Abstract
Current models suggest that the fate of the kidney epithelial progenitors is solely regulated by signals from the adjacent ureteric bud. The bud provides signals that regulate the survival, renewal and differentiation of these cells. Recent data suggest that Wnt9b, a ureteric bud-derived factor, is sufficient for both progenitor cell renewal and differentiation. How the same molecule induces two seemingly contradictory processes is unknown. Here, we show that signals from the stromal fibroblasts cooperate with Wnt9b to promote differentiation of the progenitors. The atypical cadherin Fat4 encodes at least part of this stromal signal. Our data support a model whereby proper kidney size/function is regulated by balancing opposing signals from the ureteric bud and stroma to promote renewal and differentiation of the nehron progenitors.
Introduction
Kidney development depends on reciprocal interactions between two tissues, the ureteric bud and the metanephric mesenchyme 1, 2. Signals produced by the mesenchyme promote reiterative branching morphogenesis of the ureteric bud (UB), while signals from the bud support survival and proliferation of nephron progenitor cells within the mesenchyme. Additionally, the bud produces a signal(s) that induces a subset of the progenitors to undergo a mesenchymal-to-epithelial (MET) transition to form an intermediate condensed structure known as the pre-tubular aggregate (PTA), which proceeds to an epithelial structure referred as the renal vesicle (RV). The RV will undergo morphogenesis to form the nephron, a structure consisting of the renal corpuscle, proximal tubule, loop of Henle, distal tubule and the connecting tubule. The UB will give rise to the collecting ducts and ureter.
A significant portion of the inductive activity attributed to the ureteric bud can be assigned to Wnt9b3. Previous studies have shown that Wnt9b signals to the nephron progenitor cells and activates at least two molecularly and spatially distinct programs; one that promotes progenitor cell proliferation/renewal (referred to as the Class II/progenitor signature) and another that induces their differentiation (referred to as the Class I/pre-tubular aggregate or PTA signature)4. In the absence of Wnt9b, the progenitor domain is specified correctly but does not expand and the PTAs and RVs do not form3, 4. Both programs are activated by the transcription factor beta-catenin4. A question that arises is how the same molecule promotes two seemingly contradictory, program-specific responses?
The nephron progenitors are encapsulated by a population of fibroblasts known as the stroma (Figure 1a). These cells are ideally positioned to influence the fate of the nephron progenitors. Indeed, ablation of the transcription factor Foxd1 from the stroma results in expansion of nephron progenitor cells and a severe deficit in MET/differentiation 5, 6. However, the precise mechanism underlying this phenotype is unclear.
Figure 1. The cortical stroma regulates Wnt9b target activation and differentiation of the nephron progenitors.
A: Schematic representation of the embryonic kidney indicating the Wnt9b expressing ureteric bud epithelium in red, the renewing nephron progenitors that express Wnt9b Class II targets in light gray, the differentiating nephron progenitors or pre-tubular aggregates (PTAs) that express Wnt9b Class I targets in dark gray and the Foxd1 positive stromal fibroblasts in blue. Hematoxylin and Eosin stained P1 wild type (B) and Foxd1cre; Rosa26DTA mutant (C) kidney sections. Arrows indicate nephron progenitor cells. Expression of Class II/progenitor gene Cited1 (D,E), Amphiphysin (F,G) and Class I/PTA gene Lef1 (H, I) in wild type (D, F,H) and mutant (E, G and I) kidneys at E15.5. All sections have been co-stained with antibodies to the Wnt9b independent progenitor marker Six2 (in red) and the ureteric bud epithelium marker cytokeratin (CK) in blue. Quantification of the Six2 expression domain showed that it was significantly expanded from 2.1 cell layers in the wild type to 5.3 layers in mutants (n=4, p<0.000001). Statistics source data can be found in Table 1. The ureteric bud in B–I is outlined with a white dotted line. D–I are captured at 63x magnification. Scale bar = 100 microns in B, C and 50 microns in all other panels.
Here, we show that the stromal cells produce a signal(s) that regulates progenitor cell renewal. This signal is at least in part encoded by the atypical cadherin Fat4. Fat4 normally functions to modify beta-catenin activity, promoting the differentiation program and repressing the renewal program. We hypothesize that Fat4 accomplishes this role by modulating the activity of Yap and Taz within the nephron progenitors. By providing opposing progenitor renewal and differentiation signals, the ureteric bud and the stroma provide a niche that assures proper nephron endowment and optimal kidney function.
Results
The stroma promotes differentiation
To test a potential role for the stroma in nephron progenitor fate, these cells were ablated by generating pups carrying both a Cre inducible form of the Diphtheria toxin A chain (RosaDTA)7 and Foxd1Cre8.
Foxd1Cre;RosaDTA pups died within 24 hours of birth. As expected, examination of E18.5 kidneys revealed a complete absence of the cortical stromal cells and their derivatives (Figure S1A). Foxd1 positive stromal cells were absent at E15.5 although there were still some Foxd1 derived medullary stromal cells (Figure S1A). Thus, the Foxd1Cre;RosaDTA mouse results in deletion of the cortical stroma by at least E15.5.
