Abstract
Despite the launch of a WHO European Region strategic plan 2005–2010 for eliminating measles and rubella and preventing congenital rubella (CR) infection, measles and rubella are still circulating in Europe. Increased transmission and outbreaks of measles in Europe were still observed in 2011. In Italy, the objectives of the National Plan (2003–2007) for measles elimination have not yet been achieved. The goal of measles elimination and incidence reduction of CR cases has been postponed to 2015 by the Italian Ministry of Health through the implementation of the new National Plan 2010–2015 which will require (1) the achievement of more than 95% coverage with 1 dose and two doses of measles containing vaccine (MCV), respectively, within 24 mo and within 12 y of age; (2) supplementary vaccination activities aimed at susceptible populations including adolescents, young adults and those at risk (health care and educational workers, military, groups “hard to reach” like nomads); and in addition, (3) reduction to less than 5% in the proportion of susceptible women of childbearing age (especially immigrant women). Experiences at regional level, like in Tuscany, have shown promising results in order to create an integrated surveillance system between regional and local health authorities, university and laboratory and in the future, to validate elimination. Moreover, the evaluation of all preventive activities performed in Tuscany during the last decade, immunization coverage data, sero-epidemiological population profile and incidence of measles and rubella cases has highlighted critical points which should be improved and good practices already implemented which should be maintained in the future in order to reach the new goals.
Keywords: measles, rubella, congenital rubella syndrome, elimination, vaccination, Tuscany, Central Italy
Introduction
Measles and rubella are highly contagious, but vaccine-preventable, viral diseases. Without preventive measures they can have a serious impact on people's health. Infection from measles virus can lead to serious complications, especially in infants and adults, and it is a leading cause of death in children < 5 y worldwide.1 A study estimated that the overall measles child mortality decreased in the period 2000–2010, from 0.477 million to 0.114 million.2 Measles is common and often fatal in developing areas: the World Health Organization (WHO) estimates that there were 139,300 deaths globally from measles in 2010, more than 95% of which occurred in low-income countries with weak health infrastructures.3 In the pre-vaccination era, the infection of children < 15 y of age was near universal and epidemics cycles occurred every 3–4 y.4 The measles case fatality rate (CFR) varies from 0.1%5 in industrialized countries to as high as 30% in emergency settings (e.g., among refugees).6-8
Rubella is usually a mild rash illness in children and adults, but it can cause serious consequences when a pregnant woman becomes infected.9,10 Rubella usually occurs in a cyclical pattern, with epidemics every 5–9 y. However, the extent and frequency of rubella outbreaks is highly variable in both industrialized and developing countries. In the pre-vaccination era, around 80% of subjects became infected with rubella virus within the 20th-24th mo of age. Around 10-20% of adults remained susceptible. Infection with rubella virus during pregnancy may lead to the death of the fetus or premature delivery, or may cause Congenital Rubella Syndrome (CRS), first described by an Australian ophthalmologist, Norman McAlister Gregg, in 1941, a condition in which all fetal organs may be affected, resulting for example in cataracts, deafness, heart defects, mental retardation. The severity of the damage to the fetus depends on the gestational age, the highest risk is in the first trimester of pregnancy. Congenital infections from rubella virus accounted for more than 100,000 cases worldwide in 1996 especially in countries with high rates of susceptibility to rubella among women of childbearing age.11 Many countries have introduced rubella vaccine ever since, but very few countries introduced the vaccine in Africa, South-East Asia and the Western Pacific (areas at higher risk for rubella infection) by the year 2008, therefore the current burden of CRS in these areas is thought to be similar to that estimated for 1996.12
Eradication and Elimination of Measles and Rubella
Measles is an antigenically stable virus: there is only one serotype and 23 identified genotypes, but the differences in the genotypes do not influence the vaccine protective efficacy. Measles vaccines contain live attenuated viruses, and immunity lasts several decades, probably lifelong. About 85% (range: 70–98%) of children vaccinated at 9 mo of age develop protection after being vaccinated, a proportion which increases to 95% at 12 mo.13,14 In developed countries children get vaccinated at 12 mo of age and older, often in co-administration with mumps and rubella as MMR vaccine or with mumps, rubella and varicella as MMRV vaccine. Both measles and rubella infections meet all requirements for eradication: they are caused by genetically stable microorganisms, humans are critical for the transmission and there are no environmental or animal reservoirs for the viruses, the period of infectiousness is short, accurate diagnostic tests are available, infections confer lifelong immunity and safe and effective vaccines are available.15 The World Health Organization Region of the Americas already achieved the measles elimination goal in 2002, while the 5 remaining WHO Regions have measles elimination goals set, the Western Pacific Region aiming to eliminate measles by end of 2012, the European and the Eastern Mediterranean WHO Regions by 2015. In 2011, the African Region took on the goal to eliminate measles by 2020, and in 2010 the South-East Asia Region adopted a resolution urging countries to mobilize resources to support measles elimination.
As far as rubella infection is concerned, the Americas and Europe targeted rubella and CRS elimination by 2010 and 2015, respectively and the Western Pacific Region aims to have significantly accelerated rubella and CRS prevention by 2015.16
Circulation of the Diseases in Europe
Despite the launch of a WHO European Region strategic plan 2005–2010 for eliminating measles and rubella and preventing CRS, measles and rubella are still circulating in Europe.
