Abstract
Different approaches to enhancement of electrocatalytic activity of noble metal nanoparticles during oxidation of small organic molecules (namely potential fuels for low-temperature fuel cells such as methanol, ethanol and formic acid) are described. A physical approach to the increase of activity of catalytic nanoparticles (e.g. platinum or palladium) involves nanostructuring to obtain highly dispersed systems of high surface area. Recently, the feasibility of enhancing activity of noble metal systems through the formation of bimetallic (e.g. PtRu, PtSn, and PdAu) or even more complex (e.g. PtRuW, PtRuSn) alloys has been demonstrated. In addition to possible changes in the electronic properties of alloys, specific interactions between metals as well as chemical reactivity of the added components have been postulated. We address and emphasize here the possibility of utilization of noble metal and alloyed nanoparticles supported on robust but reactive high surface area metal oxides (e.g. WO3, MoO3, TiO2, ZrO2, V2O5, and CeO2) in oxidative electrocatalysis. This paper concerns the way in which certain inorganic oxides and oxo species can act effectively as supports for noble metal nanoparticles or their alloys during electrocatalytic oxidation of hydrogen and representative organic fuels. Among important issues are possible changes in the morphology and dispersion, as well as specific interactions leading to the improved chemisorptive and catalytic properties in addition to the feasibility of long time operation of the discussed systems.
Keywords: electrocatalysis, oxidation of fuels, noble metal and alloyed nanoparticles, metal oxides, polyoxometallates
1. Introduction
Development of the science and technology of low-temperature fuel cells (FCs) is a multidisciplinary challenge. Significant progress has been made in this area, but there are still a number of fundamental problems to be resolved not only in fuel cell design but also in the choice of electrode materials and their utilization [1-7]. In particular, FC limitations are connected to (i) the poor activity/stability of conventional electrocatalysts, (ii) the poisoning of electrocatalytically active species by strong adsorption of intermediate products during reaction (i.e. CO during oxidation of alcohols); (iii) slow kinetics of electrode reactions including that of oxygen reduction at the cathode, and complexity and thus low efficiency of the oxidation processes that may be considered at the anode; and (iv) fuel cross-over through the membrane, which depolarizes the cathode and decreases its activity [1-3,8-12]. Moreover, the necessity of cost reduction and improvement of the performance of conventional Pt-based catalysts requires development of multicomponent systems capable of operating at low temperatures, certainly below 150 °C [13] but practically much lower than 100 °C. Therefore, having in mind the overall efficiencies of electrode processes, reaction rates need to be enhanced (i.e. the overvoltages need to be decreased) either by increasing the reaction temperature or by modifying the catalyst composition or structure thus producing a more active electrocatalytic material. By increasing reaction temperature, the poisoning effect of the otherwise strongly bonded CO intermediate diminishes with respect to activity of noble metal catalytic surfaces. On the other hand, performance of the proton-exchange polymer membranes lowers due the reduced ionic conductivities caused by the membrane dehydration at higher temperatures [14].
A common approach to enhancing the reactivity of platinum involves its nanostructuring to produce electrocatalysts of high surface area and dispersion [1-4]. Further optimization of Pt-based electrocatalysts has been achieved through the formation of bi- and tri-metallic alloys such as PtCr and PtCo (oxygen reduction), PtRu (oxidation of methanol), PtPd (oxidation of formic acid), and PtSn (oxidation of ethanol). With such systems, that have been recently discussed and reviewed [4-7], enhancement of the Pt catalytic activity has been understood in terms of changes in the electronic nature and morphology of the Pt surface and mutual interactions between alloy-forming metals. In the present review, we concentrate mostly on efficient electrocatalytic systems that poisoning of the Pt catalyst can be diminished by increasing the FC operating temperature and the roughness factor or dispersion of the Pt [1-3]. It has also been demonstrated that efficiency can be further improved by deliberate modification of the electrocatalytic interface or by promotion that utilize robust large-surface-area metal oxides (e.g. WO3, TiO2 or ZrO2) as supports or matrices capable of physically separating metal particles (to diminish their tendency to undergo degradation by agglomeration) in addition to interacting mutually with them, thus affecting their chemisorptive and catalytic properties. Oxides are often thought as insulating or semiconducting materials but certain nonstoichiometric oxides existing in various valance states exhibit conductivity not much lower than that of metals and possess appreciable catalytic activity [15-17]. Among other important issues is the presence of bulk and surface states that affect reactivity of oxide films [18]. It is reasonable to expect that the ideal matrix for dispersed catalytic centers would be reactive toward the fuel studied or its reaction intermediates. Finally, an overall physicochemical stability is an issue as well.
