Abstract
Premixed calcium phosphate cements can reduce handling complications that are associated with the mixing of cements in the operating room. However, to extend the clinical indication of ceramic cements their mechanical properties need to be further improved. The incorporation of a polymeric material with intrinsically high tensile properties could possibly assist in increasing the mechanical properties of calcium phosphate cement. In this study polymer microparticles made from poly(lactid-co-glycolide) plasticised with poly(ethylene glycol) 400 (PLGA/PEG microparticles) were added in amounts of up to 5 wt% to a premixed acidic calcium phosphate cement. The PLGA/PEG microparticles added undergo a shape transformation at 37 °C, which could give a better integration between polymer microparticles and ceramic cement compared with polymer microparticles lacking this property. The results showed that the incorporation of 1.25 wt% PLGA/PEG microparticles increased the compressive strength by approximately 20% up to 15.1 MPa while the diametral tensile strength was kept constant. The incorporation of PLGA/PEG microparticles increased the brushite to monetite ratio after setting compared with pure ceramic cements. In conclusion, small amounts of PLGA/PEG microparticles can be incorporated into premixed acidic calcium phosphate cement and increase their mechanical properties, which could lead to increased future applications.
Keywords: premixed, calcium phosphate, cement, mechanical properties, polymer-ceramic composites, x-ray diffraction, scanning electron microscopy
Introduction
For decades the complex architecture of bone, and the excellent mechanical properties resulting thereof,1 has proven a great challenge for researchers attempting to formulate a bone replacing material that imitates bone both in structure as well as in mechanical properties without sacrificing a good bone integration. The mechanical resistance of bone can be attributed to the hard inorganic mineral, calcium deficient hydroxyapatite, in combination with the strong organic component, collagen, and the intricate structural arrangement of all components of bone.2
There is an increasing demand to find a good material that can efficiently heal defects in bone. The first calcium phosphate based material used for this purpose was developed in 1920.3 An advantage with these materials is their chemical resemblance to the mineral phase of bone, which makes them highly biocompatible. Furthermore, due to their chemical resemblance they are degraded in the same way as bone.4 However, their inherently poor tensile and bending strengths need to be improved in order to expand their clinical use.
In the early 1980s the two first calcium phosphate cements (CPCs) were presented by LeGeros,5 and Brown and Chow.6 These cements had an alkaline setting reaction and hydroxyapatite was formed after curing.5,6 Some years later Mirtchi et. al.7 reported on a new type of bone cement made from β-tricalcium phosphate (β-TCP) and monocalcium phosphate monohydrate (MCPM). These cements have an acidic setting reaction with brushite as the end product (Eqn. 1), and have a short setting time of only a few minutes.
β – Ca3(PO4)2 + Ca(H2PO4)2•H2O + 7H2O → 4CaHPO4•2H2O(1)
It has been suggested, and tested with good results, that the mechanical properties of CPC can be improved by the addition of a polymer, which has intrinsic ductile properties, to the liquid phase of the cement. Most research has been performed on the incorporation of poly(acrylic acid) (PAA) into the CPC,8-10 giving CPCs with compressive strengths (CS) of up to 90 MPa and diametral tensile strengths (DTS) of up to 21 MPa, which is about a 10-fold increase compared with the plain cements. Although these cements show high mechanical properties they are not optimal due to the poor resorption properties of PAA, and these CPC’s can thus mostly be used as models for other composite combinations. It has also been shown that CPCs that contain particles of the degradable poly(lactide-co-glycolide) (PLGA) show good biological properties;11-13 however, the incorporation has been found to decrease the mechanical properties of the cement,14,15 which could be due to the poor bonding and blending between the cement and polymer particles.
The water-mixed CPCs described herein, have three drawbacks related to handling properties. First, the stressful, and sometimes complicated, mixing in the operating room, which is due to the short working and setting times of the water mixed cements. Second, during mixing of the powder and the liquid phase in the operating room, there could be discrepancies between the cements depending on how well the mixing is performed, which could have effects on mechanical and biological properties. Third, due to the short working time of these cements a new batch has to be mixed for each new site of operation, which increases the cost of both the material and the surgery due to the time spent. To overcome these drawbacks several research groups have investigated and evaluated the possibility of using premixed CPCs.16-18 In most premixed CPCs the powders are mixed with a water-miscible non-aqueous liquid, e.g., glycerol16 or low molecular weight poly(ethylene glycol) (PEG),17 forming a paste that starts setting in vivo, giving a long working time. The setting of the paste first starts when the paste is injected into the body and the glycerol or PEG is exchanged for body fluids, i.e., water. The long working times of the premixed CPC implies that the paste can be delivered directly in a syringe, and, hence, the differences between batches would be quite small; furthermore, it enables the repeated use of one unit during the entire operation. To the authors’ knowledge, no studies have been performed on polymers incorporated into premixed CPCs.
