Abstract
Vaccines are developed and eventually licensed following consecutive human clinical trials. Malaria is a potential fatal vector-borne infectious disease caused by blood infection of the single-cell eukaryote Plasmodium. Pathogen stage conversion is a hallmark of parasites in general and permits unprecedented vaccine strategies. In the case of malaria, experimental human challenge infections with Plasmodium falciparum sporozoites can be performed under rigorous clinical supervision. This rare opportunity in vaccinology has permitted many small-scale phase II anti-malaria vaccine studies using experimental homologous challenge infections. Demonstration of safety and lasting sterile protection are central endpoints to advance a candidate malaria vaccine approach to phase II field trials. A growing list of antigens as targets for subunit development makes pre-selection and prioritization of vaccine candidates in murine infection models increasingly important. Preclinical assessment in challenge studies with murine Plasmodium species also led to the development of whole organism vaccine approaches. They include live attenuated, metabolically active parasites that educate effector memory T cells to recognize and inactivate developing parasites inside host cells. Here, opportunities from integrating challenge experiments with murine Plasmodium parasites into malaria vaccine development will be discussed.
Keywords: vaccine, immunization, protective immunity, murine infection model, malaria, Plasmodium, Eukaryotic pathogen, whole organism vaccine, subunit vaccine
Introduction
Clinical development and licensure of a safe, affordable, accessible and protective malaria vaccine remains a fundamental medical challenge. A vaccine is urgently needed because malaria continues to be the most relevant arthropod-transmitted infectious disease despite enormous investments into malaria control programs.1-3
The causative pathogen, an obligate intracellular protist of the genus Plasmodium, is injected into the human host upon a nightly bite by a previously infected female Anopheles mosquito. However, disease onset does not occur until the parasite propagates inside erythrocytes and is preceded by a clinically and diagnostically silent population expansion phase in the liver.4-6 Since parasite stage conversion follows an intrinsic developmental program and is non-reversible, three consecutive phases in the human host can be distinguished and targeted by vaccines, (1) sporozoites and liver infection,6-8 (2) asexual blood stage infection, the exclusive cause of malaria,9-13 and (3) sexual stages that eventually colonize the insect vector.14-17
Experimental evidence is robust for all three stages as vaccine targets. Hence, an idealized, perfect malaria vaccine would elicit robust immune responses against all life cycle stages simultaneously. Experimental proof-of-principle studies in murine malaria models established that vaccination with liver arrested, metabolically active parasites could elicit lasting sterile protection against sporozoite challenge.18-20 All existing anti-sporozoite and –liver stage vaccines exploit this population bottleneck of the parasite life cycle, i.e., mosquito-to-man transmission, but need to be 100% effective to prevent a subsequent blood infection.6,8,21,22 Active immunization with purified native or recombinant blood stage antigens, exemplified by the merozoite surface protein 1 (MSP1; PF3D7_0930300; gi:929796), resulted in low blood infection upon high dose parasite challenge in murine models.23,24 Similarly, passive and active immunization against gamete and ookinete surface antigens reduced colonization and sporozoite formation in the Anopheles vector to very low levels.25,26
Despite these encouraging results from pre-clinical research in animal models only very few vaccine candidates were advanced to field-testing in malaria-endemic countries. These first generation malaria vaccines all share in common that they contain small portions of one or more selected Plasmodium falciparum antigens. In the absence of robust correlates of protection in naturally acquired immunity to malaria,12,27-29 they are based on the expectation that vaccine-induced responses against these distinct subunits can elicit lasting protection against clinical disease and malaria-related deaths after three immunizations. Because the precise mechanisms and targets of protective immunity in humans remain unverifiable, studies of Plasmodium infections in mice can establish new concepts and prevent failures in human clinical trials.
In the following, an appraisal of current and upcoming anti-malaria strategies that originate from murine infection models will be presented. Brief overviews of murine Plasmodium parasites and the current state of clinical trials with subunit vaccine candidates will introduce the pre-clinical development opportunities and limitations of malaria vaccine discovery and development.
