Abstract
In the present study we first compare immunogenicity against vaccine and heterologous circulating A(H1N1)pdm09 strains, tolerability and safety of intradermal Intanza® 15 μg and of virosomal adjuvanted, intramuscularly delivered influenza vaccine, Inflexal® V, in healthy elderly volunteers.
Five-hundred participants were enrolled in the study and randomly assigned to the two vaccine groups to receive either one dose of Intanza® 15 μg or Inflexal® V vaccine. All subjects reported solicited local and systemic reactions occurred within 7 d after vaccination and unsolicited adverse events up to 21 d post-immunization and any serious adverse event appeared during the study. A subset of 55 participants was randomly selected for immunogenicity and cross-protection evaluations. Serum samples were collected before and 1 and 3 mo after immunization. Antibody responses were measured using hemagglutination inhibition (HI) against all viruses used in the study and neutralization (NT) assays against A(H1N1)pdm09 strains.
At least one of the CHMP criteria for influenza vaccine approval in the elderly was met by virosomal vaccine against all the tested viruses; intradermal vaccine met all criteria against all strains. Several parameters of immune response against strains with a different antigenic pattern from that of vaccine A/California/04/09(H1N1)pdm09 were significantly higher in the intradermal vaccine group compared with the virosomal group.
Safety and systemic tolerability of both vaccines were excellent, but injection site reactions occurred significantly more frequently in the intradermal vaccination group.
Immunogenicity of Intanza® 15 μg intradermal vaccine tended to be higher than that of Inflexal® V against heterologous strains in healthy elderly.
Keywords: influenza, vaccine, intradermal, virosome, immunogenicity, cross-protection, safety, tolerability
Introduction
Influenza vaccination still remains the best prevention strategy to reduce the burden of the disease in people at high risk of severe influenza or associated complications, including elderly people, but some limitations of influenza vaccine are well known, affecting vaccine effectiveness and limiting its compliance.
First, immunosenescence may importantly affect immune response to influenza vaccination in the elderly, by impairing both innate and adaptive immunity and reducing antibody response to vaccine components.1-3 Antibody responses to influenza vaccination have been estimated to be 2–4 fold lower in adults aged ≥ 58 y than in younger individuals and the efficacy of vaccines decreases with age: the seroprotection rate against influenza virus strains is only 29–46% in subjects aged ≥ 75 y, compared with 41–58% in subjects aged 60–74 y.4-6
Second, antigenic drift, the process consisting in frequent amino acid changes at the antigenic sites of influenza viruses, results in virus strains that are no longer effectively neutralized by host antibodies developed by previous vaccination containing parental viruses. Antigenic drift causes an increased susceptibility of vaccinated subjects against circulating viruses. This event has important implications especially in older people: as observed by many Authors, the decrease in immunogenicity against circulating drifted strains elicited by influenza vaccination is dramatic in older people or in subjects with chronic diseases, determining a wide impact in terms of complications, hospitalizations and deaths.7-11
Finally, another aspect that heavily affects influenza vaccine diffusion and uptake is its sub-optimal tolerability and acceptability. A well-established barrier to influenza immunization is a general lack of patient acceptance of traditional intramuscular vaccination: recent studies have demonstrated that the most common reasons for missing previous vaccinations are fear of adverse events, bothered by pain during injection and dislike for injections or needles, and that other ways of vaccine administration were considered to be an encouraging alternative to implement vaccination against influenza.12-19
For all these reasons, new vaccines providing (1) a robust, effective and protective antibody response in patients with impaired innate and adaptive immunity, such as older people or patients with chronic diseases, (2) a broader and cross-reactive immune response against drifted influenza variants and (3) an excellent safety, tolerability and acceptability profile are urgently needed. Among several strategies explored to enhance immunogenicity of plain - subunit and split - vaccines, the use of adjuvants (i.e., MF59®), or carriers, (i.e., virosome), have led to licensed vaccines in Europe, while intradermal administration of influenza vaccine has emerged as a promising option, thanks to the recent availability of a new delivery device. Both MF59®- and virosomal-adjuvanted influenza vaccines were introduced into the market in 1997 and licensed for adults over the age of 64 and for all age groups, respectively. Intanza® 15 µg is the first intradermal influenza vaccine to be licensed for use in older people (> 60 y of age); it received marketing authorization in the European Union in 2009 for use in adults ≥ 60 y of age. Clinical studies in older people have shown that Intanza® 15 µg is more immunogenic than the standard intramuscular vaccine when the immune response was evaluated against the strains included in the vaccine composition.20,21 A recent study by Van Damme et al. showed that, in elderly volunteers, immunogenicity and safety of Intanza® 15 µg are largely comparable with those of a subunit vaccine adjuvanted with MF59® and administered intramuscularly.22 To our knowledge, comparison between Intanza® 15 µg and other non-conventional licensed influenza vaccines are lacking.
In the present study, we first compare the safety, tolerability and immunogenicity profiles of Intanza® 15 µg and of virosomal intramuscularly delivered vaccine, Inflexal® V, in older people and explored the persistence of antibody responses and the ability of the two vaccines to elicit an effective antibody response against circulating A(H1N1)pdm09 viruses presenting different antigenic patterns compared with that of vaccine strain.