2. Platinum as model catalytic metal
Because of its stability and activity in contact with acid electrolyte, platinum is one of the most used electrocatalysts for oxidation of organic molecules, including electrooxidation of alcohols [11,13,19]. Pt is very active toward C-H bond breaking and dissociative adsorption of alcohol; however, with respect to C-C bond breaking (e.g. in ethanol and longer chain alcohols) some difficulties are observed. During the oxidation of small organic molecules, usually CO or CO-type (CHO) intermediates are formed; they are strongly adsorbed on the Pt catalyst surface thus blocking active sites and poisoning the catalytic material. Therefore, for longer chain alcohols (including ethanol), the complete oxidation to CO2 is much more difficult than for methanol. Further, the reactions proceed at potentials where CO or other by-products are either chemisorbed or ineffectively oxidized, which results in the development of increased overpotentials and loss of efficiency [11,13,19]. Many fundamental studies have shown that poisoning of the Pt catalyst can be diminished by increasing the FC operating temperature and the roughness factor or dispersion of the Pt [1-3]. It has been demonstrated that efficiency can be further improved by deliberate modification of the electrocatalytic interface or by promotion through introduction of other components [14,20-23]. Clearly, the development of highly active electrocatalysts is of primary importance to further improvement of the FC performance. In this regard, matrices that are characterized by good stabilities, large surface areas, and high proton and electron conductivities [6,7,24,25] are often needed.
3. Pt based alloys and intermetallic compounds in electrocatalysis
Until now, various electrocatalysts including binary and ternary alloys or intermetallic compounds have been investigated to promote electrooxidation reactions [26-30]. Because ruthenium can promote catalyst (Pt) reactivity by water activation and can provide preferential sites for OH-adsorption at low potentials, Ru is one of the most popular components used as a second metal of Pt-based binary electrocatalysts [8,13,19]. It can mitigate CO or CO-type intermediate accumulation on the catalyst surface and lower the potential of oxidation to CO2. The ratio of Pt to Ru also plays an important role. Although the best results for the oxidation of CO adsorbates at bimetallic Pt-Ru electrocatalysts typically have been obtained where the ratio of Pt to Ru is 1:1, the optimum content of Ru vs. Pt as well as the optimum morphology of the system is still under debate [8,19]. For example, the preferred relative content of Ru (to Pt) was postulated for methanol oxidation to be at the 20% (at.) level [9]. The actual value for the optimum ratio of Ru to Pt is a subject of dispute, but it should be remembered that a mixed bulk face-centered cubic (fcc) PtRu phase (with a statistical distribution of Pt and Ru on the surface and related high bifunctional activity) forms when the relative atomic content of Pt to Ru exceeds 40% [31]. Due to lower stability of Ru component, a proper determination of the surface concentrations of the alloy constituents is very important.
It was also demonstrated that a highly dispersed PtSn catalyst can be as active as PtRu for methanol oxidation [12]. Unlike ruthenium in PtRu, which is largely metallic, tin is unlikely to maintain its metallic state under operating conditions, Sn can be converted to oxygenated species such Sn oxides or hydroxides [21,32]. Recent investigations, however, have revealed that complete ethanol oxidation can be performed effectively on Pt admixed with SnOx, which suggests that formation of an intermetallic platinum-tin phase is not required in the case of this reaction. It was found that the mechanism and the product distribution during electrooxidation of ethanol were dependent on the tin content, but little as 5% tin was sufficient to enhance this electrocatalytic process [21]. The presence of Sn or SnOx in the vicinity of Pt seemed to mitigate the otherwise-strong ligand effect, thus weakening the Pt-C interactions responsible for C-C bond splitting in ethanol; under such conditions, acetaldehyde rather than CO2 will be produced predominantly [19]. Overall, the results concerning PtRu and PtSn electrocatalysts are often contradictory or, at least, open to further discussion. Apparently, the final activity of these systems during the electrooxidation of ethanol is a function of the active-phase dispersion, composition (i.e. the Pt:Me ratio, where Me is Sn, Ru or Sn+Ru), and preparation method [19,30,33].