Murine Infection Models
Five Plasmodium species infectious to humans share most biological, clinical and molecular features with closely related Plasmodium species that infect other mammals.30 With the isolation and adaptation of a rodent Plasmodium species, named Plasmodium berghei, to laboratory rats and mice, an experimental infection model became available.31 Additional isolates from wild rodent hosts and Anopheles vectors in Central and West Africa yielded a repertoire of subspecies and strains of four distinct Plasmodium species infectious to inbred and outbred mice (Table 1). Despite the evolutionary distance of their hosts, the chromosomal arrangements and overall gene repertoire between the most dangerous human parasite P. falciparum and rodent Plasmodium species are remarkably conserved.32
Table 1. Murine Plasmodium infection models.
| Plasmodium species | Strains | Mouse strain | Characteristics |
|---|---|---|---|
| P. berghei | NK65,ANKA | C57bl/6 | Robust vaccine model |
| ANKA | Malaria clinical syndromes | ||
| P. yoelii | 17X | Balb/c | Modest vaccine model |
| P. chabaudi | AS | A/J, DBA/2 | Susceptible to blood infection |
| C57bl/6 | Resistant to blood infection | ||
| P. vinckei | vinckei | Balb/c | Lethal infections |
Infection experiments with P. berghei sporozoites are particularly informative since they (1) permit access to all aspects of the life cycle with the broadest selection of modified parasites,33 (2) are performed in C57bl/6 mice, the most wide-spread mouse strain, where knockout and transgenic substrains are available,34 (3) represent a robust vaccine model, i.e., C57bl/6 mice are difficult to protect35,36 and (4) recapitulate central aspects of malaria complications, including experimental cerebral malaria,37 acute lung injury38 and malarial anemia39 (Table 1). The related species, P. yoelli,40 is typically examined in Balb/c mice. In this model, sterile immunity is easily achieved36,41 (Table 1). While it offers opportunities for vaccine discovery42 this model is also prone to over-interpretation of protective efficacy. P. chabaudi displays some striking similarities to the course of blood infection by human Plasmodium species43 (Table 1). However, the value for anti-blood stage vaccine development is limited by the current lack of genetically modified P. chabaudi parasites. The fourth rodent parasite, P. vinckei, is notoriously underrated despite its exceptionally high virulence.44 Major research investments, including generation of genome data, establishment of transfection technology and adaptation to Anopheles transmission will be required toward a potentially very important murine vaccine and infection model. Together, murine Plasmodium species offer a wide range of experimental opportunities. Limitations, such as absence of species-specific antigens, can be overcome by established transgene expression in P. berghei45 or by exploring humanized mouse models46,47 for infections with human parasites.
Malaria Subunit Vaccines in Advanced Clinical Trials
The first malaria vaccine to enter phase II trials in malaria-endemic countries was a candidate synthetic malaria vaccine, termed SPf66. Designed as a low-complexity peptide, multi-subunit vaccine, it contains one small MSP1 peptide, two NANP repeat peptides of the major sporozoite surface protein, termed circumsporozoite protein (CSP; PF3D7_0304600/PFC0201c; gi: 160161) and peptides of still unknown origin.48 Human challenge studies and phase II trials in Colombia were reported surprisingly successful,49,50 prompting independent phase III trials.51-54 The first study in 1–5 y old children in Tanzania concluded that SPf66 was safe, immunogenic and reduced the risk of clinical malaria by ~30%.51 Partial vaccine efficacy reportedly extended well beyond the first year of follow-up.52 In contrast, a phase III trial in infants aged 6–11 mo in The Gambia could not detect any significant differences between the SPf66 and polio groups.53 This negative result was confirmed in a trial in Thailand,54 triggering discontinuation of SPf66 vaccine development. In the absence of a murine infection model to provide an immunological framework for a SPf66-based strategy, no improvements to the vaccine design can be made.