Results
Participants
A total of 500 participants were enrolled in the study, 250 per vaccine group. Clinical diary for safety and reactogenicity assessments reporting solicited local and systemic reactions within 7 d after vaccination and unsolicited adverse events within 21 d after vaccination were available for 227 (90.8%) participants in the intradermal group and 200 (80.0%) participants in the virosomal group.
A subset of 55 participants were selected for immunogenicity and cross-protection assessments: 28 and 27 subjects were randomly assigned to receive intradermal and virosomal vaccine, respectively. Blood samples for immunogenicity evaluation were available from 55, 47 (24 and 23 belonging to the intradermal and virosomal vaccine group, respectively) and 39 (20 and 19 belonging to the intradermal and virosomal vaccine group, respectively) subjects before, 1 mo and 3 mo after immunization, respectively.
The two groups are comparable in terms of age (mean ± SD 76.9 ± 8.4 and 75.3 ± 7.7 y in intradermal and virosomal group, respectively): 12 subjects belonged to the 60–69 y age group (6 for each vaccine arm), 27 to the 70–79 y age group (13 for intradermal and 14 for virosomal) and 16 to the > 79 y age group (9 for intradermal and 7 for virosomal).
Safety and reactogenicity
Safety and tolerability results are summarized in Table 1. Higher incidence of at least one solicited local reaction within seven days after immunization was reported in intradermal vaccine recipients compared with subjects receiving virosomal vaccine. Erythema, induration, pruritus and swelling were significantly more common with intradermal rather than virosomal vaccination, whereas the incidence of pain and ecchymosis were comparable. Most of the solicited injection-site reactions were of mild intensity. Less than 4% of the participants in each group reported reactions of moderate or severe intensity with comparable incidence in both vaccine groups. The incidence of solicited systemic reactions was similar in both the intradermal and virosomal vaccine groups.
Table 1. Safety and tolerability assessment after vaccination with intradermal and virosomal-intramuscular vaccine.
| Vaccine route of administration and adjuvant | p-value | ||
|---|---|---|---|
| Intradermal | Virosomal Intramuscular | ||
| Solicited injection site reactions, days 0–7 (%, 95% C.I) | |||
| At least one | 43 (36–49) | 20 (15–26) | < 0.01 |
| Pain | 13 (9–18) | 12 (8–17) | NS |
| Pruritus | 20 (16–26) | 5 (3–9) | < 0.01 |
| Erythema | 33 (27–39) | 7 (4–11) | < 0.01 |
| Swelling | 19 (14–24) | 5 (2–8) | < 0.01 |
| Induration | 25 (20–31) | 7 (4–11) | < 0.01 |
| Ecchymosis | 6 (4–10) | 5 (2–8) | NS |
| Solicited systemic reactions, days 0–7 (%, 95% C.I) |
|||
| At least one | 11 (7–15) | 10 (7–15) | NS |
| Fever | 2 (1–4) | 2 (1–4) | NS |
| Headache | 4 (2–8) | 6 (3–10) | NS |
| Malaise | 5 (3–9) | 6 (3–10) | NS |
| Myalgia | 6 (4–10) | 7 (4–11) | NS |
| Shivering | 3 (2–6) | 5 (3–9) | NS |
| Unsolicited events, days 0–21 (%, 95% C.I) |
2 (0,88; 0,24–3,15) | 4 (2,00; 0,78–5,03) | NS |
| SAEs, days 0–180 (%, 95% C.I) |
0 | 0 | NS |
No unsolicited adverse events occurred within 30 min of vaccination in either group. During the 21 post-vaccination days, 6 patients reported unsolicited adverse events: 2 cases (i.e., influenza-like illness and upper respiratory tract infection without fever) occurred in the intradermal group, and 4 (i.e., influenza-like illness, asthenia, phlebitis, and urogenital infection) in the virosomal group. No participants reported serious adverse events in the 6 mo following vaccination.
Immunogenicity and cross-protection
Antibody response 1 and 3 mo after immunization with intradermal and virosomal vaccine, determined using HI and NT assays, are summarized in Tables 2 and 3, respectively.
Table 2. Antibody response determined by using HI assays after vaccination with intradermal and virosomal-intramuscular vaccine.
