Abstract
The exceptional discoveries of antigen/gene delivery systems have allowed the development of novel prophylactic and therapeutic vaccine candidates. The vaccine candidates employ various antigen-delivery systems, particularly recombinant viral vectors. Recombinant viral vectors are experimental vaccines similar to DNA vaccines, but they use attenuated viruses or bacterium as a carrier “vector” to introduce microbial DNA to cells of the body. They closely mimic a natural infection and therefore can efficiently stimulate the immune system. Although such recombinant vectors may face extensive preclinical testing and will possibly have to meet stringent regulatory requirements, some of these vectors (e.g. measles virus vectors) may benefit from the profound industrial and clinical experience of the parent vaccine. Most notably, novel vaccines based on live attenuated viruses combine the induction of broad, strong and persistent immune responses with acceptable safety profiles. We assess certain technologies in light of their use against human immunodeficiency virus (HIV).
Keywords: vaccines, DNA, live attenuated viruses, HIV, measles, SIV, reverse genetics, recombinant vectors
Introduction
A vaccine works by presenting pieces of the pathogen, virus or bacteria, to the body's immune system that causes an immune reaction. Immunogens or antigens “prime” the immune system so that when it is exposed to the real pathogen, the body’s immune system can efficiently attack and neutralize the incoming pathogen.1
Almost all current vaccines work through the induction of humoral antibodies, IgG and IgA in serum and mucosa. These Antibodies neutralize “functionally” the invasion of a microbe and, thus, prevents infection at a particular level of antibody in the plasma or mucosa. Whereas the neutralizing antibodies can maintain protection, cell-mediated immunity operates as another correlate of protection against a disease.2
While it was feasible to generate vaccines for some of the world’s deadliest diseases (e.g. small pox and measles), no one have been able to find a vaccine against AIDS, caused by HIV.3 There are a number of scientific challenges that can be addressed in order to gain more prospects towards successful HIV-vaccine clinical trials.4 One reason is that scientists still need to know more about the type of immune response(s) to prevent HIV infection and control of HIV replication. Moreover, methods to induce HIV-specific mucosal immunity, optimization of HIV envelope immunogens that induce sterilizing immunity, methods to optimize antiviral T-cell and NK cell activity, and to determine the mechanism of protection of an efficacious vaccine in the nonhuman primate model need extensive optimization. Additionally, research focused on the interaction between HIV and the human immune system may also address many of these unanswered questions and can provide insight for the design and development of more effective vaccine candidates.3,4
Vaccine Approaches and Technologies
A variety of viruses have been investigated for their ability to express heterologous antigens derived from certain pathogens to induce stronger and longer-lasting humoral and cellular immune responses.5,6 Extensive experience has been gathered using subunit vaccine formulation,7 DNA, viral and bacterial vectors (Table 1). Importantly, candidate vaccines should be safe, induce humoral and cellular immune responses against the transgene, and should provide long-lasting protection.8
Table 1. Vaccine Designs and strategies in light of their use against HIV.