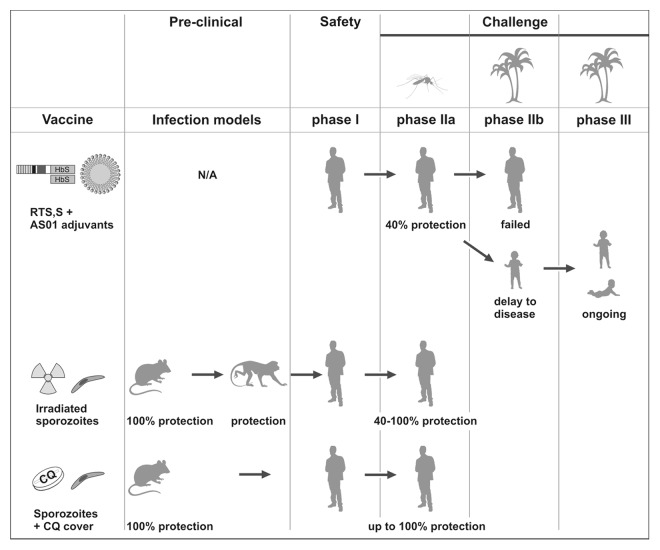
The established benchmark of ~30% protection against clinical malaria was subsequently met by another first generation subunit vaccine developed in 1980s, termed RTS,S. This vaccine consists of an enhanced EngerixTM, a pediatric hepatitis B vaccine, and includes portions of CSP as P. falciparum antigen and a proprietary adjuvant, AS01.55,56 This liposome formulation with monophosphoryl lipid A, a lipopolysaccharide derivative and ligand of toll-like receptor 4 (TLR4), and QS21, a triterpene glycoside, is a strictly essential component for efficacy. RTS,S also exemplifies the current clinical development plan for candidate malaria vaccines (Fig. 1). Since no infection models were developed for RTS,S, experimental challenge infections by a single exposure to laboratory-reared Anopheles mosquitoes infected with a homologous P. falciparum strain were the starting point for efficacy trials.57-59 40% of malaria-naïve adults were completely protected against a single challenge infection (Fig. 1). Additional tests, such as heterologous challenge with another P. falciparum strain to mimic diverse parasite populations in the field and re-challenge at a later time point to test for duration of protection, remained unexplored.
Figure 1. Clinical development of exemplary malaria vaccine candidates. RTS,S/AS01 (top) is currently the clinically most advanced subunit vaccine candidate. The recombinant proteins are shown as boxes (HbS, hepatitis B surface antigen) and the adjuvant AS01 as a lipid droplet. It was developed in the 80s and consistently protected 40% of adult volunteers against homologous challenge with infected laboratory-reared Anopheles mosquitoes (mosquito symbol). In a subsequent field trial (palm symbol) no protection was afforded in adults. Pictograms represent adults, 5–17 mo old children, and 6 weeks to 3 mo old infants. N/A, not applicable. Irradiated sporozoites (center) were first tested in a murine infection model in the 60s and shown to consistently protect non-human primates and adult volunteers against homologous challenge with infected laboratory-reared Anopheles mosquitoes. Sporozoite infection under chloroquine cover (bottom) was tested in a murine infection model in the 70s and recently shown to elicit robust and lasting protection in adult volunteers against homologous challenge with infected laboratory-reared Anopheles mosquitoes.
Instead, RTS,S was tested in a field trial with semi-immune adults in the Gambia.60 Prevalence of Plasmodium falciparum infections were indistinguishable between the RTS,S/AS02 and rabies control groups already 15 weeks later, and could not be improved by an additional vaccine boost60 (Fig. 1). RTS,S was also tested subsequently in proof-of-concept field trials in the target group, toddlers and infants.61-63 Numerous phase II trials have repeatedly shown a robust (~6 mo) delay to disease in the RTS,S vaccine group, which can be calculated again to ~30–50% protection against clinical disease (Fig. 1). Confirmation of this efficacy is currently being tested in an ongoing multi-center phase III trial. Trial completion and data analysis is scheduled toward the end of 2013. According to two interim results,64,65 the previous outcomes were fully corroborated, yielding a significant (~6 and ~3 mo, respectively) delay to clinical disease, which can be calculated as 50% and 30% protection in the first periods of follow-up. Immunological parameters, such as anti-CSP antibody titers or persistence of antigen-specific CD4+ T cells, do not correlate with the observed delay to disease afforded by RTS,S, suggesting that a murine infection model could fill an important knowledge gap. Of note, early work in the P. yoelii/Balb/c infection model demonstrated that high anti-CSP repeat antibody titers did not protect against sporozoite challenge in vivo.66
A recent CSP-based subunit vaccine,67 using the outer membrane protein complex of Neisseria meningitis as a carrier for CSP-repeat peptides, illustrates the current evidence-based pre-clinical development plan for a subunit malaria vaccine, integrating tailor-made transgenic P. berghei parasites for murine infection experiments45 and immunological assessment in primates.