| Vaccine route of administration and adjuvant | p-value | ||
|---|---|---|---|
| Intradermal | Virosomal Intramuscular | ||
| Geometric Mean Titer 1 mo post-vaccination | |||
| A(H1N1) vaccine strain | 48 (29–77) | 32 (21–50) | NS |
| A(H3N2) vaccine strain | 41 (28–60) | 26 (18–35) | NS |
| B vaccine strain | 131 (90–191) | 93 (69–125) | NS |
| A/Genoa/1/11(H1N1) | 69 (42–115) | 47 (25–87) | NS |
| A/Genoa/6/11(H1N1) | 71.3 (40–126) | 31 (19–53) | < 0.05 |
| A/Genoa/24/11(H1N1) | 42 (25–72) | 31 (15–65) | NS |
| Mean Fold Increase 1 mo post-vaccination | |||
| A(H1N1) vaccine strain | 3.4 (1.9–5.9) | 2.3 (1.3–3.8) | |
| A(H3N2) vaccine strain | 2.9 (1.8–4.8) | 2.1 (1.4–3.1) | |
| B vaccine strain | 2.7 (1.5–4.9) | 1.5 (1–2.3) | |
| A/Genoa/1/11(H1N1) | 4.1 (2.2–7.6) | 3.7 (1.8–7.6) | |
| A/Genoa/6/11(H1N1) | 4.8 (2.3–9.7) | 3.3 (1.7–6.5) | |
| A/Genoa/24/11(H1N1) | 2.8 (1.5–5.5) | 2.6 (1.1–6.1) | |
| Seroconversion rate 1 mo post-vaccination (%, 95% C.I.) | |||
| A(H1N1) vaccine strain | 50 (31–69) | 43 (26–43) | NS |
| A(H3N2) vaccine strain | 50 (31–69) | 35 (19–55) | NS |
| B vaccine strain | 46 (28–65) | 0 (0–14) | < 0.05 |
| A/Genoa/1/11(H1N1) | 63 (43–79) | 43 (26–63) | NS |
| A/Genoa/6/11(H1N1) | 67 (47–82) | 48 (29–67) | NS |
| A/Genoa/24/11(H1N1) | 42 (25–61) | 35 (19–55) | NS |
| Seroprotection rate 1 mo post-vaccination (%, 95% C.I.) | |||
| A(H1N1) vaccine strain | 63 (43–79) | 57 (37–74) | NS |
| A(H3N2) vaccine strain | 71 (51–85) | 48 (29–67) | NS |
| B vaccine strain | 100 (86–100) | 100 (86–100) | NS |
| A/Genoa/1/11(H1N1) | 79 (60–91) | 70 (49–84) | NS |
| A/Genoa/6/11(H1N1) | 79 (60–91) | 52 (33–71) | NS |
| A/Genoa/24/11(H1N1) | 75 (55–88) | 52 (33–71) | NS |
| Geometric Mean Titer 3 mo post-vaccination | |||
| A(H1N1) vaccine strain | 39 (22–67) | 26 (17–40) | NS |
| A(H3N2) vaccine strain | 36 (24–55) | 19 (14–27) | < 0.05 |
| B vaccine strain | 130 (81–209) | 93 (65–132) | NS |
| A/Genoa/1/11(H1N1) | 59 (30–115) | 30 (16–56) | NS |
| A/Genoa/6/11(H1N1) | 72 (34–152) | 23 (14–38) | < 0.05 |
| A/Genoa/24/11(H1N1) | 41 (23–76) | 31 (14–67) | NS |
| Seroprotection rate 3 mo post-vaccination (%, 95% C.I.) | |||
| A(H1N1) vaccine strain | 45 (26–66) | 47 (27–68) | NS |
| A(H3N2) vaccine strain | 60 (39–78) | 32 (15–54) | NS |
| B vaccine strain | 95 (76–99) | 100 (83–100) | NS |
| A/Genoa/1/11(H1N1) | 70 (48–86) | 53 (32–73) | NS |
| A/Genoa/6/11(H1N1) | 70 (48–86) | 47 (27–68) | NS |
| A/Genoa/24/11(H1N1) | 75 (53–89) | 58 (36–77) | NS |
Table 3. Antibody response determined by using NT assays after vaccination with intradermal and virosomal/intramuscular vaccine.