In the European Region of the World Health Organization, in 1998, the elimination of measles and reduction in the incidence of CRS to < 0.01 per 1000 live births by 2010 was endorsed. The current goal, endorsed in 2010 with Resolution EUR/RC60/R12, comprises the elimination of measles and rubella and the prevention of congenital rubella infection by 2015.
In 2011, 30,474 measles cases were reported to The European Surveillance System (TESSy) by the 29 contributing EU and European Economic Area (EEA)/ European Free Trade Association (EFTA) countries (the cases were 35,768 according to the WHO epidemiological brief).17 Most cases were reported in France, which accounted for more than half of the cases. Five countries (France, Italy, Romania, Spain and Germany) accounted for more than 90% of all measles cases in Europe.18 Outbreaks of measles in Europe were still observed in 2012 (period January–July), although the number of reported cases decreased by 40% compared with the same period in 2011 (n = 31,667).19
The regional number of rubella cases fell from 804,567 in 1999 to 10,448 in 2010. In the period 1990–2010 only 467 cases of congenital rubella syndrome, reported by 24 member states, were registered. Rubella vaccines are included in routine vaccination programs of all WHO member states in the European Region and in the period 2000–2010, around 30 million people were immunized against rubella during supplemental immunization campaigns, mostly in Newly Independent States.20 The immunization coverage, especially for first doses of measles containing vaccines, is overall high across the region, and the coverage was as high as 95% in 2010. The situation, however, is not totally clear, as many countries do not supply information over vaccination coverage (this is especially true for second doses) and surveillance systems are inadequate in other countries (this is highlighted, for example, by the too few cases of reported CRS in comparison to the rubella incidence rates during 1990-2010).
In the period January–July 2012, the number of reported rubella cases in the Region increased by 400% compared with the same period in 2011 (n = 3,794).19 There were 20,199 rubella cases among 43 reporting member states. Of the total, 99% of cases (n = 19,922) were registered in only three countries: Romania, Poland, and the Russian Federation, while 15 countries (Albania, Austria, Bulgaria, Croatia, Cyprus, Denmark, Estonia, Finland, Greece, Iceland, Malta, Portugal, Slovenia, Switzerland, and Turkmenistan) reported no cases for the whole period. The laboratory‐confirmed cases were 3,024 (15%) and 11,765 (58%) epidemiologically linked. Most of the confirmed cases (76%) concerned adolescents in the age range of 15–19 y, indicating the need for an immunization strategy targeting this age group. The immunization status was available only for < 1,000 patients (5%), of these, 562 (60%) were unvaccinated and 377 (40%) were vaccinated with at least one rubella vaccine dose.19
Historical Changes in Measles and Rubella Surveillance and Recommendations for Immunization in Italy
In Italy the implementation of the National Plan for Measles and Congenital Rubella Elimination (NPMCRE) was performed since 2003, following a large national measles epidemic, which caused 40,000 cases and the death of 8 children in 2002. During the implementation of the NPMCRE, measles and rubella surveillance has been strengthened, in order to monitor the progress made towards the elimination of the diseases and the prevention of CRS. Laboratory investigation of all suspected cases, with special attention to rubella cases in pregnant women, started in 2005, when CRS became a statutory notifiable disease,21 and a catch-up campaign of school-aged children (7–14 y old) with Measles-Mumps-Rubella (MMR) vaccine was conducted in all regions. Despite all the efforts, the objectives of the National Plan (2003–2007) for measles elimination have not yet been achieved. The goal of measles elimination and incidence reduction of CR cases has been postponed to 2015 by the Italian Ministry of Health, through the implementation of the new National Plan 2010–2015, approved in March 2011,22 which requires (1) the achievement of more than 95% coverage with one dose and two doses of measles containing vaccine within 24 mo and within 12 y of age, respectively; (2) supplementary vaccination activities aimed at susceptible populations including adolescents, young adults and those at risk (health care and educational workers, military, “hard to reach” groups like nomads); (3) the reduction to less than 5% in the proportion of susceptible women of childbearing age (especially immigrant women).
Measles containing vaccines have been available in Italy since 1985. The rubella vaccine is available in Italy since 1972. Initially, only the selective vaccination of adolescent females was recommended. Starting from the early 1990s, the universal vaccination of all newborns was recommended and offered free of charge in several Regions. In 1999 the MMR vaccine was included in the national routine childhood vaccination schedule with a dose administered at 12–15 mo of age. Since 2001, MMR vaccine has been offered free of charge in all Regions and in 2004 a two-dose vaccine schedule was adopted.
The current schedule for MMR vaccine in Italy is: 1st dose at 12–15 mo, 2nd dose at 5–6 y of age.
In 2010 the mean national coverage for the first dose of M- MMR-MMRV in children up to 2 y of age was 90.6% and only one Region (Umbria) had achieved the expected 95% coverage.23
Between July 2009 and September 2010, 2.151 measles cases were reported in Italy, 42% (n = 895) of which were laboratory confirmed.24 The median age of cases was 18 y, 92% occurred in unvaccinated subjects. Many cases with complications were reported (n = 305), three of which with encephalitis and 36% of cases were hospitalized. Molecular characterization revealed that genotypes D4, D8 and B3 were those circulating in Italy during the covered period of the analysis.
