Abstract
Asymptomatic throat carriage of Neisseria meningitidis is common in healthy individuals. In unusual cases, the bacteria become invasive, resulting in life-threatening disease. Effective meningococcal serogroup B (MnB) vaccines should provide broad protection against disease-causing strains and may confer indirect protection by impacting carriage and subsequent transmission. Factor H binding proteins (fHBPs), components of MnB vaccines in development, are classified into two immunologically distinct subfamilies (A and B). fHBP variants of MnB strains carried by adolescents are similar to those detected in infants with MnB disease. A vaccine containing subfamily A and B fHBP variants elicited bactericidal antibody responses (titers ≥ 1:4) against MnB strains expressing fHBP variants common to carriage strains and strains that cause disease in adolescents and infants in 75–100% of adolescent study subjects. This suggests that the bivalent fHBP vaccine has the potential to provide protection against invasive MnB strains and interrupt meningococcal carriage, which may also reduce infant MnB disease.
Keywords: Neisseria meningitidis serogroup B, meningococcal carriage, factor H binding protein, meningococcal vaccines, herd protection
Introduction
Neisseria meningitidis is a Gram-negative bacterium commonly found in the throat and nasal passages of healthy individuals. Transfer of bacteria between individuals is thought to occur through direct contact with respiratory and throat secretions and is more common among individuals living in crowded conditions (e.g., college dormitories or military barracks).1-3 Although meningococcal carriage is usually asymptomatic, in rare cases and for unknown reasons, the bacteria can enter the bloodstream and cause a life-threatening invasive infection. Invasive meningococcal disease progresses rapidly and, despite the availability of sophisticated medical care, continues to be associated with a 5% to 15% case fatality rate as well as devastating sequelae including limb loss, epilepsy, mental retardation and deafness.4,5 The burden of invasive meningococcal disease is often underappreciated because of the low incidence: 0.28 cases per 100,000 persons in the United States (US) and 0.89 cases per 100,000 persons in Europe.6,7
Twelve capsular serogroups of N. meningitidis have been identified; however, five (A, B, C, W and Y) are responsible for most cases of invasive disease.8,9 Analyses of carriage and disease-causing strains in Greece, Norway and the Czech Republic have demonstrated that prevalence of variation among serogroups and clonal complexes occurs in geographically distinct areas, and that the serogroups and clonal complexes associated with hypervirulent strains responsible for most cases of invasive disease are also common among carriage isolates.3,10,11 The prevalence of meningococcal carriage may vary among geographic regions, but generally is believed to increase through childhood, from 4.5% in infants to a peak at 23.7% in adolescents (19 y of age).12