| Vaccine route of administration and adjuvant | p-value | ||
|---|---|---|---|
| Intradermal | Virosomal Intramuscular | ||
| Geometric Mean Titer 1 mo post-vaccination | |||
| A(H1N1) vaccine strain | 28 (17–44) | 19 (12–31) | NS |
| A/Genoa/1/11(H1N1) | 80 (44–147) | 44 (23–84) | NS |
| A/Genoa/6/11(H1N1) | 90 (48–169) | 37 (19–69) | < 0.05 |
| A/Genoa/24/11(H1N1) | 49 (28–86) | 20 (11–37) | < 0.05 |
| A/Genoa/89/11(H1N1) | 35 (20–61) | 20 (10–38) | NS |
| Mean Fold Increase 1 mo post-vaccination | |||
| A(H1N1) vaccine strain | 3.5 (2–6) | 2.4 (1.3–4.3) | |
| A/Genoa/1/11(H1N1) | 4.9 (2.3–10.5) | 4 (1.9–8.9) | |
| A/Genoa/6/11(H1N1) | 5.8 (2.8–12) | 3.9 (1.8–8.1) | |
| A/Genoa/24/11(H1N1) | 3.7 (1.9–7.1) | 2.5 (1.2–5) | |
| A/Genoa/89/11(H1N1) | 4.2 (2.3–7.9) | 2.5 (1.3–5.2) | |
| Seroconversion rate 1 mo post-vaccination (%, 95% C.I.) | |||
| A(H1N1) vaccine strain | 54 (35–72) | 44 (26–63) | NS |
| A/Genoa/1/11(H1N1) | 54 (35–72) | 48 (30–67) | NS |
| A/Genoa/6/11(H1N1) | 58 (39–76) | 30 (16–51) | NS |
| A/Genoa/24/11(H1N1) | 58 (39–76) | 39 (22–59) | NS |
| A/Genoa/89/11(H1N1) | 50 (31–69) | 26 (13–47) | NS |
| Titer ≥ 40 rate 1 mo post-vaccination (%, 95% C.I.) | |||
| A(H1N1) vaccine strain | 58 (39–76) | 48 (29–67) | NS |
| A/Genoa/1/11(H1N1) | 75 (55–88) | 65 (45–81) | NS |
| A/Genoa/6/11(H1N1) | 75 (55–88) | 44 (26–63) | < 0.05 |
| A/Genoa/24/11(H1N1) | 79 (60–91) | 39 (22–59) | < 0.05 |
| A/Genoa/89/11(H1N1) | 54 (35–72) | 30 (16–51) | NS |
| Geometric Mean Titer 3 mo post-vaccination | |||
| A(H1N1) vaccine strain | 22 (13–37) | 13 (8–20) | NS |
| A/Genoa/1/11(H1N1) | 46 (25–85) | 27 (15–48) | NS |
| A/Genoa/6/11(H1N1) | 57 (30–105) | 22 (12–37) | < 0.05 |
| A/Genoa/24/11(H1N1) | 51 (27–95) | 14 (10–27) | < 0.05 |
| A/Genoa/89/11(H1N1) | 25 (14–43) | 11 (7–19) | < 0.05 |
| Titer ≥ 40 rate 3 mo post-vaccination (%, 95% C.I.) | |||
| A(H1N1) vaccine strain | 55 (34–74) | 32 (15–54) | NS |
| A/Genoa/1/11(H1N1) | 60 (39–78) | 42 (23–64) | NS |
| A/Genoa/6/11(H1N1) | 65 (43–82) | 37 (19–59) | NS |
| A/Genoa/24/11(H1N1) | 75 (53–89) | 21 (9–43) | < 0.05 |
| A/Genoa/89/11(H1N1) | 45 (26–66) | 21 (9–43) | NS |
In Table 2, HI immune response against A/Genoa/89/11(H1N1) is not reported, because the virus showed a reduced ability to agglutinate guinea pig red cells. The use of red blood cells from chickens and humans did not increased agglutination ability.
At least one of the CHMP criteria for influenza vaccine approval in older people was met by both vaccines against all of the viruses tested, as requested by EMA guidelines.
The CHMP MFI, seroprotection and seroconversion criteria were met against all vaccines and circulating viruses by Intanza® 15 µg, while the Inflexal® V did not elicit antibody responses that met MFI and seroconversion criteria against B vaccine strains and seroprotection criteria against A(H1N1) and A(H3N2) vaccine strains and against circulating A/Genoa/6/11(H1N1) and A/Genoa/24/11(H1N1) viruses.
Pre-vaccination HI titers against all viral strains were similar in Intanza® 15 µg and Inflexal® V groups (data not shown). Post-vaccination HI titer, MFI, seroconversion and seroprotection rates trended higher against all viruses in subjects who received the intradermal vaccine both 1 and 3 mo after vaccination. However, statistical significance of the superiority of the response after 1 mo from vaccination was reached only for GMT against A/Genoa/6/11(H1N1) and the seroconversion rate against the B vaccine strain. Furthermore, post-vaccination HI titer 3 mo after vaccination was higher in the intradermal group against the A(H3N2) vaccine strain and against the A/Genoa/6/11(H1N1) virus (Table 2).
Pre-vaccination NT titers against all viral strains were similar in the Intanza® 15 µg and Inflexal® V groups with no significant difference between vaccine groups (data not shown).
Post-immunization neutralizing antibody titers 1 mo and 3 mo after vaccination were significantly higher in subjects immunized with Intanza® 15 µg vaccine than in individuals receiving the standard intramuscular vaccine against A/Genoa/6/11(H1N1) and A/Genoa/24/11(H1N1) and after 3 mo from vaccination against A/Genoa/89/11(H1N1). Furthermore, the proportion of subjects reaching the NT titer ≥ 40 was higher in the intradermal group against A/Genoa/24/11(H1N1) 1 and 3 mo after vaccination and against A/Genoa/6/11(H1N1) when the immune response was evaluated 1 mo after vaccination (Table 3).