| Vaccine Design | How Does this Vaccine Work? | Critical issues |
|---|---|---|
| Viral Proteins or Viral Peptides 7 | Chemically synthesized or produced by mammalian cell lines or Bacteria. These pieces of HIV peptides or proteins that elicit strong T and B cell responses are formulated with lipids and adjuvant in order to induce significant immune responses. | Peptide-based preparations require the addition of an adjuvant to enhance immunogenicity. At present, alum is the only adjuvant authorized by FDA for general medical use, however many alternative products are being tested likely in clinical trials. |
| DNA 9 | Target genes are inserted into a specific site in the backbone of DNA (known as plasmid), under the control of certain promoter to efficiently express the gene. The DNA (vaccine) is injected into muscle of the recipient where the target genes are expressed into proteins. These proteins are then degraded into small peptide fragments, which are presented by MHC I and II molecules on the cell surface to activate humoral and/or cellular responses. |
Clinical trials did not show conclusive efficacy yet. Except for clinical trials, DNA vaccines have not yet been licensed for use in humans by the FDA. |
| Viral (Live or replication deficient) or bacterial vectors13-17 | The HIV or SIV genes are inserted into the genomes of live, infectious, but non-disease-causing forms of viruses (e.g., adenovirus, poxvirus, measles virus) or bacteria (e.g., Bacille Calmette-Guerin (BCG). These vectors shuttle “foreign” genes along with their own into cells. HIV proteins generated from these recombinant genes inside the cell are either secreted or displayed on the cell surface and presented to the immune system. |
The development of viral vectors has been robust, with a few entering Phase III trials. Only a few bacterial vectors are under development in small and large animal models, and some Phase I trials. The complex nature of bacteria hampers the development of bacterial vector systems. |
| Virus-like Particles (VLPs) 23 | Empty, non-infectious shells of the HIV envelope protein; they mimic the outer coat of the virus but lack a genome inside and cannot reproduce. Because VLPs resemble the virus, they can induce high titers of neutralizing antibodies to protect against viral challenge. |
VLPs represent an exciting new strategy for HIV vaccines but it has been difficult to make them reproducibly. |
DNA vaccines
DNA vaccines are circular or linear plasmids that encode pieces of viral genes of interest. After the vaccine is injected into patients the plasmid is taken up by cells at the site of injection and viral genes are expressed into proteins.9 For gene expression to occur a stretch of genetic code called the promoter is necessary. The viral proteins are degraded into small peptide fragments, which are then presented by MHC class I and class II molecules on the cell surface where T cells recognizing these complexes to generate an immune response.
DNA vaccines represent a favored vaccine strategy because they are safe, stable, easy to engineer and produce and immune responses generated pose no interference against later boost immunizations. However, DNA vaccines need to be improved significantly (e.g. by adding molecular adjuvants) as they do not induce sufficient levels of immune responses.9
In addition, optimization of both the coding sequence and the gene regulatory elements may be necessary for high levels of gene expression. Moreover, novel delivery technologies may be necessary [e.g. particle-mediated delivery into antigen presenting cells using, viral or bacterial vectors, lipid polymers and gold microparticles, or virus like particles (VLP)]10-12 and physical delivery methods such as needle-free devices such as epidermal patches.
Live vectors
Live recombinant vector vaccines are constructed by inserting HIV or simian immunodeficiency virus genes into genomes of live, infectious, but non-disease-causing forms of viruses or bacteria such as vaccinia virus13 or Bacille Calmette-Guérin (BCG).14 One can think of these viral and bacterial vectors as backpacks that shuttle the cargo of their own genes as well as “foreign” genes into cells.
Viral vectors
Viruses have evolved sophisticated structures and mechanisms to infect cells, and hence, they serve as efficient delivery vectors of various antigens,15 namely the HIV genes.15-17 Scientists use viruses other than HIV as delivery vehicles or vectors to transport and express HIV genes. The modular nature of viral genomes allows scientists to insert foreign genes and sometimes replace the vector’s own genes with the desired HIV or other genes.18-20 Such a recombinant virus is safe and cannot cause HIV infection. HIV proteins generated from the recombinant genes inside the cell are either secreted or displayed on the cell surface and presented to the immune system in the same way that proteins from a virus-infected cell would be.19,20,22
For a virus to be considered a delivery vehicle, it must have the capacity to accommodate large pieces of foreign genes, maintain genetic stability, be feasible for large-scale manufacturing and most, importantly, not cause disease or be toxic. Viruses used as vaccine vectors can be engineered to retain their replication capacity or be rendered non-replicating, e.g. measles viruses (MV) or Adenoviruses (Adv), respectively. In the replication-defective kind, one or more gene(s) that play a role in virus replication are removed or mutated, and the resultant virus will not reproduce itself as it can only infect one cell.6 Replicating viral vectors infect and reproduce in cells using the cell’s machinery.16,21 The new copies of the virus generated can subsequently infect other cells but because it has been manipulated it cannot cause disease. Several replication-incompetent viral vectors have been tested in clinical trials for HIV vaccines, including Adenovirus and adeno-associated virus, alphavirus and poxvirus.18 Replication competent viral vectors like measles virus, vesicular stomatitis and rabies viruses, have been tested with varying levels of success in preclinical studies in animals.16,19,21 Some vaccine developers are focusing on the use of replicating vectors in clinical trials, as they are more fruitful in animal studies in activating elements of innate immunity, the first responders to an invading organism, and are more likely to induce stronger cellular and mucosal immune responses and antibodies. These effects are achieved at much lower doses than the non-replicating vectors.16,17
Live attenuated viral vectors induce strong, long-lasting immune responses against the expressed proteins, including antibody and T cell responses in the blood, and many generate immune responses at mucosal surfaces, depending on their cell targets and sites of replication.24 Importantly, immune responses can be generated to the vector as well as to the incorporated immunogens. Ironically, immune responses to the vector components could limit the effectiveness of subsequent vaccinations using the same vector.24
Gatekeepers for the Processing of Recombinant Multivalent Vaccine
In order to generate a recombinant viral vector a series of gate-keepers must be set before the advancement of the vector to clinical testing. Among the major challenges are: (1) the stability of the insert and protein expression; (2) The use of properly defined vector backbone (e.g., from a clinically approved original vaccine) and the manufacturing process; (3) Efficacy and safety of the recombinant vector.