Vaccination with polysaccharide conjugate meningococcal serogroup C (MnC) vaccines is highly effective in preventing invasive disease. All European countries with routine MnC vaccination programs have substantially reduced incidence of MnC disease, particularly those that implemented a program that included vaccination of an adolescent group.13 The MnC conjugate vaccination campaign in the United Kingdom (UK), which included vaccination of individuals between 2 mo and 18 y of age when it was initiated in 1999, resulted in a 66% reduction (p = 0.004) of MnC carriage in the group 15 to 17 y of age.14 Two to three years after the vaccination campaign began, even unvaccinated individuals, 1 to 18 y of age, showed a decreased incidence of invasive disease suggesting the induction of herd protection as a result of vaccination.15,16 Herd protection extends to infants who have not received the complete MnC vaccination course and to individuals > 25 y of age who would not have been offered MnC vaccines.15
Following the introduction of polysaccharide conjugate vaccines that target serogroups A, C, W and Y, meningococcal serogroup B (MnB) remains a prominent cause of invasive meningococcal disease in the US and Europe.6,7,17 Infants and toddlers have the highest incidence of invasive MnB disease (IMBD), with a second incidence peak often noted for adolescents and young adults, resulting in significant disease burden in these age groups (Fig. 1A and C).6,18 Analyses of case numbers by age reveal that the total case numbers in the group 10 to 25 y of age are often greater than the total numbers seen in infants (Fig. 1B and D). This is evident in many European countries with the expeption of Greece and the UK where more cases in the infant age group are reported (Fig. 1D). Multiple studies across Europe have determined that MnB is a prevalent serogroup identified among carriage isolates.19 In the US, MnB and meningococcal serogroup Y are the most common identifiable serogroups of carriage isolates.20 Available evidence from Latin America also suggests that meningococcal carriage is common, and serogroup B, C, Y and W isolates have been identified.21-23
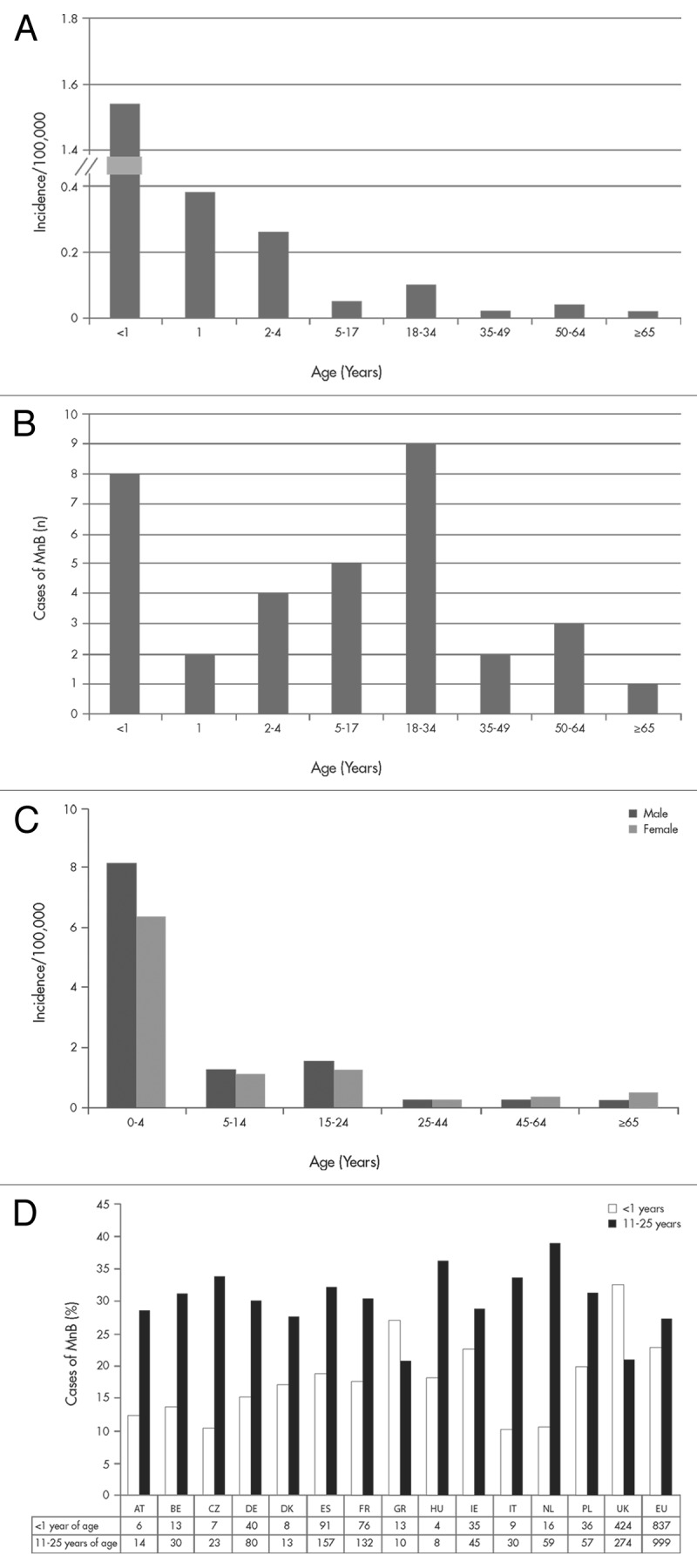
Figure 1. Summary of the age distribution of invasive meningococcal serogroup B (MnB) disease in 2009 from the United States (A and B) and the European Union (C and D). Panels A and C list incidence rate per 100,000. Panel B provides the estimated number of cases in the US. Panel D illustrates the proportion (and number of cases) in European Centre for Disease Prevention and Control (ECDC) participating countries for infants (< 1 y of age) and adolescents and young adults (11–25 y of age). US data were obtained from the Centers for Disease Control and Prevention (CDC) Active Bacterial Core Surveillance sites which includes 32 of the 174 reported cases of notifiable disease in 2009.6,17 The European data were obtained from the ECDC and European Surveillance System (TESSy),7 which included country reports from Austria (AT), Belgium (BE), Cyprus, the Czech Republic (CZ), Denmark (DK), Estonia (ES), Finland, France (FR), Germany (DE), Greece (GR), Hungary (HU), Iceland, Ireland (IE), Italy (IT), Latvia, Lithuania, Luxembourg, Malta, Netherlands (NL), Norway, Poland (PL), Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom (UK).