HI titers at 3 mo significantly decreased compared with titers at 1 mo against all the 3 strains included in the vaccine composition and against A/Genoa/1/11(H1N1) (p-value ranged between 0.012 and 0.001) with a difference of about one quarter of HI dilution (difference in titers transformed into base 2 logarithms ranged between -0.15 and -0.33). Similarly, NT titers at 3 mo significantly decreased compared with titers at 1 mo against H1N1 vaccine strain, A/Genoa/1/11(H1N1), A/Genoa/6/11(H1N1) and A/Genoa/89/11(H1N1) (p-value ranged between 0.05 and < 0.001) with difference ranged between one quarter and three quarter of NT dilution. The decrease of HI titers is significantly less in the intradermal group when the immune response was evaluated against H3N2 vaccine strain (-0.1 and -0.37 in 1 mo and 3 mo after vaccination in the intradermal and virosomal group, respectively, p-value 0.04) and A/Genoa/1/11(H1N1) (-0.1 and -0.58 in the intradermal and virosomal group, respectively, p-value 0.04).
Discussion
The need for influenza vaccines that provide an enhanced profile of immunogenicity in older people and against drifted viruses led to the development and approval of vaccines with adjuvants, carriers and a higher antigen content and the use of routes of administration other than intramuscular. Data on tolerability, safety and immunogenicity of these vaccines are usually obtained from their comparison with subunit or split vaccines and few data emerge from the comparison between “enhanced” vaccines (i.e., MF59®, virosome, high dose or intradermal vaccine). Furthermore, the advantage in terms of higher immunogenicity offered by “enhanced” vaccines usually derives from immune response evaluated 3–4 weeks after vaccination, by using HI assay and against vaccine strains. There is poor evidence on the persistence of antibody responses, on the cross-reactive response against circulating viruses presenting different antigenic patterns compared with that of vaccine strains and the functional potential of antibodies induced by these vaccines.
In this study, for the first time we used HI and NT assays to compare immune response 1 and 3 mo following vaccination in subjects immunized with intradermal and virosomal intramuscular-administered vaccines against homologous vaccine strains and heterologous A(H1N1)pdm09 viruses.
The present study showed that, in older volunteers aged ≥ 60 y, the immunogenicity of Intanza® 15 µg intradermal vaccine tended to be higher than that of the Inflexal® V virosomal influenza vaccine, especially when the immune response was evaluated against circulating strains with different antigenic patterns compared with that of vaccine A/California/04/09(H1N1)pdm09. Several parameters of immune response obtained by using HI and NT assays 1 and 3 mo following vaccination against A/Genoa/6/11, a virus belonging to the A/Christchurch/16/10(H1N1)pdm09 clade, A/Genoa/24/11, A/Genoa/89/11, the less phylogenetically and antigenically close to the vaccine strain, were significantly higher in intradermal vaccine group compared with the virosomal group.
NT 1-mo and 3-mo post-vaccination GMT was 1.8–2.5 and 2.3–3.6 fold higher, respectively, in recipients of intradermal vaccine, against the above-mentioned circulating strains. Similarly, post-vaccination HI GMT was 2.3 and 3.1 fold higher in recipients of intradermal vaccine, against A/Genoa/6/11 when the immune response was evaluated 1 and 3 mo following vaccination. The advantages offered by intradermal route of administration were weaker when the immune response was evaluated against the three vaccine strains: post-vaccination GMT ratio (intradermal/intramuscular-virosomal) was about 1.5 against all three vaccine strains both 1 mo and 3 mo following vaccination. These data are consistent with the comparison of antibody responses against heterologous circulating H3N2 strains in adults 60 y and older elicited by Intanza® 15 µg and split vaccines.23
The decrease of antibody titers at 3 mo after vaccination compared with titers at 1 mo was observed for both vaccines, but it is less evident in the intradermal vaccine group: HI titer decrease was significantly lower in the intradermal group for 2 out 6 tested viruses and seroprotection EMA criteria 3 mo following vaccination was reached for 5 out 6 viruses and for 1 out 6 viruses in intradermal and intramuscular-virosomal groups, respectively.
The safety and systemic tolerability of both vaccines were excellent, but the local reactogenicity profile is quite different in subjects immunized via the intradermal and intramuscular route. In particular, injection site erythema, induration, pruritus and swelling occurred significantly more commonly in the subjects who received intradermal vaccination as compared with virosomal group. Reactogenicity was typically mild or moderate in severity and short-lived with the majority of symptoms resolving within 3 d. An increase in local reactions is to be expected with injections close to the skin surface, as opposed to deep muscle intramuscular injections, and as reported in previous studies.24-29 Despite the higher incidence of injection site reactions, the high satisfaction with intradermal injection system emerged from several studies in young adults and in older people and the good acceptability profile of intradermal vaccine reflects the high willingness to get re-vaccinated in the following season.16,17,25,30
The present study demonstrated that the immunogenicity of Intanza® 15 µg intradermal vaccine was superior than that of Inflexal® V virosomal influenza vaccine, when the immune response was evaluated against circulating strains. Several immune response parameters obtained by using HI and NT assays 1 and 3 mo following vaccination were higher following the administration of Intanza® 15 µg as compared with the virosomal intramuscular formulation, but the small sample size due to the complexity of the assays and the number of viruses used for the cross-reactivity evaluation did not allow to reach a final conclusion.
The higher immunogenicity profile both against homologous and heterologous strains and the excellent safety results, despite the higher incidence of some injection-site reactions compared with intramuscular vaccine, suggested that intradermal vaccination might be an appropriate strategy to address the challenge of declining immune responses in older people.