Utilization of the above-mentioned bimetallic catalytic nanoparticles is often facilitated by the choice of matrix, deposition environment and fabrication procedure. It was shown that out of two systems, PtSn and PtRu, electrodeposited within polyaniline, the latter exhibited higher activity for methanol oxidation [34,35]. Enhancement of methanol oxidation also was observed at a sputtered Pt3Sn surface, but the magnitude of the effect was lower relative to that observed on PtRu alloys [36]. But under some conditions, carefully and precisely dispersed Sn-containing systems can exhibit higher electrocatalytic activities than PtRu toward methanol electrooxidation [19,37,38]. In these studies, smooth platinum, platinized platinum and PbPt electrodes modified by poly(o-phenylenediamine) were used, and Pt, PtRu and PtSn particles were electrochemically deposited. Although PtRu and PtSn systems are recognized as active and promising for the alcohol FC research (e.g. oxidation of ethanol or ethylene glycol), in practice, reaction products with C-C bonds still appear in sizeable amounts. In other words, dehydrogenation of alcohols and dissociation of C-C and C-O bonds existing in ethanol and higher alcohols still remain as key issues [39].
4. Electrocatalytic systems of trimetallic and more complex alloys
With the goal of producing more functionalized electrocatalytic systems, recent literature reports on alternative Pt alloys including binary systems (e.g. PtPd, PtAu, PtRe, PtW and PtMo) and more complex PtRu- and PtSn-based alloys such as PtRuOs, PtRuSn, PtRuNi, PtRuW, PtSnIn, PtRuSnW, PtRuOsIr have been investigated [21,23,30,40-43]. Representative results are consistent with the view that ternary electrocatalysts usually provide superior performance during alcohol oxidations than conventional binary alloyed systems. It is noteworthy that, depending on a ratio of platinum to ruthenium in a typical PtRu binary alloy, Ru and Pt can form a solid solution because they have a fcc crystal structure, but, following addition of other components, the crystal lattice structure changes and thus the arrangement of active species capable of promoting water activation and the surface -OH formation (bi-functional mechanism) is altered [30]. In another example, application of the PtRuSn system has led to some, but not significant, improvement (relative to respective binary and PtRuW systems) in the electrooxidation of methanol and ethanol [19]. In comparison to PtSn and PtRu, the PtRuSn ternary alloy is characterized by different lattice parameters which may be interpreted as indicative of interactions among the Pt, Sn, and Ru components. The superior performance of the PtRuSn system toward electrooxidation of both methanol and ethanol may be indicative of alloying resulting in the synergistic effect related to Ru serving as an activator of water molecules (bifunctional mechanism) and Sn acting as an electronic modifier of Pt [44-47]. Related to these observations is that a metal substrate can serve in a similar manner as an element combined in the nanoparticle. For example, an Au substrate can affect indirectly formation of -OH groups generated on the surface of PtRuW catalyst, thereby promoting the complete oxidation of ethanol. The use of Au is discussed in more detail in Section 3.
It has also been postulated that the presence of a separate SnO2 phase is of importance to the efficiency of the electrooxidation process [44]. The role of tin in the performance of a carbon (Vulcan) supported PtRuSn (PtRuSn/C) electrocatalyst for methanol and ethanol oxidation can be attributed its existing in two states. One is in the form of a PtSn alloy, in which the electronic properties of Pt are changed to more effectively adsorb methanol or ethanol, thereby promoting dissociation of C–H bonds. The second form is SnOx species, which facilitate oxidation of an adsorbed, passivating CO intermediate (according to the bifunctional mechanism). Efficacy in the ability to break C-H bonds, which increases catalytic activity toward ethanol oxidation, has been reported for a PtSnIn ternary alloy in which In is in the oxide form [48]. As in the case of PtRuSn, certain amounts of acetic acid, acetaldehyde and CO have been detected during applications of a PtSnIn catalyst. In general, the presence of tin favors formation of acetic acid and tends to decrease appearance of acetaldehyde and CO2 as products of the ethanol electrooxidation. Addition of Ru or In to a PtSn bimetallic alloy leads to a rather small increase in the formation of CO2 but, on the whole, the product distribution during oxidation of ethanol is largely unchanged.