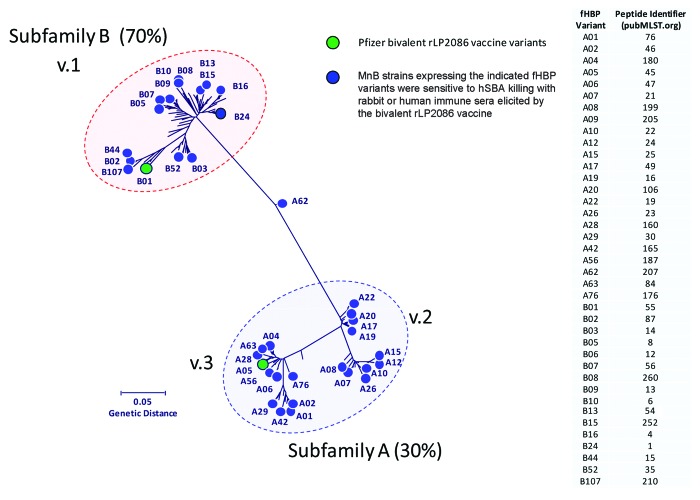
Development of serogroup B capsular polysaccharide-based vaccines has not been successful, and MnB vaccines based on the outer membrane protein porin A (PorA) have been effective only against epidemic MnB strains.24,25 No broadly protective vaccine is currently licensed for the prevention of IMBD. Two vaccines are in late-stage development and include meningococcal factor H binding proteins (fHBPs) as key antigens for the generation of serum bactericidal antibodies.26 fHBP (also known as LP2086) is a lipoprotein expressed on the surface of most meningococcal isolates regardless of serogroup.27 By binding to human factor H, fHBP downregulates complement-mediated bacterial cell lysis and allows N. meningitidis to evade host immune responses.28,29 Sequence analysis has shown that fHBP is expressed as one of two serologically distinct subfamilies (A and B).27 Researchers have also used an alternate scheme that classifies the protein into 3 variants, variant 1 corresponds to subfamily B fHBP, variants 2 and 3 to subfamily A fHBP.30 The fHBP amino acid sequences are at least 83% identical within each fHBP subfamily but are less conserved (60–75% sequence identity) between subfamilies A and B.27,31 Lipidated recombinant fHBP is capable of eliciting subfamily-specific bactericidal responses against a range of MnB strains and is protective against nasopharyngeal colonization in animal models.31,32 One lipidated protein from each fHBP subfamily was required to elicit broad serum bactericidal activity against MnB strains that express phylogenetically diverse fHBP variants (Fig. 2).33
Figure 2. The phylogenetic tree of factor H binding protein (fHBP) variants expressed by meningococcal serogroup B (MnB) strains killed in serum bactericidal antibody assay with human complement (hSBA). The phylogenetic tree of fHBP proteins is based on a clustal alignment and drawn using MEGA 5. Subfamily B (≈70% of isolates) is equivalent to variant 1, and subfamily A (≈30% of isolates) encompasses variants 2 and 3.27,41 The fHBP proteins included as components of the Pfizer lipidated bivalent rLP2086 vaccine are indicated in green. Novartis 4CMenB variant 1.1 = B24.
Evaluation of the potential impact of MnB vaccines on carriage by adolescents will be of interest to assess the possibility for indirect benefits in other age groups (herd protection).16 Understanding the molecular epidemiology of both invasive disease strains and carriage isolates may help predict the potential breadth of coverage provided by MnB vaccines. Potential coverage can be assessed by evaluating vaccine-induced immune sera in serum bactericidal antibody assays using human complement (hSBA) against diverse MnB isolates selected based on antigen diversity. hSBA responses have been correlated with protection in humans and are currently used as surrogates to determine the efficacy of MnB vaccines in late stage development.34,35 We report here that adolescent and young adult subjects immunized with the bivalent rLP2086 vaccine demonstrate high response rates (hSBA titer ≥ 1:4) against MnB strains expressing fHBP variants that are prevalent among both carriage and invasive disease isolates.
Results
Estimating the potential coverage of LP2086 in invasive meningococcal disease and efficacy of the bivalent rLP2086 vaccine
Infants, adolescents and young adults represent the most important at-risk age groups for IMBD. As such, it is useful to determine potential variation among antigen composition of invasive and carriage strains associated within these age groups. As shown in Figure 3, fHBP subfamily prevalence differs across age groups, with subfamily A fHBP variants being more commonly detected in IMBD isolates in US infants and subfamily B fHBP variants being more common in UK infants and US/UK adolescents and young adults. This suggests that for a vaccine to be considered broadly protective, serum bactericidal activity needs to be demonstrated against MnB isolates regardless of which fHBP variant they express.
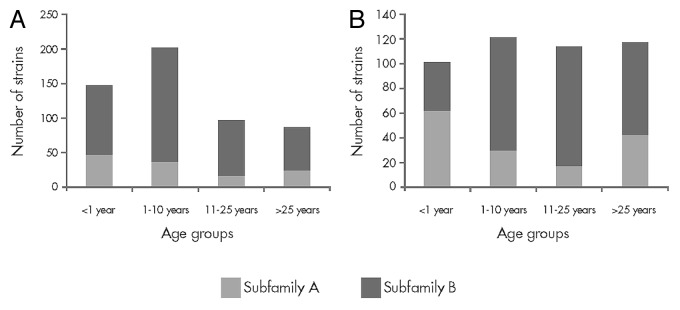
Figure 3. Factor H binding protein subfamily prevalence for invasive meningococcal serogroup B disease isolates obtained from 2001–2006 in (A) the United Kingdom and (B) the United States Active Bacterial Core Surveillance sites. Data from the UK Healthcare Protection Agency18 and the US Centers for Disease Control.6
A relatively limited number of variants have been associated with most disease-causing isolates. For example, in a collection of 445 IMBD isolates from adolescents collected in the US, Norway, Germany, Spain, the Czech Republic, France and the UK (2000–2006), only 13 fHBP variants associated with MnB isolates were responsible for 87% of disease (Fig. 4).