Materials and Methods
Study design
This phase-IV, spontaneous, multicenter, randomized, controlled, open-label and parallel-group study was performed in subjects older than 60 y who were recruited at the Department of Health Sciences, University of Genoa, Genoa, Italy, and at several vaccination centers of the Genoese Local Health Unit (ASL3 Genovese), between September and December 2010.
Subjects participating in our study were interviewed and both a thorough medical history and physical examination were conducted, investigating the criteria for inclusion and exclusion. All subjects were randomly assigned to the two vaccine groups at a ratio of 1:1 to receive either one dose of Intanza® 15 µg or Inflexal® V vaccine. In order to evaluate safety and tolerability, all subjects were asked to report on clinical diaries the solicited local and systemic reactions occurring within 7 d after vaccination and unsolicited adverse events (AE) up to 21 d post-immunization and any serious adverse event (SAE) appeared in the 6 mo after vaccination. Local (pain, erythema, swelling, induration, ecchymosis, pruritus) and systemic (fever [rectal equivalent temperature ≥ 38.0°C], headache, malaise, myalgia, shivering) reactions were directly monitored by the investigator physician immediately after immunization (observation for 30 min) and recorded by the patient, starting from the day of immunization and for the following 6 d, by completing the appropriate clinical diary. The same diary was used to report AEs and SAEs.
The immunogenicity profile against vaccine and circulating A(H1N1)pdm09 strains and the persistence of antibody responses was evaluated in a subgroup of participants. Serum samples were collected immediately before and 1 (28 ± 2 d) month and 3 (90 ± 7 d) months after immunization. All sera were stored at −20°C before testing.
The study was approved by the Ethic Committee of “San Martino” University Hospital and of ASL3 Genovese, and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. All participants gave written informed consent before enrolment. The study was registered at EUDRACT with the follow trial code: 2009–014637–24.
Inclusion and exclusion criteria
All participants were aged more than 59 y (≥ 60years). Exclusion criteria included: systemic hypersensitivity to egg or chicken proteins or any of the vaccine constituents, acute febrile illness (temperature ≥ 37.5°C) at the time of enrolment, any vaccination within the previous 4 weeks or planned vaccination within the 6 mo following vaccination, current abuse of alcohol or drug addiction, seasonal influenza vaccination in the previous 6 mo with vaccines object of the study or other influenza vaccines and unstable chronic illness that could interfere with the conduction or completion of the study. Subjects with high-risk medical conditions for influenza infection were not excluded, provided they met the above criteria.
Vaccines
Both vaccines used in the present study were formulated according to the WHO recommendations for the 2010–2011 Northern Hemisphere influenza season: A/California/7/2009 (H1N1)pdm09-like, A/Perth/16/2009 (H3N2)-like and B/Brisbane/60/2008-like strains.
The intradermal vaccine (Intanza® 15 µg; Sanofi Pasteur, Lyon, France) is an inactivated, split-virion influenza vaccine containing 15 µg HA/strain per 0.1-ml dose and was administered in the deltoid region using a pre-filled intradermal micro-needle injection system (SoluviaTM, Becton Dickinson; Franklin Lakes, NJ, USA), designed to provide an easy-to-perform, reliable and safe intradermal vaccination.20,24 The virosomal adjuvanted vaccine (Inflexal® V; Crucell, Switzerland) is an inactivated, subunit influenza vaccine containing the standard dosage of 15 μg HA/strain in each 0.5 ml dose and was injected intramuscularly into the deltoid muscle using a 25mm-long needle.
Immunogenicity assessments
Antibody responses before and 1 and 3 mo after immunization were measured using hemagglutination inhibition (HI) against all viruses used in the study and neutralization (NT) assays against A(H1N1)pdm09 strains, in line with the WHO guidelines and standardized methods of our laboratory, respectively.31-36 Guinea pig red blood cells were used in the HI assay. All samples were assayed twice.
Antibody titers were expressed as the reciprocal of the last serum dilution showing hemagglutination. Immunogenicity results from the HI assay were reported as: geometric mean titers (GMT), mean-fold increase (MFI; ratio of post- to pre-vaccination titers), seroconversion rates (percentage of subjects with a 4-fold increase in HI antibody titers after 1 mo after vaccination, providing a minimal post-vaccination titer of 40), and seroprotection rate (the percentage of subjects achieving an HI titer ≥ 40). Since there are no established immune correlates for neutralization, in that analysis we assessed GMT, the proportion of subjects showing seroconversion (a 4-fold or more increase in the antibody titer) and a microneutralization titer of 40 or more. The results from the HI assay were evaluated against the Committee for Medicinal Products for Human Use (CHMP) criteria for approval of influenza vaccines in older people, which require that at least one of the following criteria be met: MFI > 2; seroprotection rate > 60%, or seroconversion rate > 30%.31
Viruses used for cross-protection assessments
Antibody responses elicited by intradermal and virosomal-adjuvanted vaccines have been evaluated also against four A(H1N1)pdm09 circulating strains isolated during the 2010/2011 influenza season, selected because they presented a different antigenic pattern. Figure 1 shows the phylogenetic tree based on the sequence analysis of the region codifying for the globular head region of haemagglutinin of the strains used in the study, amino acid mutations onto antigenic sites with respect to the vaccine A/California/04/09(H1N1)pdm09 strain and serological characterization of isolates and reference viruses, performed with the HI test using whole viruses and post-infection ferret sera.