The aim of this study was to investigate if microparticles of 53 kDa PLGA (85:15%, < 100 µm, RegenTec Ltd.) with small amounts of PEG 400 added by a hot melt blending technique, studied by Dhillon et. al.,19 can be incorporated into an acidic premixed CPC and thereby increase its mechanical strength. The microparticles investigated herein have a PEG 400 content of 6.5 wt.%, and a Tg of 37 °C,19 equal to normal body temperature. When subjected to temperatures of 37 °C, PEG 400 is leached out and the Tg is increased until the microparticles become completely solid at 37 °C. During this process, the microparticles are molded in the micro environment within which they are trapped, giving them the ability to spread and fill voids. This feature is hypothesized to benefit the mechanical properties unlike the previously investigated PLGA particles that have decreased the strength of water-mixed CPCs.14,15 The ceramic part of the cement investigated in this study is an acidic CPC with the small difference that monocalcium phosphate anhydrous (MCPA) is used instead of MCPM. MCPA has previously been shown to be a good alternative to MCPM in premixed CPCs.20 Glycerol was used as the mixing liquid for the premixed CPCs, since it gives faster setting times than low molecular weight PEG.17 The premixed CPCs were evaluated with regard to their mechanical properties, i.e. CS, and DTS, as well as their chemical composition and microstructure.
Results
Mechanical testing
The addition of small amounts of polymer microparticles (1.25 wt%) gave a significant increase in CS (Fig. 1). In fact, the maximum value for both CS and DTS was found for compositions containing 1.25 wt% polymer microparticles. The composition containing 5 wt% microparticles showed the lowest strength, both for CS and DTS; however a significant difference to 0 wt% was only seen for DTS.
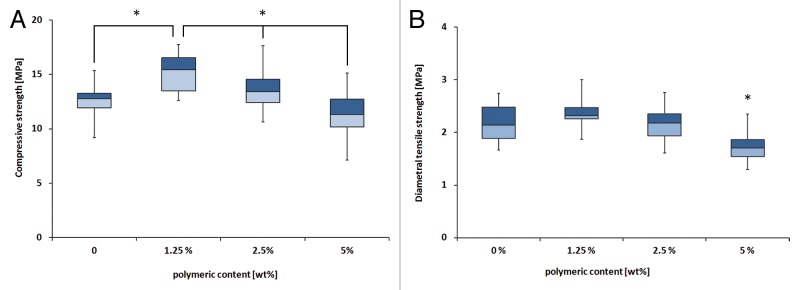
Figure 1. (A) Compressive strength of all cements. *Significant difference between the connected groups (P < 0.05), i.e., there is a significant difference between 0 and 1.25%, and between 1.25, 2.5, and 5%. (B) Diametral tensile strength of all cements. *Significant difference; i.e., 5% is significantly different to all others (P < 0.05)
SEM
The polymer microparticles were well incorporated into the ceramic matrix, see Figure 2. The small circular holes that were visible in the polymer were likely a result of the PEG leaching. These holes were not visible on the polymer microparticles before incorporating them into the cement (Fig. 2B). It was also apparent that the microparticles had been flattened and spread out during leaching of PEG and setting of the cement (Fig. 2A and B), due to the setting temperatures above Tg of the microparticles. Figure 2A also indicated that the polymer had spread in between the ceramic particles and a good integration between the two phases was visible. No obvious difference between the different compositions was seen. However, not many polymer microparticles were visible at the edge of failure, strengthening the theory that they were well integrated with the ceramic matrix.