Whole Cell-Based Vaccines
The first and some of the most effective human vaccines are live-attenuated pathogens. Live attenuated viruses, such as the vaccinia virus small pox vaccine, the oral polio vaccine and the yellow fever vaccine, are groundbreaking public health tools that were instrumental in successful eradication or ongoing elimination campaigns. The complex underlying immunological mechanisms and target antigens are only now being explored,68 and it remains speculative whether effective subunit vaccines could have been engineered against these viruses.
In recognition of the much more complex Plasmodium biology and very slow acquisition of naturally acquired partial anti-malaria immunity, scientist at New York University established the proof-of-concept for a live-attenuated malaria vaccine.69,70 Immunization of mice with irradiated sporozoites induced lasting69 and stage-specific70 immunity against sporozoite challenge (Fig. 1). These pre-clinical studies suggested that a malaria vaccine is possible and provided a rationale for advancing the irradiated sporozoite vaccine to a limited primate trial71 and small phase I/IIa trials in humans72-74 (Fig. 1). The momentum gained by the first successful human malaria vaccination72 cannot be over-estimated. Confirmation of sterile protection was consistently achieved in follow-up trials, at least in a proportion of volunteers73,74 (Fig. 1). However, apart from anecdotal evidence of lifetime protection against malaria in volunteers of the early experimental vaccine studies, no clinical data from heterologous challenge infection experiments or natural exposure in malaria-endemic countries are available.
Recent research and bioengineering investments into this benchmark malaria vaccine have resulted in the production of aseptic, purified, cryopreserved and irradiated P. falciparum for syringe delivery.75 However, intradermal or subcutaneous immunization with this sporozoite vaccine failed to elicit protection.76 In the absence of a positive control it remains unclear which of the several alteration(s) to the original protocol, i.e., exposure to irradiated infectious Anopheles mosquitoes, need attention. Ongoing clinical trials with irradiated P. falciparum and P. vivax sporozoites will further contribute to the assessment of safety and efficacy of this candidate malaria vaccine approach (Table 2).
Table 2. Update on current clinical trials testing live, attenuated parasite strategies.
| Vaccine* | Trial | Status† | ClinicalTrials.gov identifier |
|---|---|---|---|
| Pfγspz | Phase I/IIa | ongoing | NCT01441167 |
| Pvγspz | Phase I/IIa | planned | NCT01082341 |
| Pfspz + CQ | re-challenge‡ | ongoing | NCT01660854 |
| Pfspz + CQ | Phase IIa | completed | NCT01218893 |
| Pfspzcryo | Phase I | recruiting | NCT01728701 |
| Pfspz + CQ or MQ | Phase IIa | ongoing | NCT01422954 |
| Pfspz + PQ | Phase I/IIa | ongoing | NCT01500980 |
| PfGAP: p36(-)/p36p(-) | Phase I/IIa | suspended | NCT01024686 |
*Abbreviations: CQ, chloroquine; γspz, irradiated sporozoites; GAP, genetically arrested parasite; MQ, mefloquine; p36, 6-cysteine protein 36kDa; p36p, p36 paralog; Pf, Plasmodium falciparum; Pv, Plasmodium vivax; PQ, primaquine; spz, sporozoites; spzcryo, aseptic purified cryopreserved sporozoites. †Status as of January 2013. ‡re-challenge: Protected volunteers from previous study are being re-exposed to laboratory-reared Anopheles mosquitoes infected with a different Pf strain, termed heterologous re-challenge.