Stability of the vector and insert
Among the major challenges in making viral vector-based vaccine candidates, particularly the live attenuated viral vectors, relates to the chemistry, or compatibility between the vector backbone and the insert; for example (1) some vectors are simply incompatible with the insert, due to certain nucleotide sequences that render the vector inefficient; (2) The length of the insert may not be optimal and its configuration is not suitable to the viral vector, thus the vector may simply reject the insert; (3) Consequently, the virus acting as the vector may introduce mutations into the inserted gene (the HIV genes) that may prevent production of the complete protein once inside the body. These changes can ultimately impact the generation of a good immune response after vaccination. It is, therefore, necessary to analyze the stability of these vectors during the early stages of vaccine development, in cell culture and in animal model. The stability of the recombinant vector particles are tested by subjecting them to a series of stress tests, forcing them to more than 10 rounds of amplification in cell culture that determines whether they are stable enough to express foreign proteins (e.g., the HIV proteins) before clinical trials.25 This has been successfully established for MV vectors and other members of paramyxoviridae.16,22
Origin of the vector backbone
The cloning of the vector backbones has not been solely performed for clinical use. Often, replication deficient and subsequently live viral vectors were engineered in academic labs not fully conform to industrial standards and quality control systems. The stepwise development of such vectors resulted, sometimes, in the loss of authenticity8 and thus it became difficult to relate efficacy of such vectors with the authentic vaccine. The first live attenuated MV vector, for example, originated from an attenuated “lab strain” whose efficacy in clinical trials may become obscure irrespective of its success in preclinical tests.16 Therefore, it became necessary and urgent to develop vectors with the same genetic properties and manufacturing procedure as that of the authentic vaccine strain,26 especially if a “parental” vaccine strain is available. The substantial knowledge gathered in the last decade about the replication machinery of different viruses, specifically RNA viruses and the generation of defective interfering particles (DIs), shed the light on the possible errors that such RNA viruses could carry. RNA polymerases are known to be much more error-prone than DNA polymerases due to the lack of proof-reading; thus, the error-rate in RNA viruses is on average about three orders of magnitude higher than in DNA viruses.16 The so-called quasispecies concept for RNA viruses have been explored.16 Therefore, it is necessary to set quality control procedures to assure fitness and suitability of an expression vector for clinical development. Figure 1 shows a comparative study between an attenuated MV “lab strain”, the authentic vaccines and the cloned vector from an authentic-parental vaccine. This example shows that the “lab strain” induced immune responses by an order of magnitude less than the authentic vaccine and the cloned counterpart (MVb) (Fig. 1A). Moreover, the growth kinetics showed that the authentic vaccines and the cloned vector (MVb) were superior to that of the “lab strain” (Fig. 1B).