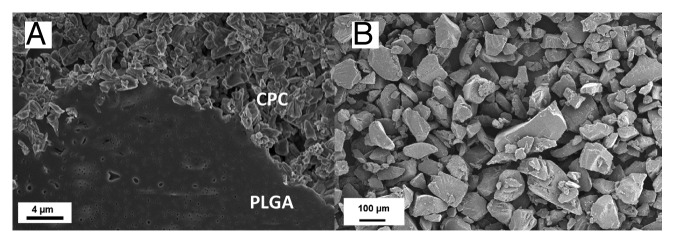
Figure 2. (A) Representative SEM micrograph of a polymer microparticle in a CPC matrix. Image is taken on a sample with 1.25 wt% polymer microparticles incorporated. (B) Polymer microparticles before incorporation in the cement. Note the different scales in the two micrographs.
XRD
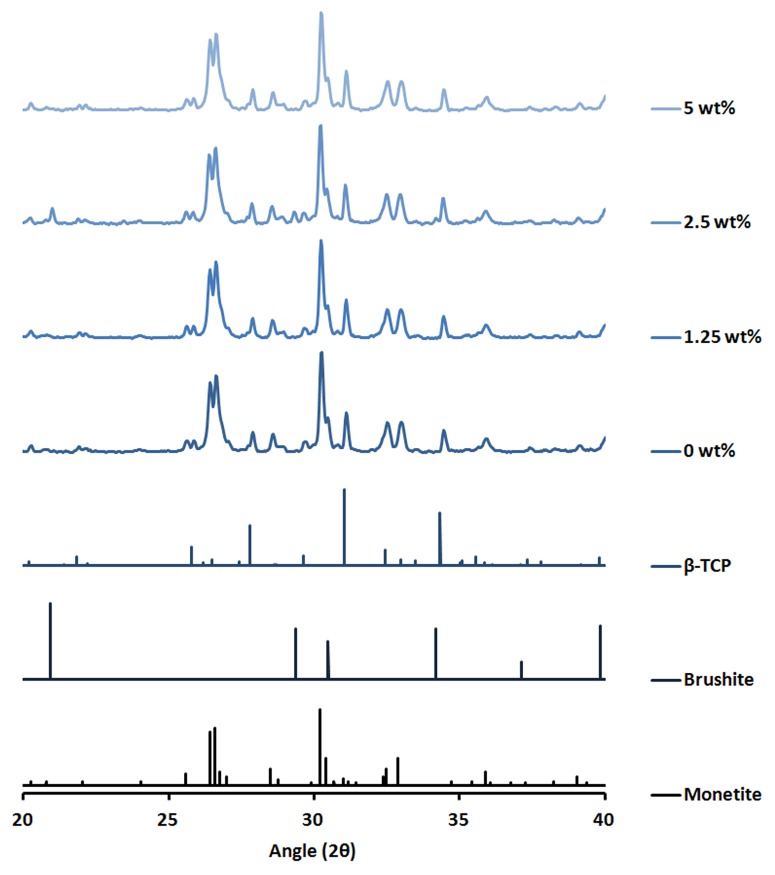
XRD plots showed that the phase composition was quite similar between the different samples (Fig. 3). The only sample that appeared slightly different was the sample containing 2.5 wt% polymer microparticles, which showed a clear brushite peak. Rietveld refinement showed that all cements contained approximately 7 mol% β-calcium pyrophosphate (β-CPP), which originates from impurities in the β-TCP used (Fig. 4). Furthermore, all cements contained between 15–18 mol% unreacted β-TCP. The only marked difference between groups was seen in the monetite vs. brushite ratio. The brushite content varied from as low as 2 mol% for the cement without added polymer to 13 mol% for the cement containing 2.5 wt% polymer microparticles.
Figure 3. Plots showing one representative XRD run for each group. Monetite, brushite, and β-TCP reference patterns are taken from respective PDF file.