The paradigm of irradiated sporozoites illustrates the power of murine infection models in informing human vaccine design and was critical for the development of alternative live attenuated malaria vaccine approaches.22 They all follow the basic principle to infect with very high sporozoite doses and prevent onset of malaria. Because liver infection is diagnostically and clinically silent, high vaccine doses can mount lasting protection. One strategy, live unaltered sporozoite administration under continuous cover with the anti-malaria drug chloroquine (CQ), which kills blood stage parasites, has already advanced from proof-of-concept studies in mice77-79 to a small human phase I/IIa clinical study80,81 (Fig. 1). The trial results were impressive and demonstrated unprecedented lasting, sterile protection against a single homologous challenge infection by only three consecutive immunizations through exposure to 15 P. falciparum-infected Anopheles mosquitoes under continuous CQ cover.80,81 These results clearly warrant major research investments and confirmation (Table 2). Ongoing clinical studies address whether (1) sporozoite exposure under CQ cover also elicits protection against challenge with a heterologous P. falciparum strain, (2) the immunization dose can be lowered, i.e., exposure to fewer Anopheles mosquitoes still induces sterile protection or (3) sporozoite exposure under cover of an alternative blood stage suppressive drug, mefloquine (MQ), elicits similar (non-inferior) or better (superior) protection. The use of antimalarial drugs that affect blood stages only, however, implies that most volunteers experience mild malaria in their first immunization round.80 This might affect compliance and requires rigorous clinical supervision during the immunization regimes.
Proof-of-concept for two alternative strategies that might overcome these limitations has recently been demonstrated in murine infection models.20,82 Sporozoite administration under cover with primaquine, a drug that acts on Plasmodium liver stages, was shown to be safe and protective.82 Based on these promising results an ongoing clinical trial in humans was designed (Table 2). However, systematic side-by-side comparison in sporozoite challenge infections showed inferior immunity as compared with irradiated sporozoites.83 It will be interesting to see if this outcome reproduces in the human trial, since it will exemplify the prognostic value of mouse infection models. It will also clarify to which extent more emphasis should be placed on preclinical evaluations before moving to proof-of-concept phase I/IIa trials in humans. Based on the comparison of vaccine efficacy in murine models,83 sporozoite infection under antibiotic treatment, including azithromycin and clindamycin, both of which are licensed for pediatric applications, would seem to be the most promising way forward toward a whole organism sporozoite vaccine.6,20,84
Tailor-made arrests at various stages in liver stage development, maturation and merozoite release can also be generated by reverse genetics. Proof-of-principle studies with these so-called genetically arrested parasites (GAPs) were first demonstrated in the P. berghei/C57bl/6 model.19 Translation of this approach to proof-of-concept studies in humans critically depends on careful preclinical studies in murine infection models in order to select multiple independent target genes that permit a complete life cycle arrest and yet induce the most potent immune responses. Two examples in the P. berghei/C57bl/6 model show that both requirements appear mutually exclusive.85,86 A tight, early and pleiotropic arrest due to deletion of a master regulator of liver stage development, termed sporozoite and liver-stage asparagine-rich protein (SLARP), resulted in incomplete and short-lived protection.85 In contrast, a very late arrest just prior to liver merozoite release elicited superior and long-lasting protection, but resulted in considerable break-through infections.86 Accordingly, selection of P. falciparum target genes that is likely to generate a safe Pf knockout line should be the first research priority. A first clinical trial with a mutant PfGAP line87 was discontinued (Table 2). Preclinical assessment in murine models demonstrated incomplete arrest in the selected target genes, providing a molecular rationale for breakthrough infections.88 Of note, this was detectable only in the robust vaccine model, P. berghei/C57bl/6 mice, lending further support for a strict requirement to assess vaccine safety and efficacy in this murine infection model. In conclusion, the decision of which whole parasite strategy to move forward to clinical trials should be evidence-based and supported by robust preclinical data.
Outlook
Strategies that led to an arrest of liver stage development or early during blood stage infection were already successfully tested in phase II clinical trials. Successful and rapid translation of vaccine strategies from murine infection models to human proof-of-concept trials demonstrates the important role of preclinical research in assessment of vaccine safety, efficacy and longevity. The proven track record of these models for malaria vaccine development warrants inclusion of similar approaches in future vaccine trials, including low-complexity subunit vaccines.
Acknowledgments
Diane Schad is acknowledged for assistance with Figure 1. Research in the author’s laboratory is supported by the Max Planck Society and the European Commission (EviMalaR, #34).