Kidneys of P1 Foxd1Cre;RosaDTA pups (which we will refer to as ‘stromaless’) were smaller than wild type and were fused to the body wall. Hematoxylin-Eosin (HE) stained sections revealed an expanded zone of mesenchymal cells capping the ureteric buds (Figure 1C). Staining with an antibody to Six2 demonstrated that the nephron progenitor domain of mutants was significantly expanded in the Foxd1cre;Rosa-DTA mutants (Figure 1E, G, I).
To determine if stromaless kidneys had a normal Wnt9b response, we assessed the expression of both Class I/PTA and Class II/progenitor target genes at E15.5. Class I targets include Pax8, Wnt4, Lef1 and C1qdc2 4. Class II targets include Cited1, phospholipase A2 group 7 (Pla2g7), amphiphysin (Amph) and expressed sequence AW049604 (Tafa5/Fam19a5) 4. At E15.5, all Class II/progenitor Wnt9b targets examined were expressed throughout the expanded progenitor domain of the stromaless mutants (Figure 1E, G, Figure S1B). However, the expression of Class I/PTA targets was significantly reduced or absent (Figure 1I and Figure S1B). Although most kidneys were largely devoid of PTAs/RVs, a very low number of PTAs/RVs did form, most likely corresponding to regions of retained stroma. These data suggest that a signal(s) from the cortical stroma suppresses renewal and/or promotes differentiation of the nephron progenitor cells.
Previous studies have found that the stroma produces secreted frizzleds and it has been hypothesized that these signals affect the activity of Wnt ligands produced by the ureteric bud and/or renal vesicles 6, 9, 10. To test if ablation of the stroma could affect the strength of ureteric bud derived Wnt9b signaling, we cultured stromaless mutants with a small molecule that inhibits Wnt production (IWP2) by repressing the fatty acyl-transferase porcupine11, 12.
Unexpectedly, Foxd1Cre;RosaDTA kidneys treated with the highest dosage of IWP2 still maintained the expression of Wnt9b Class II/progenitor targets (although at slightly reduced levels) after 48 hours of culture (Figure 2E). Interestingly, IWP2 treatment blocked expression of class I/PTA targets in both wildtype and stromaless kidneys (Figure S6). These data suggest that in the absence of stroma, Class II target expression is less dependent on a Wnt ligand.
Figure 2. Expression of Class II targets is independent of a Wnt ligand but dependent on beta-catenin in stromaless and Fat4 mutants.
A–I: Whomemount images of wild type (A–C), Foxd1cre; Rosa26DTA (D–F) and Fat4 null (G–I) kidneys cultured from E11.5 for 48 hours in the presence of DMSO (A, D, G), 5 uM IWP2 (inhibitor of Wnt production) (B, E, H) or the beta-catenin destabilizer IWR1 (C, F, I). All tissues were hybridized with an antisense probe to Pla2g7. Scale bar = 1mm. J–K:Quantitative analysis of gene expression by qrtPCR. cDNA levels of Class II/progenitor targets Tafa5 and Pla2g7 and Class I/PTA target C1qdc2 were assessed after treating wildtype or Fat4 mutant kidneys with IWP2 (J) or IWR1 (K) or DMSO. DMSO treated wildtype levels are considered baseline. Shown is the mean value of Qrt-PCR data from 3 different experiments. Error bar indicates SEM. ‘*’ indicate p<0.05 and ‘#’ indicate p<0.01.
To determine if beta-catenin was still necessary for the expression of Wnt9b targets in stromaless kidneys, Foxd1Cre;RosaDTA kidneys were cultured with a compound that destabilizes beta-catenin (inhibitor of Wnt response 1 or IWR1) (Figure 2C,F)11, 12. Both wild type and Foxd1Cre;RosaDTA mutants lost expression of Wnt9b/beta-catenin Class I and II targets in the presence of IWR1. These data indicate that removal of the stroma makes Wnt9b Class II target gene expression less dependent on a Wnt ligand, but does not lessen dependence on beta-catenin.
Stromal signals affect Yap activation
Recent studies have shown that beta-catenin activity can be regulated by crosstalk with the Hippo/Warts pathway13–16. Hippo and Warts (Mst1/2 and Lats1/2 in mice) are serine/threonine kinases that regulate cell proliferation and differentiation by activating the phosphorylation and nuclear exclusion of the transcriptional regulator Yorkie. Loss of Hippo/Warts signaling in the heart results in increased levels of nuclear Yap (a mouse ortholog of Yorkie) and inappropriate activation of the beta-catenin pathway 16. Given the observed effect on beta-catenin activity, we decided to examine the localization and activation status of Yap in stromaless kidneys using antibodies that recognize either total or phosphorylated forms of the protein17–20.