On the basis of literature reports described above, it can be concluded that selectivity of platinum towards formation of CO2 is the highest; the CO2 yield relative to other products is ca. 25% greater. In other words, Pt catalyst is capable of driving the complete 12-electron oxidation of ethanol. On the other hand, surface of Pt undergoes readily passivation with the intermediate –CO type adsorbates and even reactant molecules (acetaldehyde or acetic acid). For bimetallic and ternary Pt, Ru and Rh alloys, this CO2 selectivity is typically below 10%. Instead of CO2, acetic acid was preferentially formed (dual path mechanism). And contrary to the behavior of pure Pt catalysts, this CO2 selectivity that is observed at bimetallic and ternary systems has tended to improve the operating potential.
5. Introduction of nanostructured gold to electrocatalytic interface
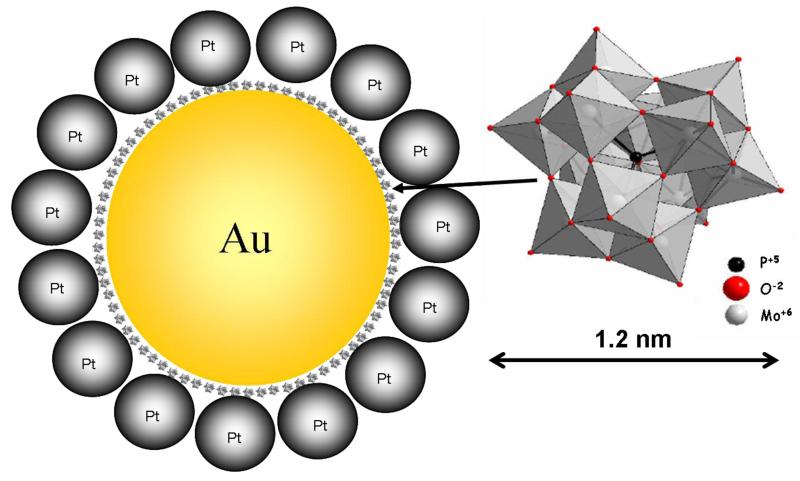
Numerous Au-based or -promoted electrocatalysts are presently considered [49,50] even though gold alone is not a good candidate for CO or alcohol oxidation in acid media because of poor adsorption of reactants and their intermediates produced upon oxidation. However, application in the form of PtAu systems yields some electrocatalytic enhancement relative to pure Pt or Au nanoparticles. Addition of gold to platinum may result not only in changes in the electronic nature of Pt but also in decrease of number of active sites available for CO adsorption; consequently, the CO poisoning effect is less pronounced [49,51,52]. The composition-dependent morphology, size, and shape features of these bimetallic PtAu nanoparticles are of importance to the electrocatalytic oxidation of alcohols [53]. Different types of bimetallic nanoparticles/systems such as alloys, core-shell and contact aggregates have been investigated [53]. An interesting example involves interfacial modification and linking of gold and platinum nanoparticles with heteropolymolybdate monolayers toward the development of catalysts for efficient electrooxidation of ethanol [49,54]. An idealized view of dispersed Pt nanoparticles and linked to larger Au submicroparticles is shown in Fig. 1. Linking presumably occurs because phosphomolybdate can interact with both gold and platinum surfaces. In another work [13], it has also been established that ultra-thin films of polyoxometallates tend to activate Pt centers toward oxidation of alcohols. More detailed discussion of catalytic features of polyoxometallates will be addressed in the next section. Independent investigations [53] have revealed that Au-containing systems are more catalytically active than the Pt alone [55]. The electrocatalytic properties of PtAu are even more enhanced when the system is supported on cationic poly(diallyldimethylammonium chloride), PDDA, or a polymeric PDDA layer on grapheme [56]. This enhancement is particularly important in the oxidation of formic acid under FC conditions. The role of Au in achieving high current densities in these cases can also be attributed to the ability of Au nanoparticles to improve conductivity and to promote electron transfers at the electrocatalytic interface [56].
Fig. 1.
Idealized view of platinum nanoparticles (diameter, ca. 6-8 nm) attached to gold submicroparticulate supports (diameter, 30-40 nm) by linking with Keggin-type polyoxometallate (phosphododecamolybdate) [54].