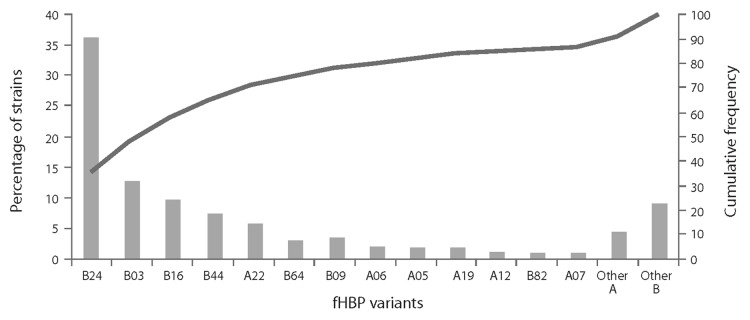
Figure 4. Distribution of factor H binding protein (fHBP) variants in invasive meningococcal serogroup B disease (IMBD). IMBD isolates were obtained from a prevalence-based collection of IMBD isolates collected from reference laboratories (US, Norway, Czech Republic, Germany, Spain, France, England, Wales and Northern Ireland spanning the period of 2000 to 2006).
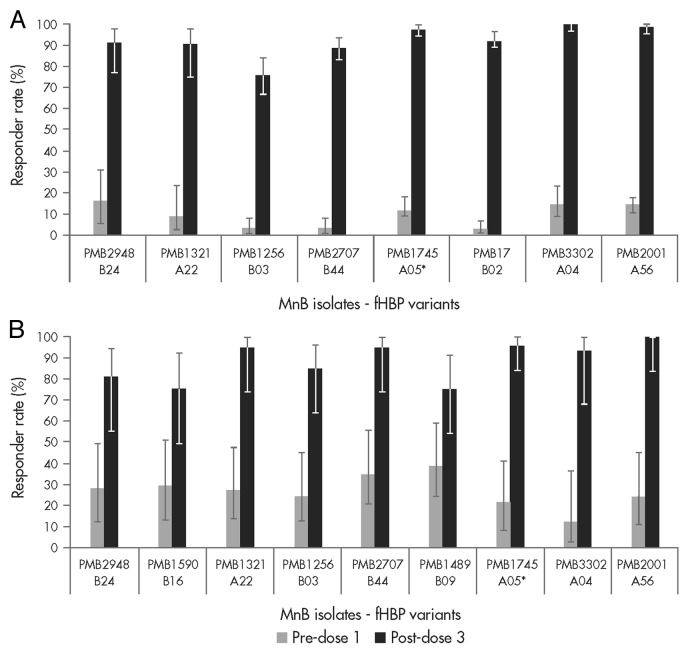
To estimate the potential coverage elicited by the bivalent rLP2086 vaccine against IMBD for adolescents and young adults, immune sera from vaccinated study subjects were tested in hSBA against a panel of invasive MnB strains.39 The panel of strains included fHBP variants that represent approximately 70% of the variants that caused disease in these populations. The post-vaccination hSBA response rates to the individual strains ranged from 75% to 100% in both the adolescent and young adult age groups (Fig. 5A and B, respectively). High hSBA responses were observed regardless of fHBP subfamily, subgroup, variant or other epidemiologic markers.
Figure 5. The bivalent factor H binding protein (fHBP) vaccine has the potential to protect against meningococcal strains expressing either subfamily A or B fHBP variants. (A) The percentage of adolescent subjects (11 to 18 y of age) with an hSBA titer of ≥ 1:4; data from hSBAs performed with 8 meningococcal serogroup B (MnB) isolates expressing different fHBP variants are shown. Sera were obtained from study B1971005.39 (B) The percentage of young adult subjects (18 to 25 y of age) with an hSBA titer of ≥ 1:4; data from hSBAs performed with meningococcal serogroup B (MnB) isolates expressing 9 different fHBP variants are shown. Sera were obtained from study B1971003.40 hSBA titers of serum samples before (light gray bars) and after vaccination (dark gray bars) are illustrated. *fHBP variants homologous to bivalent rLP2086 vaccine antigens.