In wild type kidneys, we found that total Yap protein was ubiquitously expressed. However, Yap showed differences in its sub-cellular localization with a higher nuclear to cytoplasmic ratio in differentiated cells in the more central or medullary region of the kidney (Figure 3C,D and Figure S2A). As expected, phospho- (or p-) Yap expression and nuclear Yap were largely exclusive of each other. pYap expression was strong in the cortical stromal, ureteric bud and nephron progenitor cells (Figure S2 A′). Levels of pYAP abruptly decreased in all three lineages as differentiation proceeded. The medullary region was largely devoid of pYap expression (Figure S2A′).
Figure 3. Nephron progenitors of stromal mutants show increased nuclear YAP and reduced pYAP.
P1 wild type (A–D), Foxd1cre; Rosa26DTA (E–H) and Fat4 null (I–L) kidneys were subjected to immunostaining using either an antibody to YAP or a phoshpo-YAP specific antibody (green in merged images), the progenitor marker Six2 (in red) and ureteric bud epithelium marker cytokeratin (CK in blue). Single channel images for pYap (B,F and G) and Yap (D, H and L) are shown beneath each merged image. ‘*’ indicates the progenitor domain in mutants with significantly reduced pYAP expression. All images captured at 63X magnification. Scale bars = 20 microns.
Yap protein levels were next assessed in Foxd1Cre;RosaDTA kidneys. Although total Yap levels did not change significantly in mutants, there was a striking increase in the levels of nuclear Yap in all three lineages within the nephrogenic zone of mutant kidneys (Figure 3G,H). Conversely, phosphorylation of Yap was significantly reduced in the Six2-positive cells of Foxd1Cre; RosaDTA kidneys (Figure 3E,F). These data suggest that signals from the cortical stroma regulate Yap phosphorylation and localization within the nephron progenitors.
Yap/Taz regulates Wnt9b activity
Our data suggest that nuclear Yap (and Taz) stimulate the expression of Wnt9b target genes. To test this hypothesis, nephron progenitors were isolated and cultured 21, 22. As in the stromaless mice, Yap protein was predominantly nuclear in the cultured cells although, as previously shown, the levels of nuclear Yap were inversely correlated to cell density (Figure S2B).
Cited1, Pla2g7, Tafa5 and Amphiphysin are all expressed in the isolated progenitors in the absence of Wnt9b (Figure 4A) and all tested were positively affected by Lef1/beta-catenin activity (Figure S4A). We next asked whether the expression of these genes was dependent on Wnt production. Similar to observations made in Foxd1Cre;RosaDTA kidneys, treatment of isolated progenitor cells with IWP2 had no significant effect on the expression of these progenitor target genes (Figure S4B).
Figure 4. Nuclear YAP drives Wnt9b Class II/progenitor and represses Class I/PTA target gene expression in isolated progenitor cell cultures.

A) Western blot on whole cell lysates from isolated mesenchymal cells transfected with scrambled, YAP or Taz specific siRNA for 72hrs. Blots were probed with the following antibodies: Yap, Taz, Class II target genes (Cited, Tafa5, Pla2g7, Amphiphysin), Class I target gene (Pax8), Wnt9b independent progenitor markers (Six2 and Sall1) and a marker of differentiation E-cadherin. (B) QrtPCR on cDNA from cells transfected with a scrambled siRNA or siRNAs to Taz and Yap using primers to Yap, Taz, Class II Class I targets and Wnt9b independent progenitor genes. (C) Western blot of cells 72hrs after transfection with vector, full length or Fat4-ECD plasmids. (D) MM cells (stained with CellTracker, red) were co-cultured with cells co-expressing empty vector, full-length Fat4 or Fat4-ECD and GFP (green) then stained with anti-Yap antibody (teal) and Dapi (blue). Arrows in B indicate the nucleus. Note the lack of Yap in the nucleus of mesenchymal cells adjacent to Fat4 expressing cells. Scale bar in D =20microns. In all in vitro experiments, cultured cells are isolated from 20 embryos from 3 pregnant females. The QrtPCR data shown is one representative example from 3 individual experiments.
We next asked if Wnt9b/Class II target expression was dependent on Yap/Taz activity. Cells in which Yap/Taz mRNA/protein levels were knocked-down via siRNA mediated silencing showed a significant repression of Class II/progenitor targets Cited1, Tafa5, Pla2g7, Amphiphysin (Figure 4A, B) while the levels of Wnt9b independent progenitor targets like Sall1 and Six2 were only moderately reduced (Figure 4A, B). Expression of the class I/PTA targets Pax8 and E-cadherin was significantly increased in these cells (Figure 4A, B). These findings suggest that nuclear Yap/Taz enhances the expression of Wnt9b Class II/progenitor target genes while it represses the expression of Class I/PTA targets.