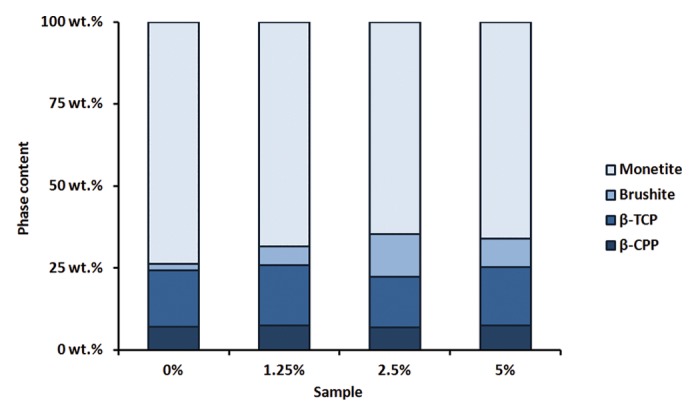
Figure 4. Composition of all cements after Rietveld refinement of the XRD plots
Discussion
This study investigated how the mechanical properties of an acidic premixed calcium phosphate cement was affected by the addition of small amounts of PLGA/PEG microparticles. Unlike other CPC/PLGA composites14,15 the CS for the investigated cements was increased, from 12.6 (±1.5) MPa to 15.1 (±1.7) MPa, with the addition of 1.25 wt% polymer microparticles, an increase of approximately 20%. The strengths of the cements lay in the range of those of cancellous bone (5–15 MPa).21 The DTS on the other hand only showed a slight (and not significant) increase from 2.2 (±0.4) MPa to 2.4 (±0.2) MPa for the same two cement compositions. These results can also be compared with the strength of cements prepared from only the polymer microparticles, which show a CS of around 2 MPa.19 Similarly, other systems have shown optimums, both for systems containing polymeric additives as well as for systems reinforced with fibers.9,22 Although these cements were not as strong as the previously mentioned cements prepared with water and PAA,8-10 there are two big advantages with this type of cement. First, the polymer is both non-toxic and degradable, and second, by utilizing the premixed system, the drawbacks of water-mixed cements, as earlier described, can be avoided. The ideal would, however, be to further increase the strength of the premixed CPC-polymer composite.
It was initially hypothesized that the polymer microparticles would be molded into the cement according to Figure 5. In fact, the SEM micrographs (Fig. 2) showed a good integration of the microparticles in the ceramic matrix, which was likely the reason for the increased mechanical strength for one of the cements. It was also clear from the SEM micrographs that the polymer microparticles were much larger than the brushite and monetite crystallites, which could also have an effect on the resulting strength of the cement. Since the polymer microparticles were produced by mechanical crushing of a solid piece,19 smaller particles are hard to produce and the yield is quite low; however, smaller particles could possibly increase the strength further, and might be good to investigate in future studies.
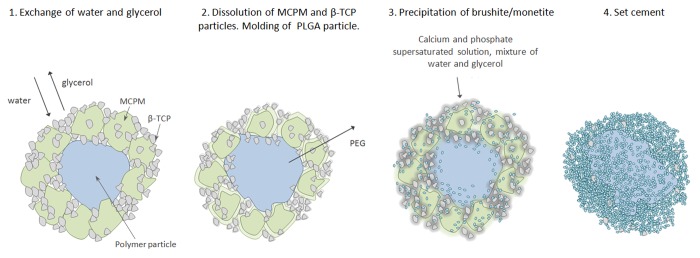
Figure 5. Conceptual drawing of the composite setting reaction. (1) An exchange of glycerol to water starts when the cement is immersed in body fluids at 37 °C. (2) The ceramic grains start to dissolve and since the temperature is around Tg for the polymer it becomes soft and leaching of PEG also starts. (3) Precipitation of crystalline brushite occurs from the supersaturated water, molding of polymer continues. (4) All PEG is leached from the polymer particle and all ceramic starting material have set to a brushite/monetite cement.
From the XRD results it could be concluded that the β-TCP content measured for all groups was slightly higher than the 10 mol% excess that was added to the mixtures. However, this was not surprising since the fast dissolving MCPA might diffuse out from the cement before the proper amount of β-TCP has been dissolved and can react to form the end product. Since β-TCP has a limited solubility at physiological pH—it needs a lower pH to dissolve—and MCPA decreases the pH in the vicinity after dissolution, the excess β-TCP will not be dissolved after all MCPA is consumed.
It has previously been observed that the main product after reaction for premixed acidic calcium phosphate cements is dicalcium phosphate anhydrous, or monetite,16,20 and not brushite, which is seen when MCPM (or MCPA) and β-TCP is mixed directly with water. Under physiological conditions monetite is the more stable phase; however, the nucleation and growth demands high energies, due to the high energies needed to dehydrate calcium, and nucleation and growth of brushite is thus favorable.23,24 In conditions where an insufficient amount of water is present two things can occur with the result of monetite being formed after setting. Either nucleation of brushite occurs, which is then decomposed to monetite to release water and continue the reaction,25 or if no water is present and the temperature is high enough to bridge the energy needed for monetite formation, it is likely that monetite is formed directly. However, in this study a large variation of the monetite vs. brushite ratio was seen. This could be explained by the PEG enclosed inside the polymer microparticles. PEG is highly hydroscopic and due to its high molecular weight compared with glycerol it is retained within the material for a longer time. In the vicinity of PEG more water will be present than anywhere else in the material, thus the brushite will not be decomposed to monetite as easily as without the PEG.