A system composed of PdAu particles deposited on a TiO2 matrix for the oxidation of formic acid comprised another example of Au-enhanced catalysis. Systems utilizing palladium are characterized by low oxidation potentials for small organic molecules and by higher resistance to surface poisoning by CO or other by-products relative to typical Pt catalysts [57]. The presence of Au in the PdAu/TiO2 system tends to improve the material’s activity, conductivity and stability; in addition, it seems to inhibit corrosive phenomena at the interface. The HCOOH oxidation current densities in a PdAu-based system that are recorded in 0.1 mol·dm−3 HCOOH (0.5 mol·dm−3 H2SO4) are the highest with 39% (at.) Au. The addition of gold to a Pd/TiO2 catalyst can under optimum conditions increase electrocatalytic currents as much as five times [57].
6. Polyoxometallates as model metal oxide supports and acidity modifiers
As mentioned above, controlled modification of catalytic surfaces can be a key issue leading to improved activity and stability. Polynuclear inorganic materials with well-defined metal oxo sites and superacidity, heteropolyacids (HPAs), have attracted particular attention in this regard [58]. HPAs can be treated as a subset of polyoxometallates. Among their characteristics are that they exhibit very strong Brønsted acidity, act as proton conductors, and undergo fast, reversible, multi-electron electron transfers leading to the formation of highly conducting, mixed-valence (e.g. tungsten(VI,V) or molybdenum(VI,V) heteropoly blue) compounds [59,60]. In addition, by changing their chemical composition the acid-base and redox behavior can be modified and adjusted to a desired level. This fact makes HPAs an attractive component of redox catalysts in electrochemical processes [13,61-69]. The role of the acid character of HPAs and their salts in their catalytic efficacy has been the topic of extensive investigations [13,61-63,70-72].
Structurally, heteropoly compounds involve a large class of coordination salts and free acids. The compounds contain a central atom, typically Si or P, tetrahedrally coordinated to oxygen so that they are oxygen–linked to 2-18 hexavalent metal atoms. These typically are Mo or W, but other transition metals, such as V, Nb, Ta, singly or in combination, may be incorporated into the polyanion structure [63,70,71].
Keggin type phosphotungstate is a common structure composed of twelve WO6 octahedrons and one central PO4 tetrahedron; nevertheless, examples of heteropoly anions with different structures are also known [63,70,71]. The Keggin cell of HPA (as in Fig. 1) is represented by the formula [XM12O40]x-8 where X is a heteroatom (X = Si, P, etc. of x oxidation state) that has four oxygen atoms attached in a tetrahedral fashion; M is the addenda atom, which is usually Mo or W. The center tetrahedron that contains the heteroatom is surrounded by twelve MO6 octahedrons. All the oxygen atoms are shared except for the twelve terminal oxygens, called the primary structure. Secondary structures are formed when the primary units are which are attached to only one atom [61,62,70,71]. The structure of the fundamental units is joined to form a solid [63,70].
With respect to the stability the heteropoly compounds, it is believed that the larger the central atom, the more stable the heteropoly anion structure [11]. In the case of secondary structures, water molecules connect the individual heteropoly anions through weak hydrogen bonds. From this arrangement, porous structures with large surface areas can be formed by the loss of some water of crystallization. The amount of water and, therefore, the porosity and thermal stability can be controlled by formation of salts with different cations [11]. Heteropoly compounds can act not only as catalytic materials themselves but also can serve as supports for various catalytic centers. They also have been considered for application in Proton Exchange Membrane Fuel Cells, PEMFCs, due to their specific reactivity, capability to modify properties of catalytic surfaces (also of importance to electrooxidation of alcohols), and high protonic conductivity [13,73]. The latter property may permit fabrication of electrode materials in such a manner that obviates the need for an ionomer [13,59,65,74]. HPAs can assist in transferring electrons in the electrochemical oxidation of CO [13,59,65,74,75]. A co-catalytic effect between an HPA and palladium that is observed on CO oxidation has been related to the presence of Pd(II) in this system [75].