Glossary
Abbreviations:
- CQ
chloroquine
- CSP
circumsporozoite protein
- GAPs
genetically arrested parasites
- MQ
mefloquine
- MSP1
merozoite surface protein 1
- PQ
primaquine
Submitted
11/26/12
Accepted
12/3/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23218
References
- 1.Okiro EA, Bitira D, Mbabazi G, Mpimbaza A, Alegana VA, Talisuna AO, et al. Increasing malaria hospital admissions in Uganda between 1999 and 2009. BMC Med. 2011;9:37. doi: 10.1186/1741-7015-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roca-Feltrer A, Kwizombe CJ, Sanjoaquin MA, Sesay SSS, Faragher B, Harrison J, et al. Lack of decline in childhood malaria, Malawi, 2001-2010. Emerg Infect Dis. 2012;18:272–8. doi: 10.3201/eid1802.111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 4.Prudêncio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol. 2006;4:849–56. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 5.Silvie O, Mota MM, Matuschewski K, Prudêncio M. Interactions of the malaria parasite and its mammalian host. Curr Opin Microbiol. 2008;11:352–9. doi: 10.1016/j.mib.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Borrmann S, Matuschewski K. Targeting Plasmodium liver stages: better late than never. Trends Mol Med. 2011;17:527–36. doi: 10.1016/j.molmed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Hafalla JC, Silvie O, Matuschewski K. Cell biology and immunology of malaria. Immunol Rev. 2011;240:297–316. doi: 10.1111/j.1600-065X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill AVS. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. doi: 10.1038/nri1746. [DOI] [PubMed] [Google Scholar]
- 9.Haldar K, Murphy SC, Milner DA, Jr., Taylor TE. Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol. 2007;2:217–49. doi: 10.1146/annurev.pathol.2.010506.091913. [DOI] [PubMed] [Google Scholar]
- 10.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7:341–54. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 11.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–41. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowkes FJI, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good MF, Stanisic D, Xu H, Elliott S, Wykes M. The immunological challenge to developing a vaccine to the blood stages of malaria parasites. Immunol Rev. 2004;201:254–67. doi: 10.1111/j.0105-2896.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 14.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooij TW, Matuschewski K. Triggers and tricks of Plasmodium sexual development. Curr Opin Microbiol. 2007;10:547–53. doi: 10.1016/j.mib.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–6. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 17.Carter R. Transmission blocking malaria vaccines. Vaccine. 2001;19:2309–14. doi: 10.1016/S0264-410X(00)00521-1. [DOI] [PubMed] [Google Scholar]
- 18.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 19.Mueller AK, Labaied M, Kappe SHI, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–7. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 20.Friesen J, Silvie O, Putrianti ED, Hafalla JCR, Matuschewski K, Borrmann S. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med. 2010;2:40ra49. doi: 10.1126/scitranslmed.3001058. [DOI] [PubMed] [Google Scholar]
- 21.Matuschewski K. Hitting malaria before it hurts: attenuated Plasmodium liver stages. Cell Mol Life Sci. 2007;64:3007–11. doi: 10.1007/s00018-007-7263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matuschewski K, Hafalla JC, Borrmann S, Friesen J. Arrested Plasmodium liver stages as experimental anti-malaria vaccines. Hum Vaccin. 2011;7(Suppl):16–21. doi: 10.4161/hv.7.0.14557. [DOI] [PubMed] [Google Scholar]
- 23.Holder AA, Freeman RR. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–4. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 24.Ling IT, Ogun SA, Holder AA. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–7. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 25.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–7. [PubMed] [Google Scholar]
- 26.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–8. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 28.Struik SS, Riley EM. Does malaria suffer from lack of memory? Immunol Rev. 2004;201:268–90. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 29.Offeddu V, Thathy V, Marsh K, Matuschewski K. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol. 2012;42:535–48. doi: 10.1016/j.ijpara.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Perkins SL, Schall JJ. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol. 2002;88:972–8. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Vincke JH, Lips M. Un noveau Plasmodium d’un Rongeur suvage du Congo Plasmodium berghei n.sp. Ann Soc Belg Med Trop. 1948;28:97–104. [PubMed] [Google Scholar]