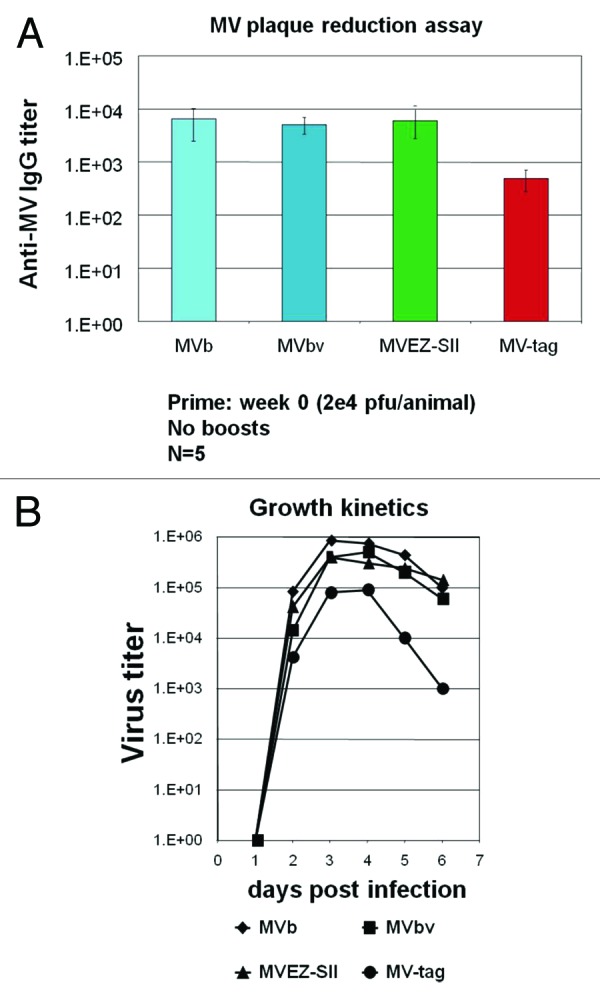
Figure 1. Comparison of the cloned MV vaccine with various commercially available MV vaccine strains and a “lab-strain.” (A) Transgenic mice expressing human CD46 were immunized i.m. with 1 × 104 pfu of an authentic cloned vaccine (rMVb), a Moraten vaccine (MVbv), Edmonston Zagreb vaccine (MVEZII) and a “lab strain” MVtag. Measles end point titers are shown on a logarithmic scale. (B) Growth kinetic analyses. Comparison of the propagation kinetics of the standard MVbv, cloned MVb and the MVEZII compared to the “lab strain” MVtag. Sub-confluent Cells were infected with the designated viruses and incubated at 31°C, media were collected every day up to 6 days. The shed viruses were titrated by plaque assays.
Efficacy and safety
Generally, killed vaccines or subunit vaccines are not necessarily safer than replication-competent vaccines as drastically shown for killed MV vaccine which proved not only to lack efficacy, but even exacerbated the effects of subsequent wild-type MV infection. Thus, there is no general increasing gradient of safety when one compares replication competent versus replication restricted versus dead vaccines. Any component delivered to humans must be planned, developed and evaluated with equal care, without a priori bias based on theoretical general and illusive classifications of danger for health.
What are the consequences of recombinant vaccines to be replication-competent? Replication-deficient vectors such as most DNA viruses in use, and RNA virus vectors based on alpha-viruses are broadly considered to be generally safer. However, replication-deficiency entails major drawback. To be efficiently immunogenic, higher doses (many orders of magnitude in comparison with replicating vehicles) have to be delivered. In contrast, attenuated live vaccines, like MV, are delivered at low doses and are highly efficacious due to systemic spread and preferential infection of professional antigen-presenting cells and lymphoid tissues. In the case of MV, replication competence appears only as an advantage both in terms of safety and efficacy. Due to the extremely good record of safety, MV vaccination is recommended even for immunocompromized patients, e.g. HIV.16 Importantly, cell targeting of MV recombinants is not altered by transgenes.27 Clinical trials with any recombinant vaccine candidate based on MV are only in the planning stage; thus, practical success is still to be awaited in view of the severe and costly hurdles imposed by regulatory agencies.
Discussion
Although recent progress in genetic engineering technique has enabled us to develop live attenuated mutants of several viruses and bacteria as potential vaccine vectors for antigen delivery, certain issues remain answered. The application routes of the vaccines, the quality of the immune responses and whether results in transgenic mice and nonhuman primates are representatives for an efficacious vaccine.