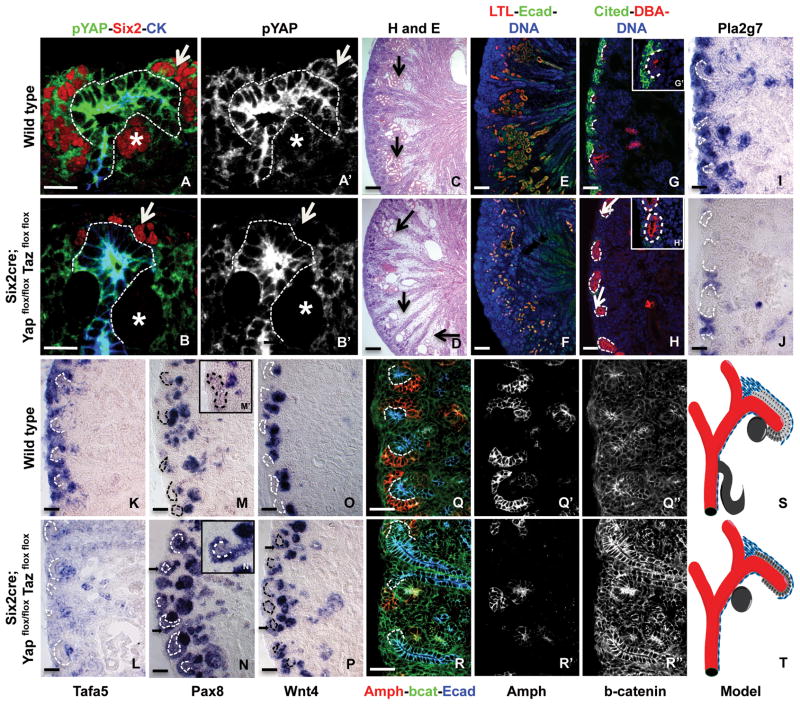
To determine if this role for Yap/Taz was conserved in vivo, we ablated both genes from the kidney nephron progenitors using Six2Cre. Both Yap and Taz are expressed in largely overlapping domains in the kidney progenitor cells (Figure S8B) and single ablation of either gene had little effect on Wnt9b target gene expression (with the exception of Cited1, which was lost in both single mutants Figure S8C). Although Six2Cre;Yapflox/flox;Tazflox/flox mutants maintained Yap protein through E13.5, levels were greatly decreased at E15.5 and by birth, the majority of the progenitor cells and their derivatives showed no detectable levels of Yap (Figure S3 and 5A–B). As expected, given the kinetics of Yap ablation, the first several days of kidney development did not appear to be affected in these mutants (Figure S3). However, Six2Cre;Yapflox/flox;Tazflox/flox mutants died within 24 hours of birth.
At E18.5, Yap/Taz double mutant kidneys were smaller than those of wild type littermates and exhibited a greatly reduced nephrogenic zone and decreased density of epithelia in the more central regions of the kidney indicative of a reduction in nephron number (Figure 5D). Staining of mutant kidneys with Lotus tetragonolobus lectin (LTL), a marker of proximal tubules, revealed a severe decrease in number and length of these structures in mutants (Figure 5E–F). Further, P1 double mutants had significantly fewer glomeruli than wild type (Table 1). However, the collecting duct epithelium, which is derived from the ureteric bud lineage, looked relatively normal.
Figure 5. Ablation of TAZ/YAP from the progenitors alters expression of Class I and Class II target genes in the nephron progenitors.
Kidney sections from E18.5 wild type (A, C, E, G, I, K, M, O, Q) or Six2cre; Yapflox/flox/Tazflox/flox mutant (B, D, F, H, J, L, N, P, R) were stained with antibodies to pYap (green), progenitor marker Six2 (red) and UB epithelium gene, cytokeratin (CK in blue) (A–B, Single channel images for pYap are shown in A′ and B′), Hematoxylin and Eosin (C–D), LTL (red), epithelial marker E-cadherin (green) and Dapi (blue) (E–F), Wnt9b/Class II target Cited1 (in green), UB epithelium marker Dolichos biflorus agglutinin (DBA, in red) and Dapi (blue) (G–H, G′-H′ shows a higher magnification with the UB outlined in white), antisense Wnt9b/ClassII target Pla2g7 (I–J), antisense Wnt9b/ClassII target Tafa5 (K–L), antisense Wnt9b/ClassI target Pax8 (M–N, M′–N′ shows the higher magnification with the UB outlined in white), antisense Wnt9b/ClassI target Wnt4 (O–P) antibodies to ClassII target Amphiphysin (in red), beta-catenin (in green) and E-cadherin (blue) (Q–R, the corresponding single channels for Amphiphysin and beta-catenin are shown in Q′–R′ are Q″–R″, respectively). Model showing the expression of progenitors in wild type and Yap/Taz double mutants (S–T). The self-renewing progenitor cells (light gray) are significantly reduced or lost in the mutants and are replaced by the differentiating progenitors (dark gray). Dotted lines indicate the UB in all panels. Arrow in A′–B′ indicate reduced Six2 positive cells and * indicate the PTAs; arrow in C–D indicates epithelial structures lost in the mutants; arrow in H indicates loss of Cited1 expression from UB tips and arrow in N and P indicates ectopic expression of target genes. Scale bar for A,B =.1mm, C-P = 10 microns, Q-R = 50 microns.