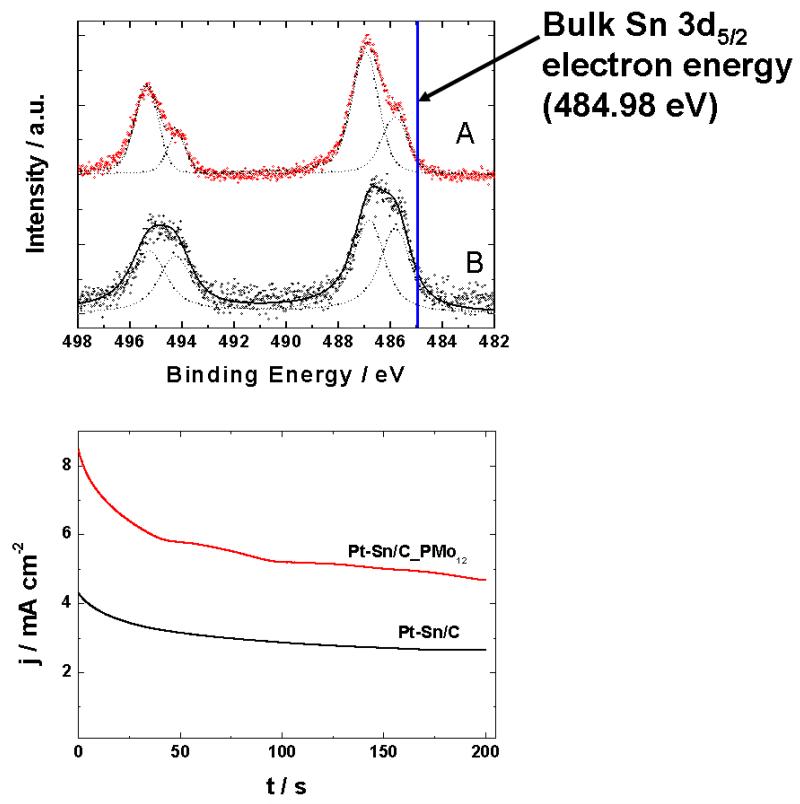
It has been demonstrated that HPAs can enhance methanol electrooxidation in aqueous acidic solution at Pt, PtRu and PtSn electrodes [13,59,65,74,76]. Interactions between HPAs and noble metal or bimetallic catalytic particles result not only in their activation but also in stabilization during electrooxidation of methanol in solution [74,77]. Both commercial PtSn/C and PtRu/C catalysts were found to be more active towards ethanol oxidation when they were modified with an ultrathin H3PMo12O40 film [13]. This modification step was especially beneficial to PtSn/C, a commonly considered electrocatalyst for ethanol electrooxidation. Using high-resolution XPS it was demonstrated that, in the presence of phosphomolybdates, the degree oxidation of a tin component in bimetallic PtSn nanoparticles is more advanced because it has existed as an oxo species of the type SnOx rather than as a metallic tin (Fig. 2).
Fig. 2.
(A) Fit of XPS spectra recorded in the Sn-3d-region [13] for carbon (Vulcan) supported PtSn nanoparticles: modified with H3PMo12O40 (PtSn/C_PMo12) and bare (PtSn/C) ones. Excitation energy: 650 eV. The Sn spectra were deconvoluted into two doublets of binding energy (BE), 485.81 and 486.94 eV, for metallic Sn and SnO, respectively. (B) Chronoamperometric responses recorded (in 0.5 M H2SO4 + 0.5 M ethanol) for bare and modified systems deposited on glassy carbon.
It is noteworthy that salts of HPAs are usually not fully stoichiometric, they could be partially reduced to form highly active mixed-valent heteropolyblue structures (in which electrons are largely delocalized) [64-68], as well as they typically include some residual protons [60,61]. The iron-substituted potassium salts of Dawson-type heteropolytungstates were found to enhance electrocatalytic properties by the presence of mobile protons and their electronic conductivity [74]. Recent studies have also shown that certain zeolite-type cesium salts of Keggin-type heteropolytungstates and heteropolymolybdates act as active supports for dispersed noble metal nanoparticles during electrooxidations of methanol and ethanol [65]. The overall activation effect reflects not only the micro and mesoporous (zeolite-type) high-surface area morphology of polyoxometallate cesium salts but also the presence of well-defined polyoxometallate clusters with reactive oxo groups at the electrocatalytic interface.