As vaccination coverage of toddlers has improved, measles and rubella cases have fallen dramatically, but the age of those infected has changed, as the main group of susceptibles is currently composed of adolescents and young adults: during the rubella epidemic of 2008, about 70% of cases occurred in individuals aged 15 y and older,25 while in the pre-vaccination era the average age of infection was five years. The higher proportion of young susceptible adults may mean a possible future increase in CRS. More efforts are therefore needed in Italy to ensure high vaccination coverage against rubella and to protect women of childbearing age. The new NPMCRE, approved in 2011, includes the following strategies for the prevention of congenital rubella: (1) the use of evidence-based interventions for improving vaccination coverage (goal: ≥ 95% routine childhood coverage for two doses of MMR vaccine); (2) the use of any opportunity to vaccinate susceptible women of childbearing age; (3) postpartum (and post-abortion) vaccination of susceptible women; (4) the promotion of awareness of CRS and its prevention among women of childbearing age; (5) improving awareness of clinicians; (6) the vaccination of healthcare workers and school personnel; (7) the vaccination of foreign-born women at their first encounter with the Italian healthcare system; (8) strengthening the surveillance systems, including laboratory confirmation of cases and molecular typing and integration with measles surveillance.
Measles and Rubella Epidemiology in Tuscany (Central Italy)
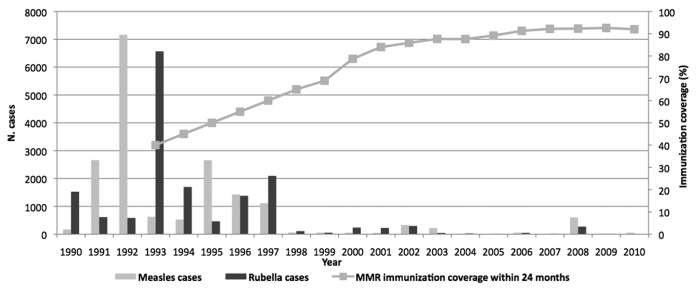
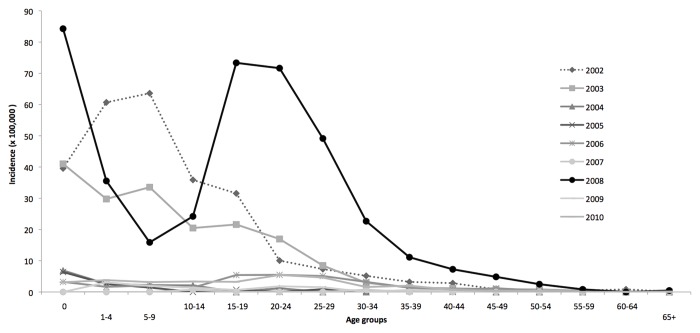
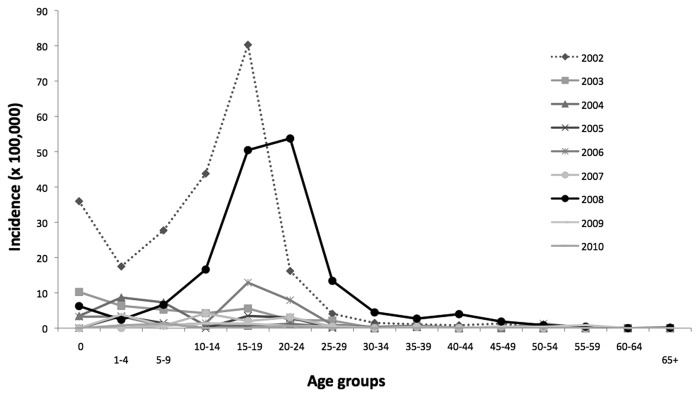
In Tuscany historical changes in vaccination strategies replicated the Italian national steps.26 Measles and rubella cases have fallen dramatically following the introduction of the immunization program in the regional territory (Fig. 1).27-29 Since the start of NPMCRE, MMR vaccination coverage with first dose at 24 mo has increased from 87.6% in 2003 to 92% in 2010. Between 2002 and 2009, the highest measles incidence was registered in 2008 (16.3 cases/100,000), with a peak of cases in the age group of 15–29 y old. The highest incidence rate was observed in children under one year of age (84.3/100,000), where 27 cases were reported (Fig. 2) in 2008, representing 5% of the overall number of measles cases (n = 599) notified in Tuscany. In the same year, the highest rubella incidence rate (7.4 cases/ 100,000) was registered as well. The highest incidence rates for rubella were seen in the age groups of 15–19 y and 20–25 y (Fig. 3), with values over 50 cases/100,000 inhabitants in both groups.
Figure 1. Number of measles and rubella cases, 1990–2010, and MMR immunization coverage within 24 mo of age in Tuscany, 1993–2010. [Measles cases ( = striped bars); rubella cases ( = gray bars); gray solid line = trend of routinely registered immunization coverage at regional level].
Figure 2. Measles incidence by age groups in Tuscany, 2002–2010.
Figure 3. Rubella incidence by age groups in Tuscany, 2002–2010.