The paucity in progenitor cell-derived structures in mutants could be caused by a deficit in progenitor cell number or progenitor cell differentiation. To test the role of Yap/Taz in progenitor cell maintenance/proliferation, we stained kidneys with an antibody to the Wnt9b Class II/progenitor target Cited1 as well as the Wnt9b independent progenitor marker Six2. The majority of ureteric bud tips lacked a Cited1 positive mesenchymal cap (arrows in Figure 5H), even though a single layer of Six2-positive cells (as opposed to 2–3 cell layers in wild type) still surrounded the ureteric bud of some mutants (Figure 5 and S3). Many tips completely lacked progenitor cells. Transcripts of Class II targets Pla2g7,Tafa5 and Amphiphysin showed similar reductions in double mutants (Figure 5J, L and R respectively). Importantly, beta-catenin protein was still expressed in mutants (Figure 5 R–R″). Thus, similar to what was observed in vitro, Yap/Taz activity is required for normal beta-catenin Class II/progenitor target gene expression in vivo.
Loss of Class II target expression correlates with low rates of progenitor cell proliferation4. Therefore, we compared the percentage of pHH3/Six2 positive cells in the Yap/Taz mutants to the wild type. Mutants showed a significant decrease in cell proliferation rates (Figure S5).
To determine if Yap/Taz activity affected the expression of the Class I/PTA targets, we assessed the expression of Pax8 and Wnt4 in P1 kidneys. Both genes showed expanded/ectopic expression in Yap/Taz mutants. Pax8 and Wnt4 showed strong ectopic expression in the cortical mesenchyme, expanding into what would normally be the expression domain of Class II/progenitor targets (Figure 5N, N′ and P).
These data suggest that Yap/Taz normally represses the expression of Class I/PTA markers in the nephron progenitors. Although we observed ectopic expression of Class I/PTA targets, the formation of renal vesicles appeared to be delayed in mutants suggesting that Yap/Taz activity is required for proper differentiation. Thus, the reduced size of the kidney and the reduction of nephron number observed in Yap/Taz mutants is most likely caused by a combination of reduced progenitor cell renewal as well as defects in differentiation.
Fat4 regulates Yap/Taz activity in the progenitors
Our data suggests that a signal(s) from the stroma regulates Yap/Taz activity within the progenitors. In flies, members of the Fat and Ds family of atypical cadherins have been shown to mediate activity of the Yap/Taz homolog Yorkie 23. Intriguingly, one member of this family, Fat4, is expressed predominantly in the stroma of the embryonic kidney 24, 25.
To determine if Fat4 regulates the phosphorylation status of Yap/Taz, we analyzed expression of Yap protein in E15.5 and P1 Fat4−/− kidneys. Similar to the observations in the stromaless kidneys, phosphorylation of YAP was normal in the stroma and the UB epithelium, but significantly reduced in the majority of Six2 positive progenitor cells in Fat4 mutant kidneys (Figure 3I,G). Further, total YAP protein was predominantly localized in the nucleus of Fat4 mutant progenitor cells (Figure 3K, L). These results suggest that Fat4 regulates the activity/localization of Yap in the mammalian kidney.
Fat4 nulls exhibit an expanded progenitor domain
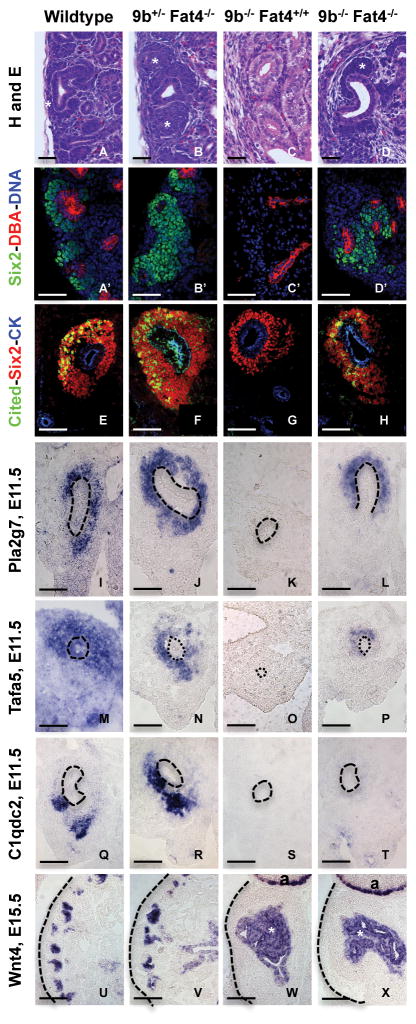
We next sought to determine if ablation of Fat4 had an impact on the nephron progenitor population. Fat4 null kidneys are smaller than their wild type littermates at birth but appear to have an expanded progenitor domain (Figure 6B). The expanded progenitor cells expressed Wnt9b nephron progenitor (Class II) targets as well as other progenitor markers (Figure 6D,F,H,J,L,N,P). Quantification of the number of Six2 positive cells revealed a significant expansion of the progenitor domain (Figure 6P). However, the number of Lef1 positive structures suggested that Fat4 mutants had significantly fewer nephrons than wildtype (Figure 6P, Table 1).
