Abstract
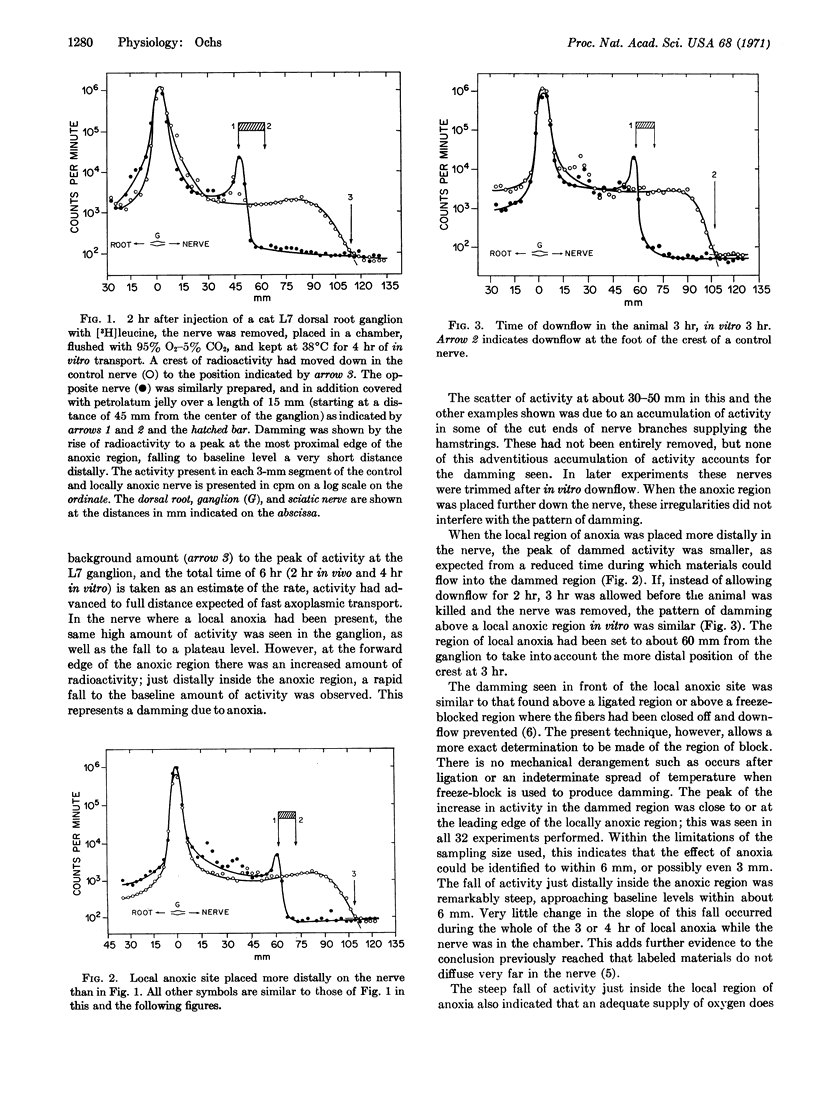
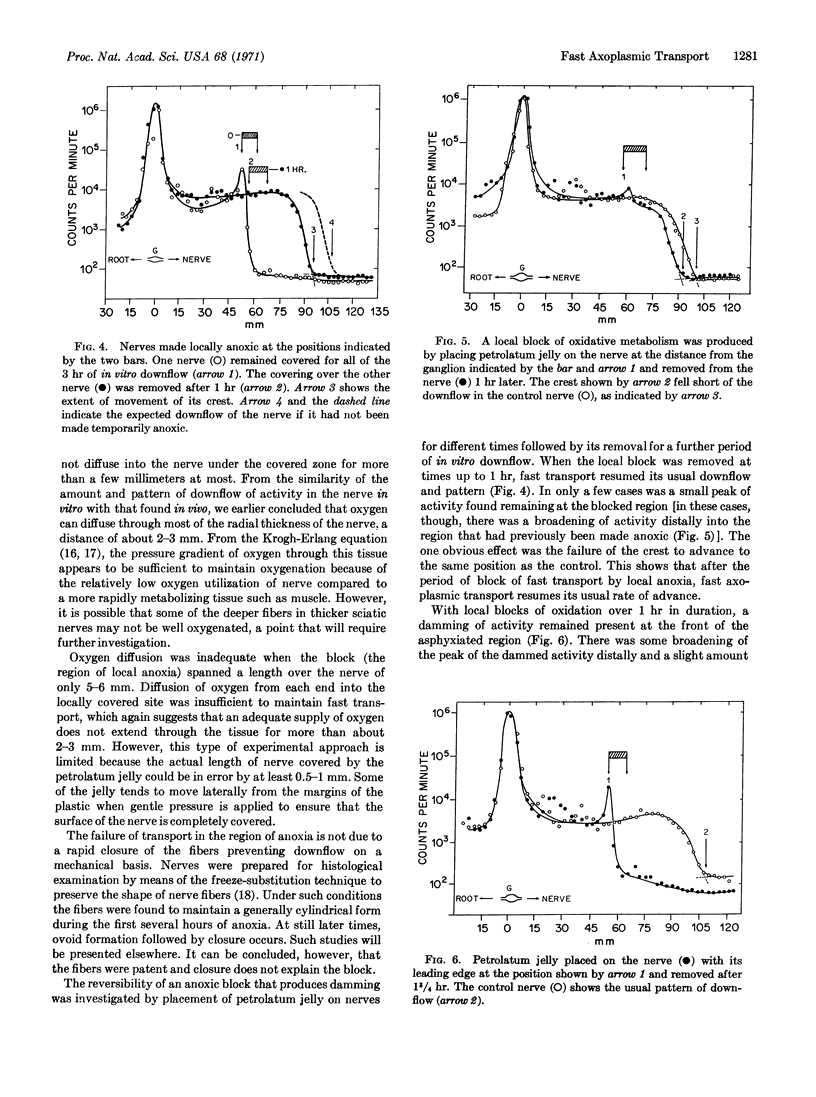
Local anoxia of a small region of cat sciatic nerves causes a block of fast axoplasmic transport in vitro. Activity becomes dammed up at the face of the anoxic region, and falls off rapidly distally just inside the anoxic region. Such blocks were reversible if they were removed within 1 hr. Earlier studies had given evidence that fast axoplasmic transport is closely dependent on ATP derived from oxidative metabolism. The present results indicate that ATP is required all along the length of the nerve fiber to supply a hypothesized “transporting filament” mechanism of fast transport.