- 32.Kooij TW, Carlton JM, Bidwell SL, Hall N, Ramesar J, Janse CJ, et al. A Plasmodium whole-genome synteny map: indels and synteny breakpoints as foci for species-specific genes. PLoS Pathog. 2005;1:e44. doi: 10.1371/journal.ppat.0010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janse CJ, Kroeze H, van Wigcheren A, Mededovic S, Fonager J, Franke-Fayard B, et al. A genotype and phenotype database of genetically modified malaria-parasites. Trends Parasitol. 2011;27:31–9. doi: 10.1016/j.pt.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, et al. The knockout mouse project. Nat Genet. 2004;36:921–4. doi: 10.1038/ng0904-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngonseu E, Chatterjee S, Wery M. Blocked hepatic-stage parasites and decreased susceptibility to Plasmodium berghei infections in BALB/c mice. Parasitology. 1998;117:419–23. doi: 10.1017/S0031182098003333. [DOI] [PubMed] [Google Scholar]
- 36.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–62. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 37.de Souza JB, Hafalla JCR, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137:755–72. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 38.Lovegrove FE, Gharib SA, Peña-Castillo L, Patel SN, Ruzinski JT, Hughes TR, et al. Parasite burden and CD36-mediated sequestration are determinants of acute lung injury in an experimental malaria model. PLoS Pathog. 2008;4:e1000068. doi: 10.1371/journal.ppat.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malarial anemia: of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 40.Killick-Kendrick R. Parasitic protozoa of the blood of rodents: a revision of Plasmodium berghei. Parasitology. 1974;69:225–37. doi: 10.1017/S0031182000048071. [DOI] [PubMed] [Google Scholar]
- 41.Weiss WR, Good MF, Hollingdale MR, Miller LH, Berzofsky JA. Genetic control of immunity to Plasmodium yoelii sporozoites. J Immunol. 1989;143:4263–6. [PubMed] [Google Scholar]
- 42.Khusmith S, Charoenvit Y, Kumar S, Sedegah M, Beaudoin RL, Hoffman SL. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science. 1991;252:715–8. doi: 10.1126/science.1827210. [DOI] [PubMed] [Google Scholar]
- 43.Stephens R, Culleton RL, Lamb TJ. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol. 2012;28:73–82. doi: 10.1016/j.pt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perlmann H, Kumar S, Vinetz JM, Kullberg M, Miller LH, Perlmann P. Cellular mechanisms in the immune response to malaria in Plasmodium vinckei-infected mice. Infect Immun. 1995;63:3987–93. doi: 10.1128/iai.63.10.3987-3993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persson C, Oliveira GA, Sultan AA, Bhanot P, Nussenzweig V, Nardin E. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169:6681–5. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 46.Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–11. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold L, Tyagi RK, Meija P, Swetman C, Gleeson J, Pérignon JL, et al. Further improvements of the P. falciparum humanized mouse model. PLoS One. 2011;6:e18045. doi: 10.1371/journal.pone.0018045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patarroyo ME, Romero P, Torres ML, Clavijo P, Moreno A, Martínez A, et al. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–32. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 49.Patarroyo ME, Amador R, Clavijo P, Moreno A, Guzman F, Romero P, et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332:158–61. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- 50.Valero MV, Amador LR, Galindo C, Figueroa J, Bello MS, Murillo LA, et al. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia. Lancet. 1993;341:705–10. doi: 10.1016/0140-6736(93)90483-W. [DOI] [PubMed] [Google Scholar]
- 51.Alonso PL, Smith T, Schellenberg JR, Masanja H, Mwankusye S, Urassa H, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet. 1994;344:1175–81. doi: 10.1016/S0140-6736(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 52.Alonso PL, Smith TA, Armstrong-Schellenberg JRM, Kitua AY, Masanja H, Hayes R, et al. Duration of protection and age-dependence of the effects of the SPf66 malaria vaccine in African children exposed to intense transmission of Plasmodium falciparum. J Infect Dis. 1996;174:367–72. doi: 10.1093/infdis/174.2.367. [DOI] [PubMed] [Google Scholar]
- 53.D’Alessandro U, Leach A, Drakeley CJ, Bennett S, Olaleye BO, Fegan GW, et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995;346:462–7. doi: 10.1016/S0140-6736(95)91321-1. [DOI] [PubMed] [Google Scholar]
- 54.Nosten F, Luxemburger C, Kyle DE, Ballou WR, Wittes J, Wah E, et al. Randomised double-blind placebo-controlled trial of SPf66 malaria vaccine in children in northwestern Thailand. Shoklo SPf66 Malaria Vaccine Trial Group. Lancet. 1996;348:701–7. doi: 10.1016/S0140-6736(96)04465-0. [DOI] [PubMed] [Google Scholar]