Figure 6. Fat4 mutants exhibit an expansion of the progenitor domain and reduced differentiation.

Wild type (A, C, E, G, I, K, M, O) and Fat4 null (B, D, F, H, J, L, N, P E18.5) kidneys stained with Hematoxylin and Eosin staining (A–B), antisense probes to Six2 (C,D), Eya1(E–F) and Wnt9b/ClassII target genes Cited1 (G–H) Tafa5 (I–J), or antibodies to Amphiphysin (green), UB epithelium marker cytokeratin (CK in red) and Dapi (K–L), Pax2 (red), Ecadherin (green) and beta-catenin (blue) (M–N), Six2 (red), Wnt9b/ClassI target gene Lef1 (green) and cytokeratin (blue) (O–P). Quantification of Six2 positive cells revealed that the progenitor domain was on average 4 cell layers thick in mutants as compared to 2.1 cell layers in wild type kidneys (n=4, p<0.000001). Wild type kidneys had an average of 51.5 Lef1 positive structures per section while Fat4 mutants had only 28 (n=4, p=0.007). Statistics source data can be found in Table 1. All images are taken at 20x magnification. Scale bars-100 microns.
To determine if the expansion of the nephron progenitor population was the result of an increased rate of proliferation or simply a failure to differentiate, we quantified the percentage of pHH3 positive progenitor cells in wild type and Fat4 mutants. Fat4 mutants showed a mild but significant increase in the percentage of pHH3 positive progenitor cells relative to wild type (Figure S5, Table 1).
We next determined if Fat4 mutant kidneys continued to express Wnt9b/Class II target genes after treatment with IWP2 (similar to stromaless kidneys). Indeed, the low level expression of Wnt9b Class II target genes was maintained, while Class I/PTA targets were lost (Figure 2H, J, K and S6). Fat4 mutants lost expression of both classes of targets after treatment with IWR1 (Figure 2I, J,K and S6). Thus, ablation of Fat4 renders expression of Wnt9b Class II/progenitor targets less-dependent on a Wnt ligand but does not relieve dependence on beta-catenin.
To determine the effect of Fat4 ablation on Wnt9b activity in vivo, we generated Fat4/Wnt9b double mutants. Fat4/Wnt9b double mutant kidneys were hypoplastic with a moderately branched ureteric bud system. Unlike Wnt9b null kidneys, double mutant buds were capped with progenitor cells although they still lacked renal vesicles and their derivatives (Figure 7D). Similar to the results observed upon culture of Fat4 mutants with IWP2, we found that expression of Wnt9b Class II/progenitor target genes Cited1, Pla2g7 and Tafa5 was rescued in Wnt9b mutants upon co-ablation of Fat4 (Figure 7H, L and P). However, class I/PTA target expression was not rescued (Figure 7T, X). These data suggest that Fat4 inhibits expression of Wnt9b Class II/progenitor targets and promotes the expression of Class I/PTA targets in vivo.
Figure 7. Fat4 mutants express Class II/progenitor genes in the absence of Wnt9b.
Wild type (A, E, I, M, Q, U, A′), Fat4 mutant (B, F, J, N, R, V, B′), Wnt9b mutant (C, G, K, O, S, W, C′) and Wnt9b/Fat4 double mutant (D, H, L, P, T, X, D′) kidneys at P1 (A–D and A′–D′), E11.5 (E–T) or E15.5 (U–X). Sections were stained with Hematoxylin and Eosin (A–D), anti-Six2 (green), DBA (red) and Dapi (blue) (A′–D′), anti-Six2 (red), Wnt9b/ClassII target gene Cited1 (green) and cytokeratin (blue, CK in E–H), antisense probes toPla2g7 (I–L),Tafa5 (M–P) and Wnt9b/Class I target gene C1qdc2 (Q–T) and Wnt4 (U–X). Dotted line indicates the UB. * in AD indicate expanded progenitor cell domains. * in W–X indicate medullary stromal expression of Wnt4. ‘a’ in W–X indicate adrenal gland. In A–D images are captured at 40x magnification and scale bar is: 20 microns. All other images are at 20x magnification and scale bars are 100 microns.