In the period 2004–2009 one case of rubella in pregnancy was notified in 2007 and 3 cases were reported in 2008: termination of pregnancy was the consequence of the rubella diagnosis in 2 cases and two children with CRS were born.
The MMR vaccination coverage with one dose in older children or adolescents and with the second dose is not measured routinely, but in Tuscany, since 2003 (Table 1), the vaccination coverage is reported for all subjects up to 15 y of age, and it is one of the highest MMR vaccination coverages with two doses in Italy.
Table 1. Immunization coverage with two doses of MMR at 6, 12 and 15 y of age in Tuscany, 2003–2011.
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |
|---|---|---|---|---|---|---|---|---|---|
| 2nd dose at 6 y (%) | 29.7 | 41.0 | 59.6 | 52.5 | 61.4 | 63.1 | 65.1 | 65.4 | 74.8 |
| 2nd dose at 12 y (%) | 18.0 | 33.2 | 61.3 | 68.2 | 68.0 | 76.1 | 80.4 | 66.8 | 79.1 |
| 2nd dose at 15 y (%) | 17.9 | 41.1 | 55.2 | 68.5 | 67.6 | 72.2 | 78.5 | 65.0 | 78.0 |
Note: In the observation period, the contribution for the calculation of immunization coverage was not provided by all 12 LHUs of Tuscany.
During the period of observation, not all Local Health Units (LHUs) of Tuscany provided the data for the calculation of immunization coverage with two doses for children older than two years or adolescents. For this reason there may be drops, instead of rises, in immunization coverage between two consecutive years (Table 1); in fact, the denominator varied according to the number of LHUs having contributed to calculating the vaccination coverage. The most reliable data are those of the last two years, 2010 and 2011, because all LHUs have provided the data for the calculation of the regional vaccination coverage. An increasing immunization coverage with two MMR doses at 6, 12 and 15 y was registered from 2003 to 2011, reaching values around 75% in children of 6 y, in the last reported year, while in adolescents of 12 and 15 y the MMR immunization coverage was 79% and 78%, respectively. According to the ICONA survey (a national vaccination coverage survey based on cluster sampling method as recommended by the World Health Organization) performed in 2008,30 the vaccination coverage in adolescents aged 16 y old in Tuscany was as high as 90.9% (CI 95%: 87.1% - 94.8%) for one dose and 75.2% (CI95%: 66.8–83.6%) for two doses, while the national average coverage, with regards to the same birth cohort, was 78.1% (CI95%: 75.9–80.1%) for one dose and 53.9% (CI95%: 51.4–56.3%) for two doses.
A study to identify, for the first time, groups of susceptible individuals in the general population of Tuscany and to evaluate the impact of the catch-up campaign in 2004–2005 was performed recently. The campaign had involved over 160,000 children attending primary and lower secondary schools (born in 1991–97, 7–14 y old), that is about 85% of the resident regional population in the same age groups.26 At the end of 2005, the MMR vaccination coverage in such population reached 88.2% for the first dose (it was 79% before the introduction of the catch-up program), and 66% for the second dose (previously 38%). About 10% of vaccination refusals was registered during the immunization campaign.31,32 Furthermore, the susceptibility to measles in the population of Tuscany (1–49 y) had been evaluated in 2003 and in 2006 by two similar sero-epidemiological surveys. No statistical significant differences were found between the two surveys, except for the age group 2–4 y old (p = 0.043). This result should be due to the increasing uptake of MMR vaccine in the pediatric population of Tuscany. An increase in susceptibility to measles in young adults from 2003 to 2006 in Tuscany was also observed. This reflects the expected epidemiological shift of mean age of infection towards older age groups when suboptimal vaccination coverage is maintained for a long period.
In Tuscany, in the period 2007–2011 measles viruses isolated from samples of 38 patients were genotyped. Five different genotypes were recognized: D4 (79%); D8 (10.5%); B3 (5.2%); H1 (2.6%) and A (2.6%). The D4 genotype had already appeared at regional level in an outbreak in the province of Grosseto in 2006.33 In this epidemic, of 40 cases, the index case was a 23 y old woman returning from a travel to India, where D4 genotypes were previously identified.34 Similar outbreaks related to importation of D4 genotype were recently reported in other Italian regions and some European countries.35-37
At the present time, the D4 genotype probably represents an endemic strain of the virus in Italy.
The D8 genotype has also circulated in Europe and India, while the B3 strains were identified in 2006 and 2007 in previous Italian outbreaks24,35,38,39
In Tuscany, B3 genotypes were isolated only in two cases, one in 2007 and one in 2011, both genotypes were isolated from patients of the province of Grosseto. In 2011 two other genotypes were isolated (H1 and A), probably due to an importation of cases from other countries.
In Tuscany, the susceptibility to rubella in fertile women (15–49 y) was also investigated.26 In 2006, the WHO-Euro threshold of susceptibility (5%) was exceeded in all female samples up to 29 y (range: 10–13% seronegative): the percentages of susceptible fertile women are still too high to reach CRS elimination. The highest incidence rate of rubella cases, in the same year, was seen in the age group 15–24 y with values ranging from 7.9 cases/100,000 inhabitants to 12.9 cases/100,000 inhabitants. (Fig. 3)
Discussion
Experiences at regional level have shown promising results in order to create an integrated surveillance system between regional and local health authorities, university and laboratories.