Characterization of N. meningitidis carriage isolates in adolescents and young adults in relation to serogroup and fhbp variant
During the mass-vaccination campaign and implementation of MnC polysaccharide-conjugate vaccine in the UK, control of MnC disease was associated with a reduction in invasive MnC disease and MnC carriage.14 Vaccination of adolescents not only reduced MnC disease in the vaccinated age group, it also reduced MnC carriage, transmission and disease incidence in unvaccinated individuals via herd protection.16 We therefore conducted an assessment of carriage isolates from the UK and the US to estimate whether the bivalent fHBP vaccine also might have the potential to decrease MnB carriage. The most common carriage serogroups in the US and UK are B and Y, although 29E also seems to be prevalent in the UK (Fig. 6).2,20 The fHBP gene was detected in all carriage isolates and expressed in most carriage isolates. When fHBP gene sequences were translated into fHBP protein sequences (variants), the variants segregated into the same two subfamilies that were previously established for IMBD isolates (Table 1).2,20
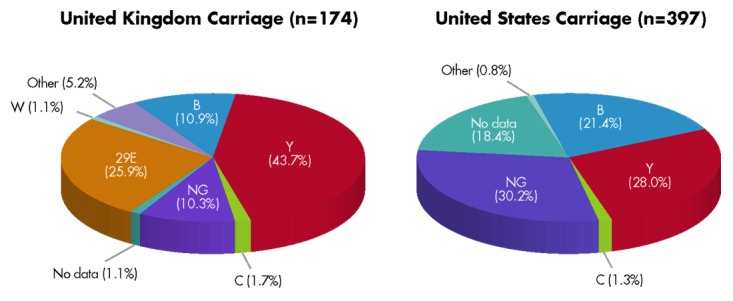
Figure 6. Distribution of carriage isolates from the United Kingdom and United States across meningococcal serogroups. Data from Bidmos et al.2 (United Kingdom, 2008–2009 academic year) and Marsh et al.20 (United States, 1998 and 2006–2007). NG represents non-groupable strains.
Table 1. fHBP subfamily and meningococcal serogroup of carriage isolates from the United Kingdom and United States.
| Serogroup (number of UK strains/number of US strains) | United Kingdom | United States | ||
|---|---|---|---|---|
| Subfamily A, % | Subfamily B, % | Subfamily A, % | Subfamily B, % | |
| B (19/85) | 36.8 | 63.2 | 91.8 | 8.2 |
| Y (76/111) | 69.7 | 30.3 | 94.6 | 5.4 |
| C (3/5) | 100.0 | 0 | 60.0 | 40.0 |
| 29E (45/0) | 0 | 100.0 | - | - |
| W (2/0) | 100.0 | 0 | - | - |
| H (1/0) | 100.0 | 0 | - | - |
| X (1/0) | 0 | 100.0 | - | - |
| Z (7/3) | 71.4 | 28.6 | 100.0 | 0 |
| NG (18/120) | 55.6 | 44.4 | 32.5 | 67.5 |
| No data (2/73) | 0 | 100.0 | 80.8 | 19.2 |
Assessment of the potential impact of the bivalent fHBP vaccine on serogroup B isolates carried in adolescents
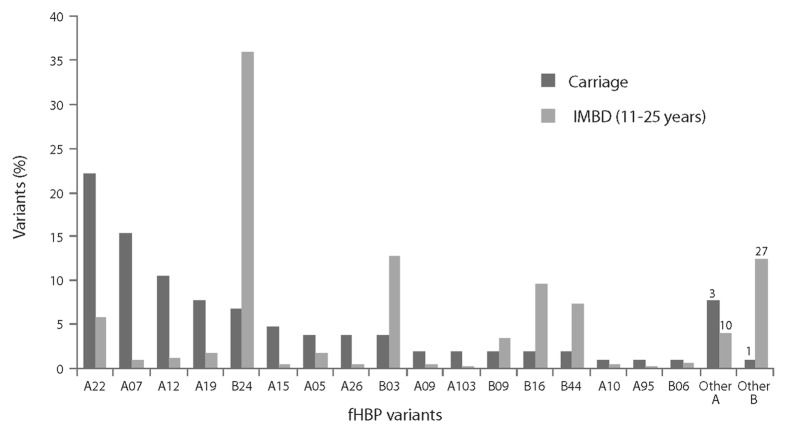
To assess whether vaccination of adolescents with the bivalent fHBP vaccine might impact meningococcal carriage, we compared fHBP variants expressed by carriage and IMBD isolates in adolescents and young adults (Fig. 7). Seventeen common fHBP variants in carriage isolates (> 90% of isolates analyzed) were also detected in 83% of IMBD isolates in this age group. While the same fHBP variants were presented in both IMBD and carriage isolates, the prevalence of the variants differed between the carriage and invasive disease isolates. For example, a relatively high proportion of subfamily A variants was detected in carriage isolates. However, fHBP B24 variants were often associated with IMBD but were less commonly identified among carriage strains (Fig. 7).
Figure 7. Factor H binding protein (fHBP) variants detected in United Kingdom (2008–2009 academic year) and United States (1998 and 2006–2007) carriage strains (n = 104) and invasive meningococcal B (IMBD) strains from adolescents and young adults (11 to 25 y of age) the United States and Europe (n = 445) from 2001–2006. The number of unique fHBP variants included in other “subfamily A” and “other subfamily B” are indicated above the bars
Assessment of potential impact of a bivalent rLP2086 vaccine on carriage of serogroup B meningococci in infants
Based upon results of vaccination campaigns with serogroup C meningococcal polysaccharide conjugates, a MnB vaccine that impacts carriage might be expected to reduce transmission across age groups and reduce disease via herd protection. Infants, who have the highest incidence of disease, might benefit greatly from a broadly protective vaccine. However, direct vaccine impact may be limited, as a high proportion of MnB disease occurs in infants under the age of 6 mo (personal communication from Amanda Cohn, CDC) and multiple doses of a MnB vaccine may need to be administered to elicit broadly protective immune responses in young infants. In this likely situation, optimal vaccine response would only be induced after 6 mo of age and would not prevent illness during the peak months of disease incidence. fHBP variants identified in carriage isolates in adolescents and young adults were compared with fHBP variants present in strains that caused IMBD in infants (Fig. 8). fHBP variants prevalent in adolescents and young adult carriage isolates were also prevalent in strains isolated from infants with invasive disease. Specifically, 16 fHBP variants representing 89% of adolescent/young adult carriage isolates accounted for 78% of the IMBD isolates from infants (< 1 y). The relative proportion of fHBP variants detected in infant IMBD isolates was similar to that seen in carriage isolates from adolescents. For example, A22 was the most prevalent fHBP variant in both adolescent carriage and infant IMBD isolates.
