Abstract
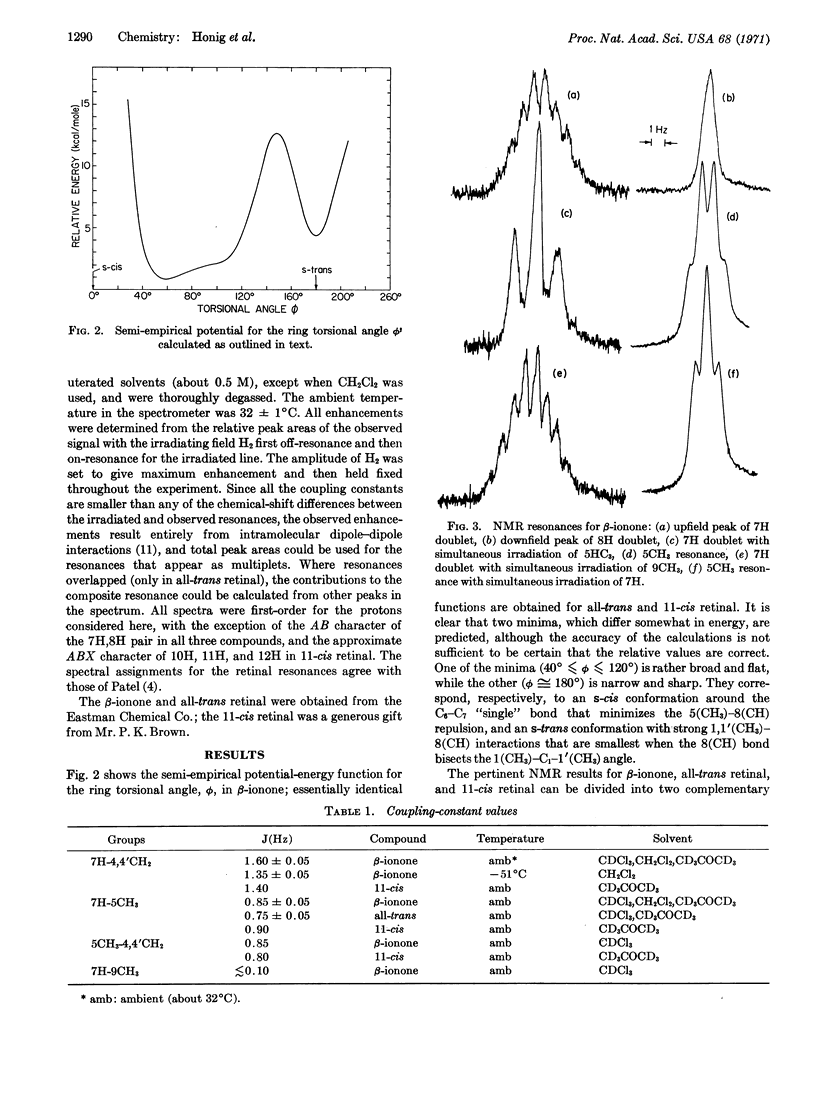
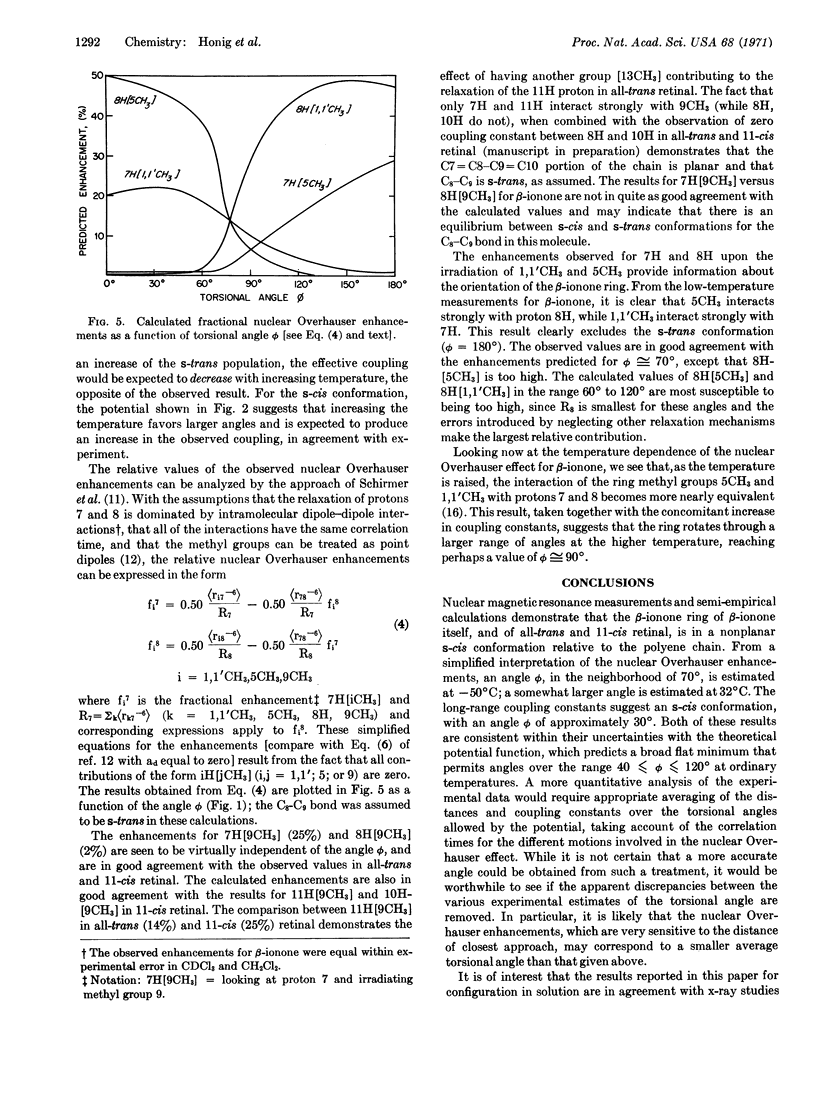
The ring orientation in β-ionone, all-trans retinal, and 11-cis retinal, relative to that of the polyene chain, has been determined by means of semi-empirical calculations and magnetic resonance measurements of the nuclear Overhauser effect and long-range coupling constants. The experimental results yield a distorted s-cis conformation about the C6-C7 “single bond”, with the torsional angle in the range 30° to 70°. This agrees well with the semi-empirical potential function, which has a broad, rather flat minimum for angles from 40° to 120°. The temperature dependence of the NMR results provide confirmation for the form of the torsional potential.
Keywords: theoretical, NMR, semi-empirical approach, Overhauser effect, s-cis conformation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Honig B., Karplus M. Implications of torsional potential of retinal isomers for visual excitation. Nature. 1971 Feb 19;229(5286):558–560. doi: 10.1038/229558a0. [DOI] [PubMed] [Google Scholar]
- Korver P. K., Kruk C., van der Haak P. J., Baas J. L., Huisman H. O. Vitamin A analogues. 3. Determination of the stereochemical configuration of some polyenes of the 4-thia-vitamin A series by NMR spectrometry. Tetrahedron. 1966 Jan;22(1):277–284. doi: 10.1016/0040-4020(66)80128-x. [DOI] [PubMed] [Google Scholar]
- Nash H. A. The stereoisomers of retinal--a theoretical study of energy differences. J Theor Biol. 1969 Feb;22(2):314–324. doi: 10.1016/0022-5193(69)90008-3. [DOI] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968 Oct 11;162(3850):230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]