Conclusions
This study showed that it was possible to incorporate PLGA/PEG microparticles into an acidic premixed calcium phosphate cement and thereby increase its strength. The compressive strength was increased with approximately 20% to 15.1 MPa, similar to cancellous bone, while the diametral tensile strength was maintained, just above 2 MPa. The SEM micrographs showed a good integration of the polymer microparticles with the surrounding ceramic matrix. Finally, the incorporation of the polymer microparticles was found to increase the brushite to monetite ratio in the cements.
Materials and Methods
Cement preparation
For the ceramic part of the cement β-tricalcium phosphate (β-TCP, 90% <50 µm, Sigma Aldrich batch no. BCBH 6869V) and monocalcium phosphate hydrate (MCPH, Alfa Aesar, >97%, 90% >200 µm, batch no. 10154036) were used. The β-TCP used consists of approximately 90 wt% β-TCP, and 10 wt% β-calcium pyrophosphate (results from X-ray diffraction (XRD) and Rietveld refinement). In order to decrease the amount of excess water in the final paste, the MCPH was dried at 110 °C for three days to remove all water,26 resulting in monocalcium phosphate anhydrous (MCPA, 100% from XRD and Rietveld refinement). The PLGA/PEG microparticles (6.5 wt% PEG 400) were used as received from RegenTec Ltd.
In the cements a 45:55 molar ratio of MCPA and β-TCP was used. To the powders 0 wt.%, 1.25 wt.%, 2.5 wt.%, or 5 wt.% of PLGA/PEG microparticles was added. Glycerol (anhydrous, Sigma, viscosity approximately 1.4 Pa s at RT27) was used as the mixing liquid in a powder to liquid ratio of 3.6 g/ml, which gives completely injectable CPCs with extrusion forces between 50 and 65 N through a syringe with an outlet diameter of 1.90 mm. The mixing was performed using a vacuum mixer (Twister, Renfert) and a homogenous paste was achieved after three minutes of mixing. All cements prepared had a final setting time between 30–35 min measured with the Gilmore needle method at 37 °C.28
Mechanical testing
For CS measurements cylindrical rubber molds, Ø 6 mm and height 12 mm according to ASTM F451 standard,29 were filled with cement and immersed in 40 ml of phosphate buffered saline (PBS, Sigma) at 37 °C in a sealed container. The samples were set and cured in an oven at 37 °C for 24 h, after which they were removed from the molds and carefully polished to make the samples sides parallel and obtain the correct height. CS was measured using a universal testing machine (Shimadzu AGS-X), with a cross-head speed of 1 mm/min. A thin plastic film was placed between the sample and the cross-head in order to reduce the effect of potential surface defects from the molding. A minimum of 20 samples were made for each group. The DTS samples were treated in the same way as the CS samples; however, cylindrical rubber molds of Ø 8 mm and height 3 mm were used. A minimum of 23 samples were made for each group.
A statistical analysis was done with IBM SPSS Statistics 19 (IBM) using one-way ANOVA at a significance level of α = 0.05. Tamhane’s post-hoc test for multiple comparisons was used since equal variances could not be confirmed for all groups.
Scanning electron microscopy
The microstructure of the cross section of the samples was analyzed with scanning electron microscopy (SEM, LEO 1550, Zeiss). Before analysis the samples were dried in vacuum for three days to remove all remaining glycerol. A thin gold/palladium coating was subsequently sputtered onto the surface to avoid charging of the surface.
X-ray diffraction
The phases after setting of the cements were analyzed using X-ray diffraction (XRD) (D8, Bruker) in a theta-theta setup with Cu-kα radiation and Ni-filter. Diffraction angles (2θ) of 5–60 degrees were analyzed at 0.17 deg/min. The samples were thoroughly crushed using a mortar prior to analysis and three samples were analyzed for each group. The composition of the samples was analyzed using Rietveld refinement, with the Rietveld software from BGMN. The structures used for the Rietveld refinement was monetite from PDF #04-009-3755,30 brushite from PDF #04-013-3344,31 β-TCP from PDF #04-008-871432 and β-calcium pyrophosphate (β-CPP) from PDF #04-009-3876.33
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to acknowledge RegenTec Ltd, Nottingham, UK, for providing materials. The European project FP7-NMP project Biodesign and the Swedish Research Council are gratefully acknowledged for financial support.