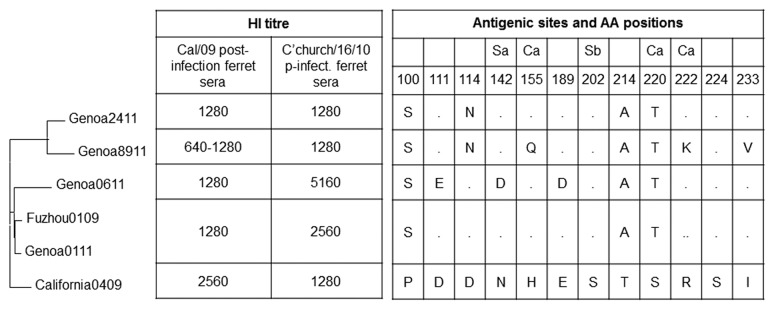
Figure 1. Molecular and serological characterization of influenza strains used in the study. Phylogenetic tree based on sequence analysis of the region codifying for the globular head region of haemagglutinin of strains used in the study, amino acid mutations onto-antigenic sites with respect to vaccine A/California/04/09(H1N1)pdm09 strain and serological characterization of isolated and reference viruses, performed with the HI test using whole viruses and post-infection ferret sera.
A/Genoa/01/11 appeared as the phylogenetically closest one to the vaccine strain showing an HA sequence identical to that of A/Fuzhou/1/09, the reference strain of clade 7, and amino acid changes onto antigenic sites in position 100 (P100S), 214 (T214A) and 220 (S220T) as compared with the A/California/04/09(H1N1)pdm09 strain. A/Genoa/06/11 presented three more amino acid mutations, in position 111 (D111E), 142 (N142D) and 189 (E189D), showing an antigenic and molecular pattern close to that of A/Christchurch/16/10(H1N1)pdm09. A/Genoa/24/11 differs from A/Genoa/01/11 only for one mutation in position 114 (D114N). A/Genoa/89/11 is the least phylogenetically and antigenically close to the vaccine strain, due to amino acid changes in position 100 (P100S), 114 (D114N), 155 (H155Q), 214 (T214A), 220 (S220T), 222 (R222K) and 233 (I233V) as compared with the A/California/04/09(H1N1)pdm09 strain. At antigenic characterization, A/Genoa/89/11 showed a 2-fold decrease of the HI titer with respect to that against homologous vaccine strain.
Statistical analysis
The method used to calculate a confidence interval for a proportion was the Wilson score method without continuity correction. Comparisons of rates between IM and ID vaccine groups were analyzed using Fischer’s exact test. The comparison of post-vaccination titers transformed into binary logarithms was analyzed using the Wilcoxon test. The comparison of titers between different timing was performed using the matched pair test. Other immunogenicity and safety variables were summarized with descriptive statistics.
Glossary
Abbreviations:
- AE
adverse events
- CHMP
Committee for Medicinal Products for Human Use
- EMA
European Medicines Agency
- GMT
geometric mean titer
- HI
hemagglutination inhibition
- ID
intradermal
- IM
intramuscular
- MFI
mean-fold increase
- NT
neutralization
- SAE
serious adverse event
- WHO
World Health Organization
Disclosure of Potential Conflicts of Interest
F.A., P.D. and G.I. have previously participated at speaker’s bureaus and advisory board meetings sponsored by GSK, Novartis, Pfizer and Sanofi Pasteur and have received research funding as principal investigators or co-investigators from Crucell Berna, Novartis, GSK, Pfizer and Sanofi Pasteur. A.O., D.D.F., V.P. and E.R. have no conflicts of interest. No other relationships/conditions/circumstances that present a potential conflict of interest exist.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23240
References
- 1.Weksler ME, Szabo P. The effect of age on the B-cell repertoire. J Clin Immunol. 2000;20:240–9. doi: 10.1023/A:1006659401385. [DOI] [PubMed] [Google Scholar]
- 2.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–46. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 3.Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- 4.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–84. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- 7.de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–9. doi: 10.1002/(SICI)1096-9071(200005)61:1<94::AID-JMV15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–62. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Baldo V, Baldovin T, Floreani A, Carraro AM, Trivello R, Family Medicine Group of Pianiga MF59-adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18-60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine. 2007;25:3955–61. doi: 10.1016/j.vaccine.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Monto AS, Ansaldi F, Aspinall R, McElhaney JE, Montaño LF, Nichol KL, et al. Influenza control in the 21st century: Optimizing protection of older adults. Vaccine. 2009;27:5043–53. doi: 10.1016/j.vaccine.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Ansaldi F, Canepa P, Parodi V, Bacilieri S, Orsi A, Compagnino F, et al. Adjuvanted seasonal influenza vaccines and perpetual viral metamorphosis: the importance of cross-protection. Vaccine. 2009;27:3345–8. doi: 10.1016/j.vaccine.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 12.Müller D, Szucs TD. Influenza vaccination coverage rates in 5 European countries: a population-based cross-sectional analysis of the seasons 02/03, 03/04 and 04/05. Infection. 2007;35:308–19. doi: 10.1007/s15010-007-6218-5. [DOI] [PubMed] [Google Scholar]