Vaccination programs reduce the risk of measles and as a consequence, an increase in susceptibility to measles among adults could be observed due to the fact that some adults have never been exposed to measles or have never been vaccinated, some adults were vaccinated but did not respond (primary vaccine failure), and vaccine-induced immunity may decline (secondary vaccine failure).15
People who received the last dose of measles vaccine more than 10 y ago have a small but significantly increased risk of becoming infected compared with people who received the last vaccine dose more recently.18 In Italy, the median age of reported measles cases was 17 y in 2008 and has further increased to 18 y in 2009–2010, when a high percentage of cases (61%) was observed in subjects aged 15–44 y.24 The mean age of cases, during a recent measles epidemic in Tuscany, was 27 y and more than 90% of subjects were over 15 y.40 Epidemiological data are consistent with the results of the two sero-epidemiological surveys performed in Tuscany in 2003 and 2005–2006, during which an increasing susceptibility to measles in adolescents and young adults (aged 15–29 y) was observed comparing the two periods.26 A new method for the interpretation of serological surveys is the mixture model approach. Mixture models can give a better idea of the complexity behind the distribution of the population immunity, which in many cases cannot be reduced to the “simple cut-off” separation between susceptible and immune persons.41 The unexpected result of the model applied to our regional data are the large group of weakly immune individuals or “low responders,” classified as “equivocal” by the ELISA method. The overall proportion of equivocal sera was 2.2% (21 sera, mainly in the age groups 10–24 y) in 2005–06 sero-epidemiological survey and 5.8% (32 sera, mainly in the age groups 5–24 y) in the 2003 sero-epidemiological measles survey.26 Surely these subjects belong to cohorts with a moderate vaccine uptake but which also faced the decline in incidence due to the increasing vaccine coverage. The increase in herd immunity might have sharply reduced the effects of natural boosting, thereby reducing antibody counts. An important question is whether “low responders” might sooner or later become “individuals at risk.” Maybe the current knowledge about immunity in highly vaccinated populations is still insufficient and therefore careful monitoring of such occurrences is important. The model accurately identifies the ages where pockets of susceptible exist, mostly between 10 and 20 y, and which therefore represent the target for further catch-up activities in Tuscany.41
As observed in other Italian serosurveys, when considering results of 2004, compared with 1996, a decrease in seroprevalence rates is observed from age group 2–4 y to all age groups up to 19 y. This finding can be explained by the waning of measles vaccine-induced antibodies, faster than the decaying with natural immunity, mostly in Northern and Central regions, which historically had the highest vaccination coverage.42
A relatively small percentage (1%) of measles genotypes isolated in the period 2007–2011 in Tuscany derived from children of one year or younger. In 2008 the highest measles incidence rate was registered in children < 1 y, representing 5% of reported cases. In Italy, during the latest outbreaks, the proportion of measles cases in infants varied from 4.3%43 to 10–11%39,44 and to 25%.45 In Europe the last measles outbreaks involved infants even in higher percentages, from 33% to 83%.46-48 According to the Italian National Plan for Elimination of Measles and Congenital Rubella one dose of MMR vaccine to children from six months of age was recommended in particular circumstances, for example, following exposure or in outbreak settings, when immediate protection against measles is required.22,49 Specific strategies to address measles epidemics in the population under one year of age were implemented in some EU countries. Vaccination of contacts as early administration of a first dose of the measles vaccine from 6 mo of age was offered not only in Italy but even in Greece, Romania and Spain, while Denmark, Norway, Poland, Switzerland, UK recommended the first vaccine dose from 9 mo of age. Other general control measures applied during outbreaks included: enhanced surveillance, active case findings, early administration of the second dose, post-exposure prophylaxis with immunoglobulins in pregnant women and specific information campaigns in nurseries and kindergartens.50 Vaccination of mothers, family members and primary caregivers could reduce the risk of unvaccinated infants to become infected.18 The changing pattern in the epidemiology of measles in developed countries and the presence of susceptible adolescents or adults require special attention to surveillance system activities aiming to identify possible sources of infection for children too young to be immunized. Health care workers in general, and particularly those involved in the assistance of newborns or infants, should be protected against measles and rubella in order to prevent new cases in these vulnerable subjects and to avoid nosocomial transmission. Healthcare workers were involved during the last outbreaks in Italy24 and nosocomial transmission was observed in Tuscany in 30% of cases.40
The most effective way to stop the measles virus circulation is to decrease the susceptible cohorts by timely administration of the measles vaccines according to national vaccination programs and reaching high vaccination coverage with the recommended 2-dose schedule.51
The evaluation of all preventive activities performed in the last decade in Tuscany, the immunization coverage data, the sero-epidemiological population profile and measles and rubella incidence rates highlighted critical points which should be improved and good practices which should be maintained in the future in order to reach new goals. Pockets of susceptibility to rubella and measles in the population of Tuscany were discovered and target populations for catch-up strategies with MMR vaccine have been identified. It is well known that the higher the immunization coverage is, the harder it is to further increase vaccination uptake. Vaccination coverage, even for second dose, needs to be improved with tailored strategies, especially with respect to the population > 15 y and urging the screening of women of childbearing age, to identify, before they become pregnant, those who lack rubella antibodies acquired either as the result of vaccination or natural infection. The immunization of all susceptible women in childbearing age in all possible occasions (e.g., the postpartum days), with a special focus on immigrant women, is indeed a priority.