Previous studies suggest that Fat4 interacts with Vangl2 to regulate PCP during tubule diameter maintenance in the kidney26. However removal of Vangl2 from mice carrying either a null or hypomorphic allele of Wnt9b27 (Wnt9b−/−;Vangl2 lp/lp and Wnt9bneo/neo;Vangl2 lp/lp) did not rescue the progenitor domain or expression of Wnt9b Class II/progenitor targets suggesting this phenotype is independent of the Vangl2/PCP pathway (Figure S7B).
The cortical stroma and mural cells of Fat4 mutant kidneys were molecularly and phenotypically indistinguishable from wild type through birth (Figure S7A). Further, we did not observe ectopic expression of progenitor markers within the stromal compartment of Fat4 mutants (Figure S7A). These data suggest that the expansion in progenitors was not caused by a transformation of the stroma into progenitors.
Reciprocally, lineage tracing of progenitor cells in kidneys revealed that the Six2Cre expressing derivatives were present in mutants until E15.5 (Figure S8A). Co-labeling of Six2Cre;RosaYFP; Wnt9b−/− with antibodies to GFP and stromal markers Foxd1, Meis1/2 or Slug demonstrated that the progenitors of Wnt9b mutants were not mis-specified or trans-fated toward a stromal fate (Figure S8A). These findings rule out the possibility that the phenotypes observed in Wnt9b, Fat4 or Wnt9b/Fat4 double mutants were the results of defects in nephron progenitor or stromal cell specification or fate.
Fat4 acts non-autonomously
Our model suggests that stromal Fat4 acts on the adjacent progenitors. To determine if Fat4 can act non-autonomously, we co-cultured isolated progenitors with cells transfected with full length Fat4. Yap localization shifted from predominantly nuclear to cytoplasmic when progenitor cells were cultured adjacent to Fat4 expressing cells (Figure 4D). A construct lacking the cytoplasmic domain of Fat4 (Fat4-ECD) had the same effect (Figure 4C). Both constructs resulted in increased levels of phosphorylated Yap. All results occurred independent of cell density indicating that Fat4 is capable of activating Yap/Taz in a non-autonomous manner.
We next assessed the effect of Fat4 on progenitor cell differentiation. Both full length and the extra-cellular domain of Fat4 had a similar effect on gene expression as knockdown of Yap/Taz, repressing class II/progenitor gene expression (with the exception of Pla2g7) and enhancing differentiation/MET as assessed by E-cadherin (Figure 4C). In sum, these data suggest that stromally-derived Fat4 non-autonomously regulates Yap/Taz activity within a subset of the nephron progenitors, which promotes their differentiation.
Discussion
The embryological studies performed by Clifford Grobstein during the 1950s established one of the central tenets of metanephric kidney development: nephron progenitor maintenance and differentiation rely on signals produced by the ureteric bud epithelium1, 2. However, there is growing evidence that final kidney form relies on inductive and inhibitory crosstalk between multiple cell types present in the organ anlagen5, 6, 28–30. In this study, we provide evidence the cortical stroma inhibits nephron progenitor cell expansion and promotes its differentiation. We propose that Fat4, produced by the stroma, provides a signal that antagonizes nephron progenitor renewal and promotes differentiation by modulating response to Wnt9b.
We suggest that Yap/Taz plays a crucial role in regulating Wnt9b signature activation. Precisely how this is accomplished is not clear. Several recent studies have shown direct interaction between beta-catenin and Yap/Taz signaling 13–16. Our data are consistent with the idea that Fat4 signaling promotes the expression of Wnt9b/beta-catenin differentiation targets, inhibits the expression of the progenitor renewal targets. We propose that nuclear Yap/Taz and beta-catenin cooperate to activate the nephron progenitor signature and loss of nuclear Yap/Taz (and possibly gain of cytoplasmic pYap/Taz) is necessary for the expression of the differentiation signature (Figure 8). As we have not identified conserved Tead (the DNA binding co-factor for Yap and Taz) binding sites within either class of target gene, the effect of Yap/Taz on beta-catenin targets may not be direct. Nevertheless, Yap/Taz must impinge on Wnt9b/beta-catenin activity at some point.
Figure 8. Model for the interaction between the stroma and the ureteric bud on the progenitor cells.
The boxed area of the nascent kidney is magnified on the right. Wnt9b, produced by the ureteric bud (red) signals canonically to both the renewing progenitors (gray cells) and to the differentiating progenitors (green cells). Fat4, produced by the stroma, promotes differentiation by enhancing the nuclear export of Yap/Taz within the progenitors (green cells), which allows expression of Class I beta-catenin targets. In the renewing progenitors levels, of pYAP are low. Un-phosphorylated Yap is localized predominantly in the nucleus and activates expression of Class II beta-catenin targets and promotes progenitor renewal. We hypothesize that cells induced to differentiate by Fat4 are only transiently located next to the stroma the migrate to the stalk side of the UB where they initiate MET.