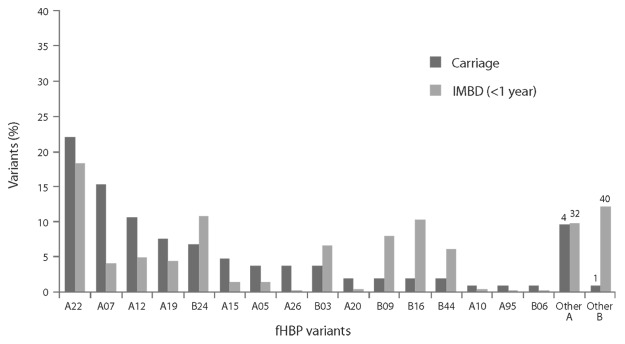
Figure 8. Factor H binding protein (fHBP) variants detected in meningococcal serogroup B (MnB) carriage isolates (n = 104) from adolescents and young adults (11 to 25 y of age; 1998, 2006–2007 and the 2008–2009 academic year) and MnB invasive disease (IMBD) isolates (n = 469) from infants (< 1 y of age) from 2001–2006. The number of unique fHBP variants included in “other subfamily A” and “other subfamily B” are indicated above the bars.
Methods
Data sources
Research presented in this manuscript is based on a comparison of fHBP variant prevalence for both invasive and carriage meningococcal strains. fHBP sequences from IMBD isolates were obtained as described in Hoiseth et al. (manuscript in preparation; also presented by Zlotnick et al.36) and Murphy et al.27 fHBP sequences were surveyed from a prevalence-based collection of IMBD isolates collected from reference laboratories in the US, Norway, the Czech Republic, Germany, Spain, France and the UK (England, Wales and Northern Ireland) spanning the period of 2000 to 2006. Variants were grouped by the age of the subject from whom the isolates were obtained. Specifically, the age stratifications used were < 1 y (infants) and 11 to 25 y (adolescents and young adults).
fHBP and serogrouping data from N. meningitidis carriage isolates were retrieved from two studies conducted in the US and the UK. The US data were obtained from Marsh et al.20 who characterized N. meningitidis carriage isolates from high school students. Serogrouping recognized serogroups B, C, Y and Z; other strains for which data were available were considered non-groupable. The UK data were collected in college students and encompassed carriage isolates characterized according to fHBP variant and meningococcal serogroup.2,37 Serogroups recorded were B, C, 29E, Y, H, X, W, Z and other non-groupable strains.
To estimate potential breadth of vaccine protection, serum samples from healthy individuals immunized with the bivalent rLP2086 vaccine were assayed for bactericidal activity using hSBA.38 Subjects who had an hSBA titer ≥ 1:4 were considered responders. Serum samples from subjects enrolled in two clinical studies were considered and are presented here as a summary of results previously published. The first study was a randomized, single-blind, placebo-controlled trial of the bivalent rLP2086 vaccine in adolescents conducted at 25 sites in Australia, Poland and Spain.39 As part of this study, 198 adolescents (11 to 18 y of age) were immunized using a 0, 2 and 6 mo vaccination schedule with 120 µg of the bivalent rLP2086 vaccine. hSBA responses presented in this study were assessed before the first vaccination and 1 mo postdose 3. The second study was a randomized, phase 1/2, open-label study of the bivalent rLP2086 vaccine conducted in healthy adults (18 to 40 y of age) in Australia.40 As part of this study, healthy young adults (18 to 25 y of age) were immunized using a 0, 1 and 6 mo vaccination schedule with 120 µg of the bivalent rLP2086 vaccine. Serum samples were taken before vaccination and 29 to 50 d postdose 3. SBA assays were performed on samples from 19 to 26 subjects.