Conclusion
Italy is still in a stage of limited control of measles, especially as far as adolescents and young adults are concerned, and women of childbearing age are not adequately protected against rubella infection: the incidence of congenital rubella in our country is greater than 1/100,000 live births. The success achieved in other countries shows that the objectives of elimination are technically possible with the availability of vaccines and targeted vaccination strategies.
The current paradox in prevention is that immunisation programs can become victims of their own success. Some vaccine-preventable diseases have become so sporadic that people, and even health professionals, fail to appreciate the benefits of vaccination. In Western Europe, measles and rubella are not perceived as a serious problem; in fact anti-vaccine movements have gained popularity in the last decades, dangerously publicizing unfounded vaccine safety concerns. As a matter of fact, fears for a causal relationship between measles containing vaccines and autism led to decreasing vaccination coverage in Western Europe. Researchers need to better understand how anti-vaccination lobbies operate and how to intervene in order to counteract effectively their messages and conduct operational research on communication strategies with the use of adequate communication aimed at addressing parents’ concerns.
Specific efforts need to be put to target vulnerable and high-risk populations as well as health care workers, through targeted strategies, for example through advocacy and outreach campaigns like the annual European Immunization Week and using any additional opportunity for immunization of susceptible children, adolescents, adults and women of childbearing age without documented evidence of immunity.
Acknowledgments
The authors wish to thank Regional and Local Health Authorities of Tuscany for providing data on mandatory notified measles and rubella cases and on immunization coverage with MMR vaccine. The authors are also grateful to Fabio Magurano and Loredana Nicoletti (National Institute of Health - Istituto Superiore di Sanità, Rome) for providing results on molecular characterization of measles genotypes for the period 2007–2011.
Glossary
Abbreviations:
- CFR
case fatality rate
- CR
Congenital rubella
- CRS
Congenital rubella syndrome
- EEA
European Economic Area
- EFTA
European Free Trade Association
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- LHUs
Local Health Units
- MCV
measles containing vaccine
- MMR
measles-mumps-rubella
- MMRV
measles-mumps-rubella- varicella
- NPMCRE
National Plan for Measles and Congenital Rubella Elimination
- TESSy
The European Surveillance System
- WHO
World Health Organization
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/23261
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Measles - Fact sheet N°286. 2012. Available from: http://www.who.int/mediacentre/factsheets/fs286/en/
- 4.Centers for Disease Control and Prevention. Measles. In: Atkinson W, Wolfe C, Hamborsky J, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th edition. Washington DC: Public Health Foundation, 2012: 173–92. [Google Scholar]
- 5.Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(Suppl 1):S4–16. doi: 10.1086/377712. [DOI] [PubMed] [Google Scholar]
- 6.Shears P, Berry AM, Murphy R, Nabil MA. Epidemiological assessment of the health and nutrition of Ethiopian refugees in emergency camps in Sudan, 1985. Br Med J (Clin Res Ed) 1987;295:314–8. doi: 10.1136/bmj.295.6593.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter JD, Gastellu-Etchegorry M, Navarre I, Lungu G, Moren A. Measles outbreaks in the Mozambican refugee camps in Malawi: the continued need for an effective vaccine. Int J Epidemiol. 1990;19:1072–7. doi: 10.1093/ije/19.4.1072. [DOI] [PubMed] [Google Scholar]
- 8.Cairns KL, Nandy R, Grais RF. Challenges in measuring measles case fatality ratios in settings without vital registration. Emerg Themes Epidemiol. 2010;7:4. doi: 10.1186/1742-7622-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011;86:301–16. [PubMed] [Google Scholar]
- 10.Who Publication Rubella vaccines: WHO position paper--recommendations. Vaccine. 2011;29:8767–8. doi: 10.1016/j.vaccine.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 11.Reef SE, Strebel P, Dabbagh A, Gacic-Dobo M, Cochi S. Progress toward control of rubella and prevention of congenital rubella syndrome--worldwide, 2009. J Infect Dis. 2011;204(Suppl 1):S24–7. doi: 10.1093/infdis/jir155. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Immunization, Vaccines and Biologicals - Rubella. 2012. Available from: http://www.who.int/immunization/topics/rubella/en/index.html
- 13.European Centre for Disease Control and Prevention. Surveillance report European monthly measles monitoring (EMMO). Stockholm 2012;10:1-10. Available from: http://ecdc.europa.eu/en/publications/Publications/1205-SUR-Measles-monthly-monitoring.pdf
- 14.Cutts FT, Grabowsky M, Markowitz LE. The effect of dose and strain of live attenuated measles vaccines on serological responses in young infants. Biologicals. 1995;23:95–106. doi: 10.1016/1045-1056(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 15.Orenstein WA, Strebel PM, Papania M, Sutter RW, Bellini WJ, Cochi SL. Measles eradication: is it in our future? Am J Public Health. 2000;90:1521–5. doi: 10.2105/AJPH.90.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Global measles and rubella strategic plan: 2012-2020.Geneva (Switzerland) 2012:1-44. [Google Scholar]
- 17.World Health Organization Regional Office for Europe. WHO Epidemiological brief. Copenhagen; 2012;21:5-9. Available from: http://www.euro.who.int/en/what-we-do/health-topics/disease-prevention/vaccines-and-immunization/publications/2012/vpd-data-summary-table-january-december-2011-as-of-1-march-2012
- 18.European Centre for Disease Control and Prevention. Surveillance report European monthly measles monitoring (EMMO). Stockholm 2012;9:1-10. Available from: http://ecdc.europa.eu/en/publications/Publications/SUR-EMMO-European-monthly-measles-monitoring-March-2012.pdf
- 19.World Health Organization Regional Office for Europe. WHO Epidemiological brief. Copenhagen; 2012;26:2-11 Available from: http://www.euro.who.int/__data/assets/pdf_file/0017/173060/EpiBrief-Issue-26-Sept-2012.pdf
- 20.Jankovic D. Rubella and CRS overview for WHO Europe. Progress Toward Rubella Elimination and CRS Prevention in Europe. Rome, Italy; 2012. [Google Scholar]
- 21.Italia. Decreto del Ministero della Salute 14 ottobre 2004 [Ministry of Health. Decree 14 October 2004]. Notifica obbligatoria della infezione da rosolia in gravidanza e della sindrome/infezione da rosolia congenita [Compulsory reporting of rubella infection in pregnancy and of congenital rubella syndrome/infection]. Gazzetta Ufficiale n. 259, 4 novembre 2004. Italian.