Glossary
Abbreviations:
- β-TCP
beta-tricalcium phosphate
- CPC
calcium phosphate cement
- CS
compressive strength
- DTS
diametral tensile strength
- MCPA
monocalcium phosphate anhydrous
- MCPH
monocalcium phosphate hydrate
- MCPM
monocalcium phosphate monohydrate
- PBS
phosphate buffered saline
- PAA
poly(acrylic acid)
- PEG
poly(ethylene glycol)
- PLGA
poly(lactide-co-glycolide)
- SEM
scanning electron microscopy
- XRD
X-ray diffraction
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/27249
References
- 1.Rho J-Y, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. doi: 10.1016/S1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 2.Wright TM, Hayes WC. Tensile testing of bone over a wide range of strain rates: effects of strain rate, microstructure and density. Med Biol Eng. 1976;14:671–80. doi: 10.1007/BF02477046. [DOI] [PubMed] [Google Scholar]
- 3.Albee FH. Studies in Bone Growth: Triple Calcium Phosphate As A Stimulus to Osteogenesis. Ann Surg. 1920;71:32–9. doi: 10.1097/00000658-192001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theiss F, Apelt D, Brand B, Kutter A, Zlinszky K, Bohner M, Matter S, Frei C, Auer JA, von Rechenberg B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials. 2005;26:4383–94. doi: 10.1016/j.biomaterials.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Legeros R, Chohayeb A, Shulman A. Apatitic calcium phosphates: possible dental restorative materials. J Dent Res. 1982;61:343. [Google Scholar]
- 6.Brown WE, Chow LC. A New Calcium-Phosphate Setting Cement. J Dent Res. 1983;62:672. [Google Scholar]
- 7.Mirtchi AA, Lemaitre J, Terao N. Calcium phosphate cements: study of the β-tricalcium phosphate--monocalcium phosphate system. Biomaterials. 1989;10:475–80. doi: 10.1016/0142-9612(89)90089-6. [DOI] [PubMed] [Google Scholar]
- 8.Khashaba RM, Moussa MM, Mettenburg DJ, Rueggeberg FA, Chutkan NB, Borke JL. Polymeric-calcium phosphate cement composites-material properties: in vitro and in vivo investigations. Int J Biomater. 2010;2010:691452. doi: 10.1155/2010/691452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majekodunmi AO, Deb S. Poly(acrylic acid) modified calcium phosphate cements: the effect of the composition of the cement powder and of the molecular weight and concentration of the polymeric acid. J Mater Sci Mater Med. 2007;18:1883–8. doi: 10.1007/s10856-007-3026-5. [DOI] [PubMed] [Google Scholar]
- 10.Chen W-C, Ju C-P, Wang J-C, Hung C-C, Chern Lin J-H. Brittle and ductile adjustable cement derived from calcium phosphate cement/polyacrylic acid composites. Dent Mater. 2008;24:1616–22. doi: 10.1016/j.dental.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Link DP, van den Dolder J, van den Beucken JJJP, Cuijpers VM, Wolke JGC, Mikos AG, Jansen JA. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A. 2008;87:760–9. doi: 10.1002/jbm.a.31831. [DOI] [PubMed] [Google Scholar]
- 12.Ruhé PQ, Hedberg EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG. Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J Biomed Mater Res A. 2005;74:533–44. doi: 10.1002/jbm.a.30341. [DOI] [PubMed] [Google Scholar]
- 13.Ruhé PQ, Hedberg-Dirk EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789–800. doi: 10.1089/ten.2006.12.789. [DOI] [PubMed] [Google Scholar]
- 14.Habraken WJEM, Wolke JGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17:1057–74. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 15.Fei Z, Hu Y, Wu D, Wu H, Lu R, Bai J, Song H. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med. 2008;19:1109–16. doi: 10.1007/s10856-007-3050-5. [DOI] [PubMed] [Google Scholar]