Conclusion
The bivalent rLP2086 vaccine contains lipidated recombinant versions of both subfamily A and subfamily B fHBP variants. In clinical trials, this vaccine induced robust serum bactericidal antibody responses against a broad range of MnB isolates. In the current study, most fHBP variants were noted to be common between IMBD and meningococcal carriage isolates, and all fHBP variants studied, irrespective of whether they are expressed by adolescent carriage or infant invasive disease isolates, reside in the same phylogenetic space. The proportion of individuals with hSBA titers ≥ 1:4 among subjects vaccinated with the bivalent rLP2086 vaccine ranged from 75% to 100% against MnB strains with fHBP variants common to both IMBD and meningococcal carriage isolates. These findings suggest that the bivalent rLP2086 vaccine has promise for direct protection against a broad range of IMBD strains expressing diverse subfamily A and B fHBP variants in adolescents and young adults, as well as for indirect protection in unvaccinated individuals. Future assessments of the ability of the bivalent vaccine to impact carriage directly among vaccinated individuals and the potential effect on meningococcal disease and carriage in non-vaccinated individuals through herd protection is of interest. If the bivalent rLP2086 vaccine is effective at interrupting MnB carriage in adolescents, additional benefits may be seen in unvaccinated adults, adolescents and infants through the effects of herd protection.
Acknowledgments
Clinical Investigators: Peter Richmond, The Telethon Institute for Child Health Research, Subiaco, Australia; Helen Marshall, Vaccinology and Immunology Research Trials Unit, Women’s and Children’s Hospital and School of Paediatrics and Reproductive Health, University of Adelaide, North Adelaide, Australia; Michael Nissen, Queensland Paediatric Infectious Diseases Laboratory, Queensland Children’s Medical Research Institute, Royal Children’s Hospital and University of Queensland, Queensland, Australia; Maria Garcés-Sánchez, C.S. Nazaret, Valencia, Spain; Federico Martinón-Torres, Hospital Clínico Universitario de Santiago de Compostela and Vaccine Research Unit, Instituto de Investigación Sanitaria de Santiago, Santiago de Compostela, Spain; Leszek Szenborn, Department of Pediatric Infectious Diseases, Wroclaw University of Medicine, Wroclaw, Poland; Jacek Wysocki, Poznań University of Medical Sciences, Poznań, Poland; Graham Reynolds, Australian National University Medical School, Department of Paediatrics and Child Health, Canberra, Australia; John Ziegler, Sydney Children’s Hospital, Randwick, New South Wales, Australia.
Collaborators providing clinical IMBD isolates include R. Borrow and J. Findlow (Health Protection Agency, Manchester Royal Infirmary, Manchester, UK); M.K. Taha and A.E. Deghmane (Institut Pasteur, Invasive Bacterial Infections Unit, Paris, France); P. Kriz, M. Musilek and J. Kalmusova (National Institute of Public Health, Prague, Czech Republic); D.A. Caugant and T. Alvestad (Norwegian Institute of Public Health, Oslo, Norway); L.W. Mayer, A. Cohn and J. McNeil and X. Wang (Centers for Disease Control and Prevention, Atlanta, Georgia, USA); D. Martin (Institute of Environmental Science and Research, Porirua, New Zealand).
Collaborators providing carriage isolates include D. Ala'Aldeen, N. Oldfield, S. Orner and D. Turner (University of Nottingham, UK).
Glossary
Abbreviations:
- fHBP
factor H binding protein
- hSBA
serum bactericidal antibody assay with human complement
- IMBD
invasive MnB disease
- MnB
meningococcal serogroup B
- MnC
meningococcal serogroup C
- UK
United Kingdom
- US
United States
Disclosure of Potential Conflicts of Interest
All authors are employees of Pfizer Inc. Editorial/medical writing support was provided by John Clinton Earnheart, PhD and Robert Glover, PhD, at Scientific Strategy Partners, and was funded by Pfizer Inc.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23222
References
- 1.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B71–7. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala’Aldeen DA, Bayliss CD. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol. 2011;49:506–12. doi: 10.1128/JCM.01322-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jounio U, Saukkoriipi A, Bratcher HB, Bloigu A, Juvonen R, Silvennoinen-Kassinen S, et al. Genotypic and phenotypic characterization of carriage and invasive disease isolates of Neisseria meningitidis in Finland. J Clin Microbiol. 2012;50:264–73. doi: 10.1128/JCM.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girard MP, Preziosi MP, Aguado MT, Kieny MP. A review of vaccine research and development: meningococcal disease. Vaccine. 2006;24:4692–700. doi: 10.1016/j.vaccine.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Granoff DM, Harrison LH, Borrow R. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, Offit P, eds. Vaccines. Philadelphia: Saunders Elsevier, 2008:399-434. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network, Neisseria meningitidis, 2009. Available via the Internet: http://www.cdc.gov/abcs/reports-findings/survreports/mening09.pdf 2010.
- 7.European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011. Reporting on 2009 surveillance data and 2010 epidemic intelligence data. Stockholm: ECDC. http://ecdc.europa.eu/en/publications/Publications/1011_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf 2011.