- 22.Presidency of the Council of Ministers. Rep.n. 66/ Conferenza Stato-Regioni del 23 marzo 2011. Piano nazionale per l’eliminazione del morbillo e della rosolia congenita (PNEMoRc) 2010-2015. [Rep. N. 66/State–Regions Conference of 23 March 2011. Italian national plan for the elimination of measles and congenital rubella (PNEMoRc) 2010-2015. Italian]
- 23.Italian Ministry of Health. Vaccinazioni dell’età pediatrica: coperture vaccinali (per 100 abitanti) in Italia. Rome; 2012. Italian. [Google Scholar]
- 24.Filia A, Tavilla A, Bella A, Magurano F, Ansaldi F, Chironna M, et al. Measles in Italy, July 2009 to September 2010. Euro Surveill. 2011;16:pii: 19925. [PubMed] [Google Scholar]
- 25.Salmaso S. Surveillance and control of rubella and congenital rubella in Italy. Progress Toward Rubella Elimination and CRS Prevention in Europe. Rome, Italy; 2012. [Google Scholar]
- 26.Bechini A, Boccalini S, Tiscione E, Pesavento G, Mannelli F, Peruzzi M, et al. Progress towards measles and rubella elimination in Tuscany, Italy: the role of population seroepidemiological profile. Eur J Public Health. 2012;22:133–9. doi: 10.1093/eurpub/ckq134. [DOI] [PubMed] [Google Scholar]
- 27.The Italian Vaccine Coverage Survey Working Group Childhood vaccination coverage in Italy: results of a seven-region survey. Bull World Health Organ. 1994;72:885–95. [PMC free article] [PubMed] [Google Scholar]
- 28.Salmaso S, Rota MC, Ciofi Degli Atti ML, Tozzi AE, Kreidl P, ICONA Study Group Infant immunization coverage in Italy by cluster survey estimates. Bull World Health Organ. 1999;77:843–51. [PMC free article] [PubMed] [Google Scholar]
- 29.Ciofi Degli Atti ML, Rota MC, Bella A, Salmaso S, ICONA Study Group Do changes in policy affect vaccine coverage levels? Results of a national study to evaluate childhood vaccination coverage and reasons for missed vaccination in Italy. Vaccine. 2004;22:4351–7. doi: 10.1016/j.vaccine.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Gruppo di lavoro ICONA. ICONA 2008: Indagine di COpertura vaccinale NAzionale nei bambini e negli adolescenti. Roma: Istituto Superiore di Sanità; 2009. (Rapporti ISTISAN 09/29).