The limitations of the animal models to evaluate AIDS vaccine are now more defined, especially since the choice of challenge viruses affected the outcome of vaccine studies in nonhuman primates.28 Through the study of infection with simian immunodeficiency virus (SIV) - a monkey virus that is similar but not identical to HIV - researchers have uncovered several clues about how the virus is transmitted following infection and disease progression or pathogenesis.29 There is also much to be learned from the study of SIV infection in species of nonhuman primates that can successfully control SIV infection and not develop the monkey equivalent of AIDS.
Before a vaccine candidate can be tested in humans, it is first evaluated extensively in laboratory tests, cell culture and animal models. Animal models help scientists gain important insights into the diseases and how to prevent them and to determine if a candidate vaccine is safe to administer in people.
The industrial partners, involved in the manufacturing and production of clinical lots, have contributed substantially to the understanding of the vector intrinsic requirement, vector genetic’s background, the cell substrate necessary for an efficient growth and the maintenance of stable protein expression upon multiple rounds of amplification. Not only the genetic structure of the insert is the sole determinant for an efficient and stable protein expression but the genetic stability of the vector backbone determines vector fitness and the subsequent stable amplification of the progeny; especially the RNA live viral vectors (e.g. mononegavirales).
The pre-existing immunity to most of the used vectors is a major concern, especially since multiple immunization doses are used in clinical trials. There are various studies that propose heterologous prime-boost scenarios, e.g. use of two different vectors carrying the same insert,30 or heterologous immunization routes e.g. intramuscular-intranasal.24 Scientists have, also, begun to shed light on analyzing data from various HIV vaccine trials conducted, where some (Thailand, RV144) have shown modest success in preventing HIV infection.31 These analyses are providing some hint as to what type of immune responses may be needed and will help inform future clinical trial design.
Acknowledgements
This study was supported by NIH grant AI-46007 to H.Y.N. I would like to thank all members of my Group who contributed to the success of MV vector. During the preparation of this review, H.Y.N. was a part-time faculty member at the Lebanese American University.
Glossary
Abbreviations:
- MV
measles virus
- rMV
recombinant measles viruses
- HIV
human immunodeficiency virus
- VLP
virus like particles
Disclosure of Potential Conflicts of Interest
The contents of this review can be used or disclosed for commercial purposes after a written consent from the author.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23220
References
- 1.Plotkin SA. Vaccines: past, present and future. Nat Med. 2005;11(Suppl):S5–11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis. 2012;54:1615–7. doi: 10.1093/cid/cis238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girard MP, Plotkin SA. HIV vaccine development at the turn of the 21st century. Curr Opin HIV AIDS. 2012;7:4–9. doi: 10.1097/COH.0b013e32834ddc96. [DOI] [PubMed] [Google Scholar]
- 4.Johnston MI, Fauci AS. An HIV vaccine--challenges and prospects. N Engl J Med. 2008;359:888–90. doi: 10.1056/NEJMp0806162. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Sandoval A, Harty JT, Todryk SM. Viral vector vaccines make memory T cells against malaria. Immunology. 2007;121:158–65. doi: 10.1111/j.1365-2567.2006.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, et al. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J Gen Virol. 2006;87:2135–43. doi: 10.1099/vir.0.81956-0. [DOI] [PubMed] [Google Scholar]
- 7.Glück R, Burri KG, Metcalfe I. Adjuvant and antigen delivery properties of virosomes. Curr Drug Deliv. 2005;2:395–400. doi: 10.2174/156720105774370302. [DOI] [PubMed] [Google Scholar]