- 16.Aberg J, Brisby H, Henriksson HB, Lindahl A, Thomsen P, Engqvist H. Premixed acidic calcium phosphate cement: characterization of strength and microstructure. J Biomed Mater Res B Appl Biomater. 2010;93:436–41. doi: 10.1002/jbm.b.31600. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Ma P-W, Zhang L-L, Yin Y-J, Yao K-D, Zhang F-J, Zhang YD, Li XL, Nie W. beta-TCP/MCPM-based premixed calcium phosphate cements. Acta Biomater. 2009;5:3165–77. doi: 10.1016/j.actbio.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Heinemann S, Rössler S, Lemm M, Ruhnow M, Nies B. Properties of injectable ready-to-use calcium phosphate cement based on water-immiscible liquid. Acta Biomater. 2013;9:6199–207. doi: 10.1016/j.actbio.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Dhillon A, Schneider P, Kuhn G, Reinwald Y, White LJ, Levchuk A, Rose FR, Müller R, Shakesheff KM, Rahman CV. Analysis of sintered polymer scaffolds using concomitant synchrotron computed tomography and in situ mechanical testing. J Mater Sci Mater Med. 2011;22:2599–605. doi: 10.1007/s10856-011-4443-z. [DOI] [PubMed] [Google Scholar]
- 20.Engstrand J, Aberg J, Engqvist H. Influence of water content on hardening and handling of a premixed calcium phosphate cement. Mater Sci Eng C. 2013;33:527–31. doi: 10.1016/j.msec.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 21.McCalden RW, McGeough JA, Court-Brown CM. Age-related changes in the compressive strength of cancellous bone. The relative importance of changes in density and trabecular architecture. J Bone Joint Surg Am. 1997;79:421–7. doi: 10.2106/00004623-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Xu HHK, Eichmiller FC, Barndt PR. Effects of fiber length and volume fraction on the reinforcement of calcium phosphate cement. J Mater Sci Mater Med. 2001;12:57–65. doi: 10.1023/A:1026753020208. [DOI] [PubMed] [Google Scholar]
- 23.Elliott JC. Calcium phosphate biominerals. Rev Mineral Geochem. 2002;48:427–53. doi: 10.2138/rmg.2002.48.11. [DOI] [Google Scholar]
- 24.Lundager Madsen HE, Thorvardarson G. Precipitation of calcium phosphate from moderately acid solution. J Cryst Growth. 1984;66:369–76. doi: 10.1016/0022-0248(84)90220-3. [DOI] [Google Scholar]
- 25.Gbureck U, Dembski S, Thull R, Barralet JE. Factors influencing calcium phosphate cement shelf-life. Biomaterials. 2005;26:3691–7. doi: 10.1016/j.biomaterials.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Larson HWE. Preparation and Properties of Mono-, Di-,and Tricalcium Phosphates. Ind Eng Chem. 1935;7:401–6. [Google Scholar]
- 27.Segur JB, Oberstar HE. Viscosity of Glycerol and Its Aqueous Solutions. Ind Eng Chem. 1951;43:2117–20. doi: 10.1021/ie50501a040. [DOI] [Google Scholar]
- 28.Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles. ASTM C266–99: ASTM International, 1999. [Google Scholar]
- 29.ASTM. Standard Specification for Acrylic Bone Cement. ASTM F451: ASTM International, 2008. [Google Scholar]
- 30.Dickens B, Brown WE, Bowen JS. A Refinement of Crystal-Structure of CaHPO4 (Synthetic Monetite) Acta Cryst. 1972;28:797–806.. [Google Scholar]
- 31.Curry NA, Jones DW. Crystal Structure of Brushite, Calcium Hydrogen Orthophosphate Dihydrate - Neutron-Diffraction Investigation. J Chem Soc A. 1971;0:3725–9. doi: 10.1039/j19710003725. [DOI] [Google Scholar]
- 32.Dickens B, Schroeder LW, Brown WE. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J Solid State Chem. 1974;10:232–48. doi: 10.1016/0022-4596(74)90030-9. [DOI] [Google Scholar]
- 33.Boudin S, Grandin A, Borel MM, Leclaire A, Raveau B. Redetermination of the Beta-Ca2P2O7 Structure. Acta Crystallogr C Cryst Struct Commun. 1993;49:2062–4. doi: 10.1107/S0108270193005608. [DOI] [Google Scholar]