- 8.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 9.Zollinger WD, Boslego J. Immunologic Methods for Diagnosis of Infections by Gram-Negative Cocci. In: Rose NR, ed. Manual of clinical laboratory immunology. Washington, DC: ASM Press, 1997:473-83. [Google Scholar]
- 10.Germinario C, Tafuri S, Napoli C, Montagna MT, Balducci MT, Fortunato F, et al. Young-adult carriers of Neisseria meningitidis in Puglia (Italy): will the pattern of circulating meningococci change following the introduction of meningococcal serogroup C conjugate vaccines? Hum Vaccin. 2010;6:1025–7. doi: 10.4161/hv.6.12.13145. [DOI] [PubMed] [Google Scholar]
- 11.Yazdankhah SP, Kriz P, Tzanakaki G, Kremastinou J, Kalmusova J, Musilek M, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol. 2004;42:5146–53. doi: 10.1128/JCM.42.11.5146-5153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:853–61. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 13.Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31:101–7. doi: 10.1111/j.1574-6976.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 14.Maiden MC, Stuart JM, UK Meningococcal Carraige Group Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359:1829–31. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–7. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Summary of notifiable diseases—United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1–100. [PubMed] [Google Scholar]
- 18.UK Health Protection Agency. Meningococcal Reference Unit: Isolates of Neisseria menengitidis; England and Wales, by serogroup & calendar year, 1998-2009. http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1272032921946?pri 2010.
- 19.Soriano-Gabarró M, Wolter J, Hogea C, Vyse A. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev Anti Infect Ther. 2011;9:761–74. doi: 10.1586/eri.11.89. [DOI] [PubMed] [Google Scholar]
- 20.Marsh JW, Shutt KA, Pajon R, Tulenko MM, Liu S, Hollick RA, et al. Diversity of factor H-binding protein in Neisseria meningitidis carriage isolates. Vaccine. 2011;29:6049–58. doi: 10.1016/j.vaccine.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Núñez N, Martínez I, Izquierdo L, Álvarez N, López O. Portadores de Neisseria meningitidis y Neisseria lactamica en tres grupos de edades diferentes. VacciMonitor. 2007;16:1–6. [Google Scholar]
- 22.Parodi V, Allende F, Torres E, Macedo M, Maglione R, Algorta G. Portadores de Neisseria meningitidis en una poblaci6n de Montevideo. Rev Med Uruguay. 1998;14:221–5. [Google Scholar]
- 23.Ibarz-Pavón AB, Lemos AP, Gorla MC, Regueira M, Gabastou JM, SIREVA Working Group II Laboratory-based surveillance of Neisseria meningitidis isolates from disease cases in Latin American and Caribbean countries, SIREVA II 2006-2010. PLoS One. 2012;7:e44102. doi: 10.1371/journal.pone.0044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22:1087–96. doi: 10.1016/j.vaccine.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Tondella ML, Popovic T, Rosenstein NE, Lake DB, Carlone GM, Mayer LW, et al. The Active Bacterial Core Surveillance Team Distribution of Neisseria meningitidis serogroup B serosubtypes and serotypes circulating in the United States. J Clin Microbiol. 2000;38:3323–8. doi: 10.1128/jcm.38.9.3323-3328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AS, Jansen KU, Eiden J. New frontiers in meningococcal vaccines. Expert Rev Vaccines. 2011;10:617–34. doi: 10.1586/erv.11.50. [DOI] [PubMed] [Google Scholar]
- 27.Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200:379–89. doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- 28.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–75. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 29.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajon R, Beernink PT, Harrison LH, Granoff DM. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine. 2010;28:2122–9. doi: 10.1016/j.vaccine.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088–100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu D, Zhang Y, Barniak V, Bernfield L, Howell A, Zlotnick G. Evaluation of recombinant lipidated P2086 protein as a vaccine candidate for group B Neisseria meningitidis in a murine nasal challenge model. Infect Immun. 2005;73:6838–45. doi: 10.1128/IAI.73.10.6838-6845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086–93. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 34.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 36.Zlotnick G, Hoiseth SK, Jiang HQ, Murphy E, Andrew L, McNeil LK, et al. Epidemiology of the serogroup B Neisseria meningitidis (MnB) factor H binding protein in Strains Samples from Spain and Germany in the years 2001-2006 (poster P045). Presented at the 10th Meeting of the European Meningococcal Disease Society; Manchester, UK 2009. [Google Scholar]
- 37.Ala’aldeen DA, Flint M, Oldfield NJ, Omer SA, McNeil LK, Jiang Q, et al. Human antibody responses to the meningococcal factor H binding protein (LP2086) during invasive disease, colonization and carriage. Vaccine. 2010;28:7667–75. doi: 10.1016/j.vaccine.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 38.McNeil LK, Murphy E, Zhao XJ, Guttmann S, Harris SL, Scott AA, et al. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine. 2009;27:3417–21. doi: 10.1016/j.vaccine.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 39.Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garcés-Sánchez M, et al. 2001 Study Investigators Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:597–607. doi: 10.1016/S1473-3099(12)70087-7. [DOI] [PubMed] [Google Scholar]
- 40.Marshall H, Richmond P, Nissen M, Jiang Q, Anderson AS, Jansen KU, et al. A phase 1/2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults (poster P046). Presented at: 11th European Meningococcal Disease Society Congress, Ljubljana, Slovenia 2011. [Google Scholar]
- 41.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–99. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]