7. Application of metal oxides as active matrices
In electrocatalysis, the reactivity of surfaces is of particular importance. Redox reactions of metal oxides involve both ion and electron transfer processes. The electron transfer reactions, which could be outer-sphere (less specific) or inner-sphere (more specific) are influenced by the distribution of electronic states in the electrolyte and within the oxide [18]. When oxides are in contact with aqueous solutions, their surfaces are covered with –OH groups; their actual population depends on the nature of the oxide and its specific crystal face [17]. Obviously some metal oxides are more hydrous than the others. The hydrous behavior, which favor proton mobility and affect overall reactivity, reflects not only chemical structure but the oxide tecture as well. Recently, there has been growing interest in the utilization of certain non-noble metals, such as W, Mo, Mn, V, Zr and Co, and their oxides, including applications in mixtures, solid solutions or perovskite/perovskite-like materials [41,49,78-83]. The respective systems offer a large variety of oxidation states, the potential for mixed electronic/ionic conduction, and the possibility of generation of highly functionalized oxy-species. For example, the importance of formation of multiple hydroxyl groups within an anodically generated MnO2 layer deposited on Pt was demonstrated during the electrocatalytic oxidation of alcohols in neutral media [27]. It was suggested that successive generation and consumption of a Mn(V) species is a crucial factor in the overall reaction mechanism. Manganese possesses a wide range of high oxidation states, and such oxo-manganese species are generally strong chemical oxidants. Similar results were obtained with a chemically prepared MnO2 deposit [79]. It was characterized by considering the generation of a reactive Mn species with an oxidation state greater than IV, some of which undergo disproportionation to oxidation state V that is believed to be an important intermediate that is reactive toward alcohols [27,79]. This concept can be extended to the electrooxidation carbohydrates [27,79].
There also have been attempts to utilize such metal oxides as CeO2 [83], MgO [78], Pt-MoOx [40], WO3 [21], ZrO2 [26] and TiO2 [84] in the preparation of catalysts for direct alcohol oxidation. Consequently, significant improvement in electrode performance for alcohol oxidation, both in terms of an enhanced reaction activity and of diminution of poisoning [80] has been observed. In the presence of these oxides, both platinum- and palladium-based catalysts were found to be more active than the bare metals alone; that is, the onset potentials for oxidation of alcohols were moved to less positive values [81]. Ceria-based catalysts were investigated historically for water-gas shift reactions [85] and for the direct electrochemical oxidation of methane in solid oxide fuel cells (SOFCs) [86]. In the latter case, a ceria-based anode was used. In addition, CeO2-based catalysts in combination with Pt-group metals have received considerable attention because of their use as combustion promoters in automobile catalytic converters [83,87-89].
Recently, Pt centers dispersed over carbon-supported ceria, CeOx/C (1.5 ≤ x ≤ 2), have been proposed as an electrocatalytic material for PEMFCs, specifically for DMFC [90]. In addition to methanol, the system has exhibited improved electrocatalytic performance for electrooxidation of ethanol, glycerol, and ethylene glycolin alkaline media [83]. Many approaches have been proposed to fabricate ceria nanostructures for electrocatalysis; they include homogenous precipitation, hydrothermal synthesis, and solid-state reaction under microwave irradiation [76]. But instability still seems to be a problem with utilization of CeO2-based catalysts [90]. Further, in acid media (e.g. in H2SO4), the ceria supports tends to dissolve, particularly when in contact with platinum, causing its partial reduction [90]. Nevertheless even small amount of ceria are sufficient to effectively prevent the growth and sintering of Pt particles, to contribute to the system’s CO tolerance, and to exhibit higher catalytic activities in comparison to bare platinum investigated under analogous conditions. Among other important issues is the Pt to CeO2 ratio. In alkaline solutions, the best electrocatalytic results were obtained when the Pt to CeO2 mole ratio was 1.2 to 1 at 303 °K [83]. A further increase of the CeO2 content results in a decrease in the performance of the catalyst, presumably due to a decrease in the electrode conductivity. Overall, a synergistic effect for systems containing both Pt and CeO2 has been postulated [83]. It was speculated that CeO2 can function in a manner analogous to that of Ru in PtRu catalysts (bifunctional mechanism).
Similar promoting effects on the activity of noble metal electrocatalysts were reported for systems utilizing magnesium oxide. The Pt–MgO system has been used as a catalyst for the water–gas shift reaction [78], but an electrocatalyst composed of platinum dispersed over carbon-supported magnesia, Pt–MgO/C, also has been used for direct oxidation of ethanol. It generally is thought that MgO functions in an analogous manner to Ru in Pt–Ru/C catalysts where adsorption and dissociation of alcohols occur mainly on Pt sites [78]. At low MgO contents, there are not enough MgO sites to effectively assist the release of adsorbed intermediates, so the oxidation current remains at about the same level as that seen with Pt alone. Upon increasing the MgO content, the current density increases. The best result was reported for a Pt–MgO/C electrode in which the weight ratio of Pt to MgO was 4:1 [78]. The decrease in the ethanol oxidation upon increasing the MgO content above that level was explained in terms of inhibition of alcohol adsorption, of a relatively lower availability of Pt sites, and of a decrease the overall conductivity of the system in that MgO is a semiconductor [78].