- 55.Ballou WR. The development of the RTS,S malaria vaccine candidate: challenges and lessons. Parasite Immunol. 2009;31:492–500. doi: 10.1111/j.1365-3024.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 56.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6:90–6. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 57.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 58.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, et al. RTS,S Malaria Vaccine Evaluation Group Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis. 2001;183:640–7. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 59.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Jr., Hall T, et al. RTS,S Malaria Vaccine Evaluation Group A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naïve adults. Vaccine. 2007;25:5359–66. doi: 10.1016/j.vaccine.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Bojang KA, Milligan PJM, Pinder M, Vigneron L, Alloueche A, Kester KE, et al. RTS, S Malaria Vaccine Trial Team Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–34. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 61.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 62.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–8. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 63.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. RTS,S Clinical Trials Partnership First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 65.Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BGNO, Kawende AL, et al. A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med. 2012;367:2284–95. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lal AA, de la Cruz VF, Good MF, Weiss WR, Lunde M, Maloy WL, et al. In vivo testing of subunit vaccines against malaria sporozoites using a rodent system. Proc Natl Acad Sci U S A. 1987;84:8647–51. doi: 10.1073/pnas.84.23.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Przysiecki C, Lucas B, Mitchell R, Carapau D, Wen Z, Xu H, et al. Sporozoite neutralizing antibodies elicited in mice and rhesus macaques immunized with a Plasmodium falciparum repeat peptide conjugated to meningococcal outer membrane protein complex. Frontiers Cell Infect Microbiol. 2012;2:146. doi: 10.3389/fcimb.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 70.Nussenzweig RS, Vanderberg JP, Most H, Orton C. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 1969;222:488–9. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- 71.Gwadz RW, Cochrane AH, Nussenzweig V, Nussenzweig RS. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57(Suppl 1):165–73. [PMC free article] [PubMed] [Google Scholar]
- 72.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266:169–77. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 74.Epstein JE, Rao S, Williams F, Freilich D, Luke T, Sedegah M, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. 2007;196:145–54. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 75.Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 76.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⁺ T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 77.Beaudoin RL, Strome CPA, Mitchell F, Tubergen TA. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp Parasitol. 1977;42:1–5. doi: 10.1016/0014-4894(77)90054-6. [DOI] [PubMed] [Google Scholar]
- 78.Orjih AU, Cochrane AH, Nussenzweig RS. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans R Soc Trop Med Hyg. 1982;76:57–61. doi: 10.1016/0035-9203(82)90019-0. [DOI] [PubMed] [Google Scholar]
- 79.Belnoue E, Costa FTM, Frankenberg T, Vigário AM, Voza T, Leroy N, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172:2487–95. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 80.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 81.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377:1770–6. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 82.Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J Infect Dis. 2009;199:899–903. doi: 10.1086/597121. [DOI] [PubMed] [Google Scholar]
- 83.Friesen J, Matuschewski K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine. 2011;29:7002–8. doi: 10.1126/scitranslmed.3001058. [DOI] [PubMed] [Google Scholar]
- 84.Borrmann S, Matuschewski K. Protective immunity against malaria by ‘natural immunization’: a question of dose, parasite diversity, or both? Curr Opin Immunol. 2011;23:500–8. doi: 10.1016/j.coi.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Silvie O, Goetz K, Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 2008;4:e1000086. doi: 10.1371/journal.ppat.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haussig JM, Matuschewski K, Kooij TW. Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Mol Microbiol. 2011;81:1511–25. doi: 10.1111/j.1365-2958.2011.07787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.VanBuskirk KM, O’Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci U S A. 2009;106:13004–9. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Annoura T, Ploemen IH, van Schaijk BC, Sajid M, Vos MW, van Gemert GJ, et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine. 2012;30:2662–70. doi: 10.1016/j.vaccine.2012.02.010. [DOI] [PubMed] [Google Scholar]