Although Fat4 is expressed in the stroma (24, 25 and this study), we cannot rule out the possibility that low levels of Fat4 present in the progenitors constitute the active pool of protein. However, the observation that DTA deletion of the stromal cells results in a similar phenotype to Fat4 ablation would seem to support a stromal source. We hypothesize that Fat4 binds to another factor produced by the progenitors to activate Yap/Taz in the progenitors. The mouse ortholog of Dachsous and Fat3, a paralog of Fat4, are both expressed in the progenitor cells where they could be acting as receptors for stromal Fat4 24, 25, 31. It will be interesting to determine whether either of these factors mediates Yap/Taz activity.
The Foxd1Cre;RosaDTA kidneys elicits a more severe phenotype than mutation of Fat4 alone. Inappropriate production of other signals (such as Bmps, 6) produced by the ectopic endothelial or capsular cells observed in Foxd1 6 mutants may lead to the more severe phenotype observed in these mutants. In support of this model, we did not detect ectopic pSmad1/5/8 activity in Fat4 or SixCre;Yapflox/flox;Tazflox/flox mutants (Figure S7C), although they are detectable in Foxd1 mutants 6. These data suggest that the phenotypes resulting from ablation of Fat4 are a subset of those observed in Foxd1 and stromaless kidneys.
In summary, we have revealed that ureteric bud derived Wnt9b and stromal derived Fat4 provide opposing signals that regulate kidney progenitor cell maintenance and differentiation. Although this interaction is clearly essential for kidney development, we feel that the crosstalk between the Wnt and Fat/Yap pathways may be representative of a more general mechanism underlying stromal/epithelial interactions in multiple tissues. Given the increasing evidence of stromal involvement in numerous human pathological conditions, these findings are likely to have significant impact on our understanding of human disease.
Supplementary Material
Acknowledgments
Thanks to Yingzi Yang for providing the Vangl2 mutant mice, Masatoshi Takeichi for providing the Fat4 full length and ECD plasmids, Jose Cabera for artwork and Ondine Cleaver, Quanxi Li and Denise Marciano for reading and commenting on the manuscript. This work was supported by a post-doctoral fellowship from the A.H.A to A.D and from the Japanese Society for the Promotion of Science to S.T., the NIH (R01DK080004 and R01DK095057 to T.C.) and National Cancer Institute’s Center for Cancer Research (A.P.). This work was supported by the George O’Brien Kidney Research Center at UTSW.
Footnotes
Author contributions:
Experiments were designed by AD, ST, CMK, AOP, and TJC. Experiments were performed by AD, ST, CMK. Data was interpreted by AD, ST, CMK, AOP, and TJC. MX, LL, CC, ENO provided mice or other reagents. The paper was written by AD, ST, AOP, and TJC.
The authors declare that they have no competing financial interests.
References
- 1.Grobstein C. Inductive interaction in the development of the mouse metanephros. J Exp Zool. 1955;130:319–340. [Google Scholar]
- 2.Grobstein C. Inductive epithlio-mesenchymal interaction in cultured organ rudiments of the mouse metanephros. Science. 1953;118:52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- 3.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Karner CM, et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 6.Levinson RS, et al. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 7.Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol. 2008;180:4742–4753. doi: 10.4049/jimmunol.180.7.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, et al. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshino K, et al. Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev. 2001;102:45–55. doi: 10.1016/s0925-4773(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 10.Lescher B, Haenig B, Kispert A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Dev Dyn. 1998;213:440–451. doi: 10.1002/(SICI)1097-0177(199812)213:4<440::AID-AJA9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Karner CM, et al. Tankyrase is necessary for canonical Wnt signaling during kidney development. Dev Dyn. 2010;239:2014–2023. doi: 10.1002/dvdy.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varelas X, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008;68:2789–2794. doi: 10.1158/0008-5472.CAN-07-6205. [DOI] [PubMed] [Google Scholar]
- 19.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanigawa S, et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol. 2011;352:58–69. doi: 10.1016/j.ydbio.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L. BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development. 2009;136:3557–3566. doi: 10.1242/dev.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willecke M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Rock R, Schrauth S, Gessler M. Expression of mouse dchs1, fjx1, and fat-j suggests conservation of the planar cell polarity pathway identified in Drosophila. Dev Dyn. 2005;234:747–755. doi: 10.1002/dvdy.20515. [DOI] [PubMed] [Google Scholar]
- 25.Mao Y, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 27.Karner CM, et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yallowitz AR, Hrycaj SM, Short KM, Smyth IM, Wellik DM. Hox10 genes function in kidney development in the differentiation and integration of the cortical stroma. PLoS One. 2011;6:e23410. doi: 10.1371/journal.pone.0023410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126:1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- 30.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–2134. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 31.Brunskill EW, Lai HL, Jamison DC, Potter SS, Patterson LT. Microarrays and RNA-Seq identify molecular mechanisms driving the end of nephron production. BMC Dev Biol. 2011;11:15. doi: 10.1186/1471-213X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.