- 31.Bonanni P, Bechini A, Pesavento G, Boccalini S, Tiscione E, Graziani G, et al. Implementation of the plan for elimination of measles and congenital rubella infection in Tuscany: evidence of progress towards phase II of measles control. J Prev Med Hyg. 2005;64:111–7. [Google Scholar]
- 32.Bechini A, Pesavento G, Boccalini S, Tiscione E, Balocchini E, Graziani G, et al. Implementazione del Piano per l’eliminazione del morbillo e della rosolia congenita inToscana: progressi verso la seconda fase di controllo dell’infezione. Not Ist Super Sanità. 2006;19:iii–v. [Available from: http://www.epicentro.iss.it/ben/2006/aprile/aprile.pdf] [Google Scholar]
- 33.Boncompagni G, Incandela L, Bechini A, Giannini D, Cellini C, Trezzi M, et al. Measles outbreak in Grosseto, central Italy, 2006. Euro Surveill. 2006;11:E060803.4. doi: 10.2807/esw.11.31.03015-en. [DOI] [PubMed] [Google Scholar]
- 34.Ramamurty N, Raja D, Gunasekaran P, Varalakshmi E, Mohana S, Jin L. Investigation of measles and rubella outbreaks in Tamil Nadu, India-2003. J Med Virol. 2006;78:508–13. doi: 10.1002/jmv.20569. [DOI] [PubMed] [Google Scholar]
- 35.Filia A, Curtale F, Kreidl P, Morosetti G, Nicoletti L, Perrelli F, et al. Cluster of measles cases in the Roma/Sinti population, Italy, June-September 2006. Euro Surveill. 2006;11:E061012.2. doi: 10.2807/esw.11.41.03062-en. [DOI] [PubMed] [Google Scholar]
- 36.Siedler A, Tischer A, Mankertz A, Santibanez S. Two outbreaks of measles in Germany 2005. Euro Surveill. 2006;11:131–4. [PubMed] [Google Scholar]
- 37.van Treeck U. Measles outbreak in Germany: over 1000 cases now reported in Nordrhein Westfalen. Euro Surveill 2006;11(5):E060511.1. Available from:http://www.eurosurveillance.org/ew/2006/060511.asp#1 [DOI] [PubMed]
- 38.Chironna M, Prato R, Sallustio A, Martinelli D, Germinario C, Lopalco P, et al. Genetic characterization of measles virus strains isolated during an epidemic cluster in Puglia, Italy 2006-2007. Virol J. 2007;4:90. doi: 10.1186/1743-422X-4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtale F, Perrelli F, Mantovani J, Ciofi degli Atti M, Filia A, Nicoletti L, et al. Description of two measles outbreaks in the Lazio Region, Italy (2006-2007). Importance of pockets of low vaccine coverage in sustaining the infection. BMC Infect Dis. 2010;10:62. doi: 10.1186/1471-2334-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonanni P, Bechini A, Boccalini S, Peruzzi M, Tiscione E, Boncompagni G, et al. Progress in Italy in control and elimination of measles and congenital rubella. Vaccine. 2007;25:3105–10. doi: 10.1016/j.vaccine.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Del Fava E, Shkedy Z, Bechini A, Bonanni P, Manfredi P. Towards measles elimination in Italy: monitoring herd immunity by Bayesian mixture modelling of serological data. Epidemics. 2012;4:124–31. doi: 10.1016/j.epidem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Rota MC, Massari M, Gabutti G, Guido M, De Donno A, Ciofi degli Atti ML. Measles serological survey in the Italian population: interpretation of results using mixture model. Vaccine. 2008;26:4403–9. doi: 10.1016/j.vaccine.2008.05.094. [DOI] [PubMed] [Google Scholar]
- 43.Cova M, Cucchi A, Turlà G, Codecà B, Buriani O, Gabutti G. Spotlight on measles 2010: Increased measles transmission in Ferrara, Italy, despite high vaccination coverage, March to May 2010: increased measles transmission in Ferrara, Italy, despite high vaccination coverage, March to May 2010. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 44.Prato R, Chironna M, Caputi G, Sallustio A, Martinelli D, Falco A, et al. An outbreak of measles in Apulia, Italy, November 2006-January 2007. Euro Surveill. 2007;12:E070405–, 1. doi: 10.2807/esw.12.14.03168-en. [DOI] [PubMed] [Google Scholar]
- 45.Caputi G, Tafuri S, Chironna M, Martinelli D, Sallustio A, Falco A, et al. An outbreak of measles including nosocomial transmission in Apulia, south-east Italy, January-March 2008--a preliminary report. Euro Surveill. 2008;13 [PubMed] [Google Scholar]
- 46.Domínguez A, Torner N, Barrabeig I, Rovira A, Rius C, Cayla J, et al. Working Group for the Study of the Measles Outbreak in Catalonia Large outbreak of measles in a community with high vaccination coverage: implications for the vaccination schedule. Clin Infect Dis. 2008;47:1143–9. doi: 10.1086/592258. [DOI] [PubMed] [Google Scholar]
- 47.Stanescu A, Muscat M, Romaniuc A, Pipirigeanu R, Lupulescu E, Necula G, et al. Spotlight on measles 2010: an ongoing measles outbreak in the district of Neamt, Romania, August–September 2010. Euro Surveill. 2011;15:pii: 19682. doi: 10.2807/ese.15.40.19682-en. [DOI] [PubMed] [Google Scholar]
- 48.Groth C, Bottiger B, Plesner A, Christiansen A, Glismann S, Hogh B. Nosocomial measles cluster in Denmark following an imported case, December 2008-January 2009. Euro Surveill. 2009;14 [PubMed] [Google Scholar]
- 49.Department of Health - UK. Immunisation against infectious disease. Chapter 21. Measles. 2010th ed. London: TSO (The Stationery Office); 2010. p. 209–34. [Google Scholar]
- 50.Leuridan E, Sabbe M, Van Damme P. Measles outbreak in Europe: susceptibility of infants too young to be immunized. Vaccine. 2012;30:5905–13. doi: 10.1016/j.vaccine.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 51.Machaira M, Papaevangelou V. Current measles outbreaks: can we do better for infants at risk? Pediatr Infect Dis J. 2012;31:756–8. doi: 10.1097/INF.0b013e31825ad11b. [DOI] [PubMed] [Google Scholar]