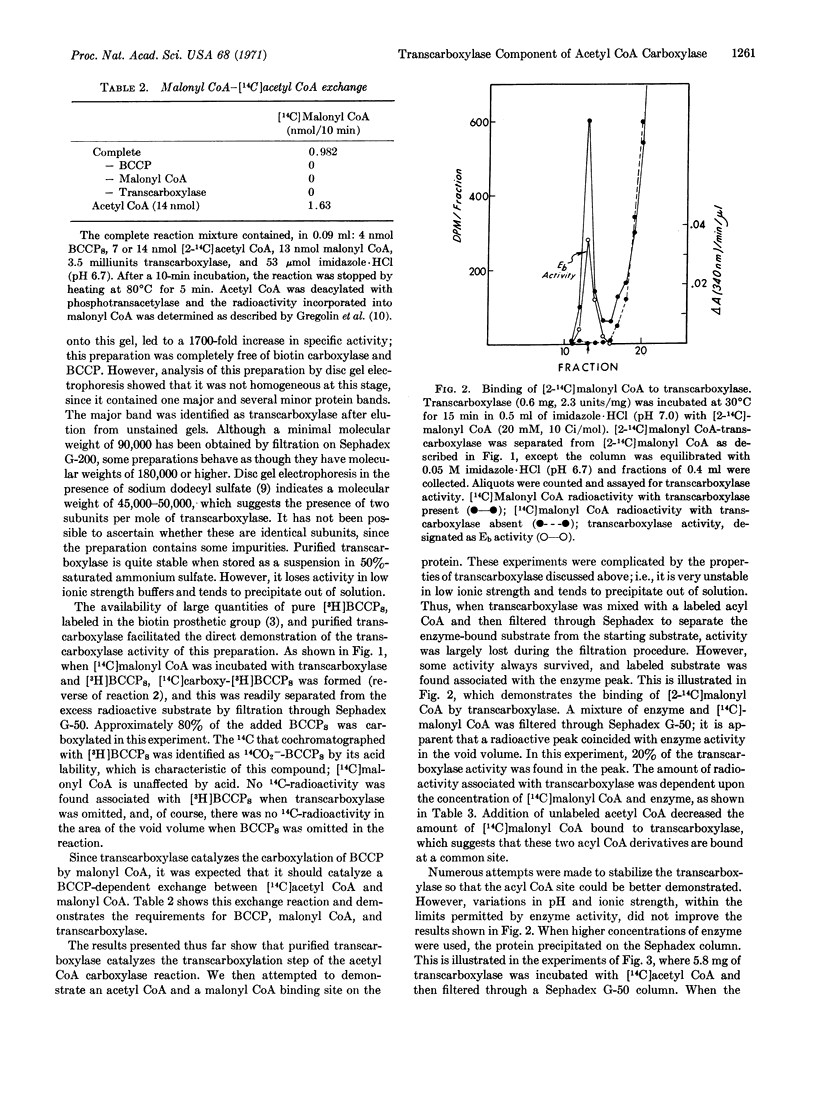
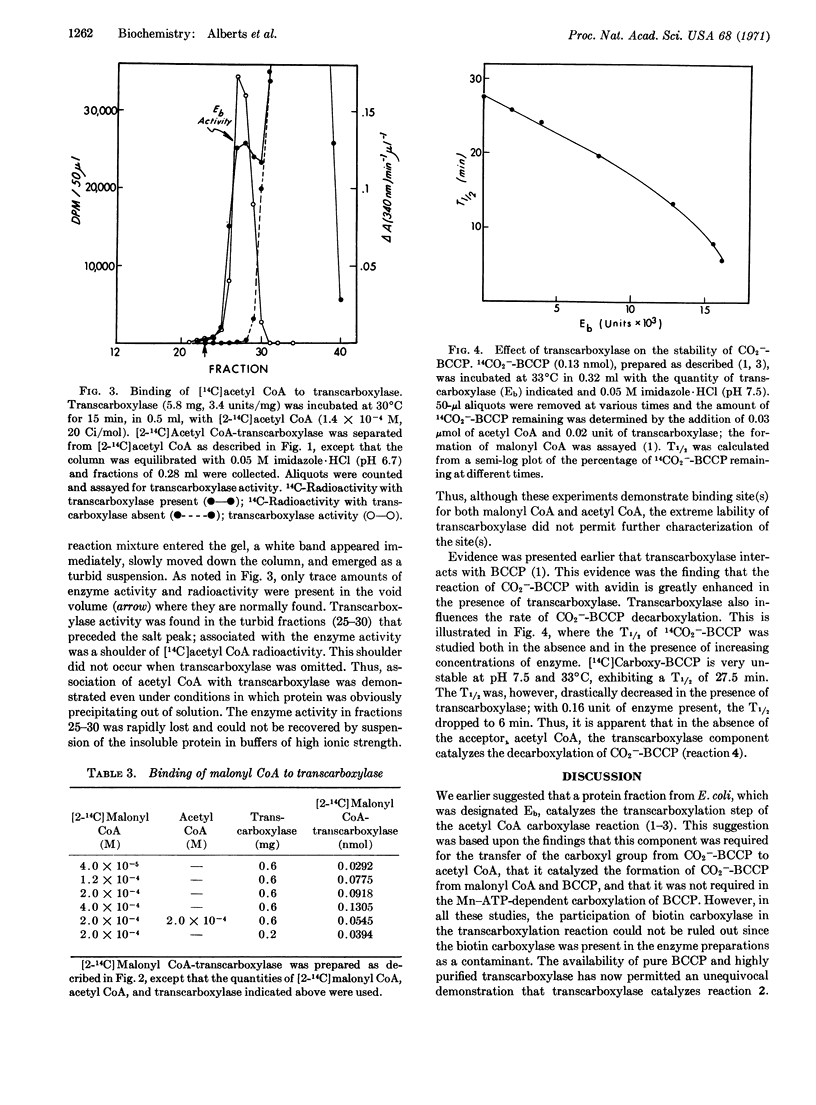
The transcarboxylase has been purified 1700-fold. Evidence that this protein catalyzes the transcarboxylase step includes the demonstration that it (a) catalyzes the carboxylation of BCCP, (b) catalyzes the BCCP-dependent exchange between [14C]acetyl CoA and malonyl CoA, (c) binds labeled acetyl CoA and malonyl CoA, and (d) catalyzes the decarboxylation of CO2--BCCP. On the basis of this evidence, it is concluded that the transcarboxylase component contains sites for the acyl CoA group and for biotin, the covalently bound prosthetic group of BCCP.
Keywords: acyl CoA binding, carboxylation, exchange reactions, biotin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Nervi A. M., Vagelos P. R. Acetyl CoA carboxylase, II. Deomonstration of biotin-protein and biotin carboxylase subunits. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1319–1326. doi: 10.1073/pnas.63.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts A. W., Vagelos P. R. Acetyl CoA carboxylase. I. Requirement for two protein fractions. Proc Natl Acad Sci U S A. 1968 Feb;59(2):561–568. doi: 10.1073/pnas.59.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dimroth P., Guchhait R. B., Stoll E., Lane M. D. Enzymatic carboxylation of biotin: molecular and catalytic properties of a component enzyme of acetyl CoA carboxylase. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1353–1360. doi: 10.1073/pnas.67.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Lane M. D. Liver acetyl coenzyme A carboxylase. I. Isolation and cat- alytic properties. J Biol Chem. 1968 Aug 25;243(16):4227–4235. [PubMed] [Google Scholar]
- Guchhait R. B., Moss J., Sokolski W., Lane M. D. The carboxyl transferase component of acetyl CoA carboxylase: structural evidence for intersubunit translocation of the biotin prosthetic group. Proc Natl Acad Sci U S A. 1971 Mar;68(3):653–657. doi: 10.1073/pnas.68.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinstein P. F., Stumpf P. K. Fat metabolism in higher plants. 38. Properties of wheat germ acetyl coenzyme A carboxylase. J Biol Chem. 1969 Oct 10;244(19):5374–5381. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