- 13.Blank PR, Schwenkglenks M, Szucs TD. Influenza vaccination coverage rates in five European countries during season 2006/07 and trends over six consecutive seasons. BMC Public Health. 2008;8:272. doi: 10.1186/1471-2458-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollmeyer HG, Hayden F, Poland G, Buchholz U. Influenza vaccination of health care workers in hospitals--a review of studies on attitudes and predictors. Vaccine. 2009;27:3935–44. doi: 10.1016/j.vaccine.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Keeton VF, Chen AK. Immunization updates and challenges. Curr Opin Pediatr. 2010;22:234–40. doi: 10.1097/MOP.0b013e328337685b. [DOI] [PubMed] [Google Scholar]
- 16.Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: Patient reported outcomes from phase 3 clinical trials. Hum Vaccin. 2010;6:336–45. doi: 10.4161/hv.6.4.10753. [DOI] [PubMed] [Google Scholar]
- 17.Arnou R, Frank M, Hagel T, Prébet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther. 2011;28:555–65. doi: 10.1007/s12325-011-0035-z. [DOI] [PubMed] [Google Scholar]
- 18.Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza® 9 μg intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther. 2011;28:640–9. doi: 10.1007/s12325-011-0042-0. [DOI] [PubMed] [Google Scholar]
- 19.Durando P, Alicino C, Alberti M, Sticchi L, Turello V, Marensi L, et al. Italian Intradermal Influenza Vaccine Working Group Acceptance and safety of the intradermal influenza vaccine among the elderly in Italy: an on-field national study. Adv Ther. 2012;29:312–26. doi: 10.1007/s12325-012-0012-1. [DOI] [PubMed] [Google Scholar]
- 20.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 21.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Van Damme P, Arnou R, Kafeja F, Fiquet A, Richard P, Thomas S, et al. Evaluation of non-inferiority of intradermal versus adjuvanted seasonal influenza vaccine using two serological techniques: a randomised comparative study. BMC Infect Dis. 2010;10:134. doi: 10.1186/1471-2334-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansaldi F, Canepa P, Ceravolo A, Valle L, de Florentiis D, Oomen R, et al. Intanza(®) 15 mcg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine. 2012;30:2908–13. doi: 10.1016/j.vaccine.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–42. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 26.Gelinck LB, van den Bemt BJ, Marijt WA, van der Bijl AE, Visser LG, Cats HA, et al. Intradermal influenza vaccination in immunocompromized patients is immunogenic and feasible. Vaccine. 2009;27:2469–74. doi: 10.1016/j.vaccine.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 27.Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010;50:1331–8. doi: 10.1086/652144. [DOI] [PubMed] [Google Scholar]
- 28.Frenck RW, Jr., Belshe R, Brady RC, Winokur PL, Campbell JD, Treanor J, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone®) administered by intradermal and intramuscular route in healthy adults. Vaccine. 2011;29:5666–74. doi: 10.1016/j.vaccine.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito S, Daleno C, Picciolli I, Tagliaferri L, Scala A, Prunotto G, et al. Immunogenicity and safety of intradermal influenza vaccine in children. Vaccine. 2011;29:7606–10. doi: 10.1016/j.vaccine.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: Randomized, controlled, phase III trial. Hum Vaccin. 2010;6:346–54. doi: 10.4161/hv.6.4.10961. [DOI] [PubMed] [Google Scholar]
- 31.Committee for Proprietary Medicinal Products. (1997). Note for Guidance on Harmonisation of Requirements for Influenza Vaccines. CPMP/BWP/214/96. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf
- 32.Zambon M. Laboratory diagnosis of influenza. In: Textbook of influenza. Edited by Nicholson KG, Webster RG, Hay AJ. Oxford: Blackwell Science; 1998:291–313. [Google Scholar]
- 33.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization, Department of Communicable Disease Surveillance and Response, Global Influenza Programme. (2002): WHO manual on animal influenza diagnosis and surveillance. WHO/CDS/NCS/2002/5. http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf
- 35.Ansaldi F, Bacilieri S, Amicizia D, Valle L, Banfi F, Durando P, et al. Antigenic characterisation of influenza B virus with a new microneutralisation assay: comparison to haemagglutination and sequence analysis. J Med Virol. 2004;74:141–6. doi: 10.1002/jmv.20157. [DOI] [PubMed] [Google Scholar]
- 36.Beyer WEP, Palache AM, Lüchters G, Nauta J, Osterhaus ADME. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103:125–32. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]