- 8.Tangy F, Naim HY. Live attenuated measles vaccine as a potential multivalent pediatric vaccination vector. Viral Immunol. 2005;18:317–26. doi: 10.1089/vim.2005.18.317. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162:171–82. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeltins A. Construction and Characterization of Virus-Like Particles. Rev Mol Biotechnol. 2012 doi: 10.1007/s12033-012-9598-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24:4062–81. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elena Gómez C, Perdiguero B, Garcia-Arriaza J, Esteban M. Poxvirus vectors as HIV/AIDS vaccines in humans. Hum Vaccin Immunother. 2012;8:1192–207. doi: 10.4161/hv.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch A, Meyers AE, Williamson AL, Rybicki EP. Stability studies of HIV-1 Pr55gag virus-like particles made in insect cells after storage in various formulation media. Virol J. 2012;9:210. doi: 10.1186/1743-422X-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauter SL, Rahman A, Muralidhar G. Non-replicating viral vector-based AIDS vaccines: interplay between viral vectors and the immune system. Curr HIV Res. 2005;3:157–81. doi: 10.2174/1570162053506900. [DOI] [PubMed] [Google Scholar]
- 16.Billeter MA, Naim HY, Udem SA. Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr Top Microbiol Immunol. 2009;329:129–62. doi: 10.1007/978-3-540-70523-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liniger M, Zuniga A, Naim HY. Use of viral vectors for the development of vaccines. Expert Rev Vaccines. 2007;6:255–66. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- 18.Spielhofer P, Bächi T, Fehr T, Christiansen G, Cattaneo R, Kaelin K, et al. Chimeric measles viruses with a foreign envelope. J Virol. 1998;72:2150–9. doi: 10.1128/jvi.72.3.2150-2159.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mebatsion T, Conzelmann KK. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci U S A. 1996;93:11366–70. doi: 10.1073/pnas.93.21.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mebatsion T, Finke S, Weiland F, Conzelmann KKA. A CXCR4/CD4 pseudotype rhabdovirus that selectively infects HIV-1 envelope protein-expressing cells. Cell. 1997;90:841–7. doi: 10.1016/S0092-8674(00)80349-9. [DOI] [PubMed] [Google Scholar]
- 21.Schnell MJ. Viral vectors as potential HIV-1 vaccines. FEMS Microbiol Lett. 2001;200:123–9. doi: 10.1111/j.1574-6968.2001.tb10703.x. [DOI] [PubMed] [Google Scholar]
- 22.Zuniga A, Wang Z, Liniger M, Hangartner L, Caballero M, Pavlovic J, et al. Attenuated measles virus as a vaccine vector. Vaccine. 2007;25:2974–83. doi: 10.1016/j.vaccine.2007.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye L, Wen Z, Dong K, Wang X, Bu Z, Zhang H, et al. Induction of HIV neutralizing antibodies against the MPER of the HIV envelope protein by HA/gp41 chimeric protein-based DNA and VLP vaccines. PLoS One. 2011;6:e14813. doi: 10.1371/journal.pone.0014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knuchel MC, Marty RR, Azzouz Morin TN, Ilter O, Zuniga A, Naim HY. Relevance of a pre-existing measles immunity prior immunization with a recombinant measles vector. Hum Vaccin Immunother. 2012 doi: 10.4161/hv.23241. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Hangartner L, Cornu TI, Martin LR, Zuniga A, Billeter MA, et al. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine. 2001;19:2329–36. doi: 10.1016/S0264-410X(00)00523-5. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga A, Liniger M, Azzouz Morin TN, Marty RR, Wiegand M, Ilter O, et al. Sequence and immunogenicity of a clinically approved novel measles virus vaccine vector. Hum Vaccin Immunother. 2012 doi: 10.4161/hv.23242. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liniger M, Zuniga A, Morin TN, Combardiere B, Marty R, Wiegand M, et al. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine. 2009;27:3299–305. doi: 10.1016/j.vaccine.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee EM, Chung HK, Livesay J, Suschak J, Finke L, Hudacik L, et al. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J Virol Methods. 2010;163:287–94. doi: 10.1016/j.jviromet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Watkins DI. HIV vaccine development. Top HIV Med. 2010;18:35–6. [PubMed] [Google Scholar]
- 30.Bolton DL, Santra S, Swett-Tapia C, Custers J, Song K, Balachandran H, et al. Priming T-cell responses with recombinant measles vaccine vector in a heterologous prime-boost setting in non-human primates. Vaccine. 2012;30:5991–8. doi: 10.1016/j.vaccine.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEnery R, Kresge KJ. First Evidence of Efficacy from Large-Scale HIV Vaccine Trial Results from a trial in Thailand show that the combination of two vaccine candidates provides some protection against HIV infection. VAX, 2009; Vol. 07, No. 09 [Google Scholar]