Keywords: cat, in vitro
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Taylor E. W. Further purification and characterization of slime mold myosin and slime mold actin. Biochemistry. 1969 Dec;8(12):4976–4988. doi: 10.1021/bi00840a047. [DOI] [PubMed] [Google Scholar]
- Bowler K., Duncan C. J. Actomyosin-like protein from crayfish nerve: a possible molecular explanation of permeability changes during excitation. Nature. 1966 Aug 6;211(5049):642–643. doi: 10.1038/211642a0. [DOI] [PubMed] [Google Scholar]
- Hatano S., Oosawa F. Isolation and characterization of plasmodium actin. Biochim Biophys Acta. 1966 Oct 31;127(2):488–498. doi: 10.1016/0304-4165(66)90402-8. [DOI] [PubMed] [Google Scholar]
- Jahn T. L., Bovee E. C. Protoplasmic movements within cells. Physiol Rev. 1969 Oct;49(4):793–862. doi: 10.1152/physrev.1969.49.4.793. [DOI] [PubMed] [Google Scholar]
- James K. A., Austin L. The binding in vitro of colchicine to axoplasmic proteins from chicken sciatic nerve. Biochem J. 1970 May;117(4):773–777. doi: 10.1042/bj1170773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwai A. M., Ochs S. Components of fast and slow phases of axoplasmic flow. J Neurochem. 1969 Jul;16(7):1105–1112. doi: 10.1111/j.1471-4159.1969.tb05955.x. [DOI] [PubMed] [Google Scholar]
- Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol. 1919 May 20;52(6):457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livett B. G., Geffen L. B., Austin L. Proximo-distal transport of [14C]noradrenaline and protein in sympathetic nerves. J Neurochem. 1968 Sep;15(9):931–939. doi: 10.1111/j.1471-4159.1968.tb11635.x. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Grafstein B. Fast and slow components in axonal transport of protein. J Cell Biol. 1968 Sep;38(3):494–508. doi: 10.1083/jcb.38.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias V. T., Huxley H. E. Electron microscope observations on actomyosin and actin preparations from Physarum polycephalum, and on their interaction with heavy meromyosin subfragment I from muscle myosin. J Mol Biol. 1970 May 28;50(1):83–90. doi: 10.1016/0022-2836(70)90105-1. [DOI] [PubMed] [Google Scholar]
- OCHS S. BEADING OF MYELINATED NERVE FIBERS. Exp Neurol. 1965 May;12:84–95. doi: 10.1016/0014-4886(65)90100-7. [DOI] [PubMed] [Google Scholar]
- Ochs S., Hollingsworth D. Dependence of fast axoplasmic transport in nerve on oxidative metabolism. J Neurochem. 1971 Jan;18(1):107–114. doi: 10.1111/j.1471-4159.1971.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Ochs S., Johnson J., Ng M. H. Protein incorporation and axoplasmic flow in motoneuron fibres following intra-cord injection of labelled leucine. J Neurochem. 1967 Mar;14(3):317–331. doi: 10.1111/j.1471-4159.1967.tb09529.x. [DOI] [PubMed] [Google Scholar]
- Ochs S., Ranish N. Characteristics of the fast transport system in mammalian nerve fibers. J Neurobiol. 1969;1(2):247–261. doi: 10.1002/neu.480010211. [DOI] [PubMed] [Google Scholar]
- Ochs S., Ranish N. Metabolic dependence of fast axoplasmic transport in nerve. Science. 1970 Feb 6;167(3919):878–879. doi: 10.1126/science.167.3919.878. [DOI] [PubMed] [Google Scholar]
- Ochs S., Sabri M. I., Ranish N. Somal site of synthesis of fast transported materials in mammalian nerve fibers. J Neurobiol. 1969;1(3):329–344. doi: 10.1002/neu.480010308. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Shelton E., Weihing R. R., Korn E. D. Ultrastructural characterization of F-actin isolated from Acanthamoeba castellanii and identification of cytoplasmic filaments as F-actin by reaction with rabbit heavy meromyosin. J Mol Biol. 1970 May 28;50(1):91–97. doi: 10.1016/0022-2836(70)90106-3. [DOI] [PubMed] [Google Scholar]
- Puszkin S., Berl S. Actin-like properties of colchicine binding protein isolated from brain. Nature. 1970 Feb 7;225(5232):558–559. doi: 10.1038/225558a0. [DOI] [PubMed] [Google Scholar]
- Schmitt F. O. Fibrous proteins--neuronal organelles. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1092–1101. doi: 10.1073/pnas.60.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W., Lymn R. W., Moll G. Myosin-product complex and its effect on the steady-state rate of nucleoside triphosphate hydrolysis. Biochemistry. 1970 Jul 21;9(15):2984–2991. doi: 10.1021/bi00817a008. [DOI] [PubMed] [Google Scholar]