Recently, promising results were obtained with electrocatalysts utilizing platinum admixed with molybdenum oxide; the Pt-MoOx composite electrode exhibited an enhancement effect during on electrocatalytic oxidation of methanol [91]. It was postulated that molybdenum or, more precisely an oxo-molybdenum species, showed a beneficial co-catalytic effect towards CO oxidation. The co-catalytic effect also was responsible for increases in current densities during electrooxidations of small organic molecules [14,19,40,91]. The activity of MoOx may reflect the existence of the Mo(VI)/Mo(IV) redox system and its involvement in the reaction mechanism [40,91]. A potential limitation is that during operation molybdenum oxide may be lost from the electrode surface [92,93].
In acidic media, redox reactions of MoOx system involve formation of hydrogen bronzes, HxMoO3(x>0), that co-exist with MoO3 species and may undergo dissolution and re-deposition on a Pt surface at potentials lower than 0.4 V. Thus, HxMoO3 may act in a manner analogous to underpotential deposition of adatoms influencing the electrode catalytic effect. The fully oxidized species, in particular semiconducting MoO3 is likely to somewhat block active catalytic centers [93]. On the other hand, lower molybdenum oxides, (MoO2, MoO2(OH)), may be mixed-valence and, therefore, support electronic conductivity. Moreover, these oxides can activate interfacial water molecules, thereby producing hydroxyls at lower potentials. Nevertheless, acetic acid was the main product of the electrooxidation of ethanol over a broad potential range.
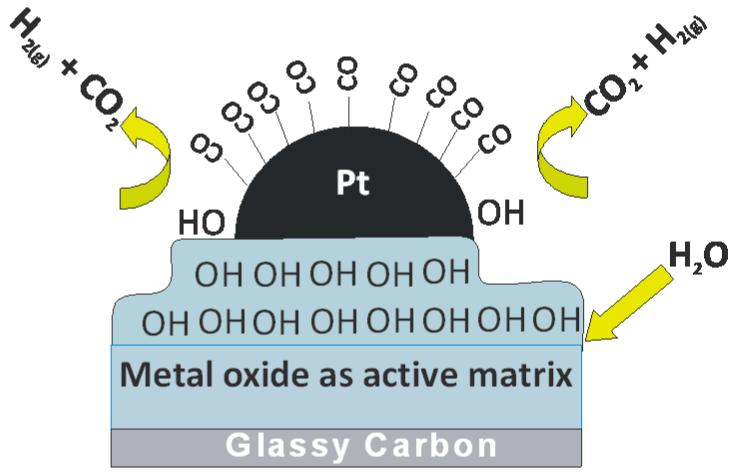
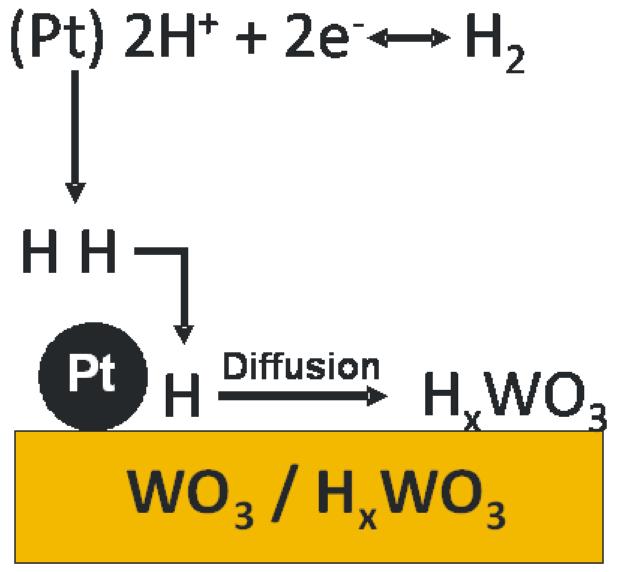
Application of WO3 is another example of an oxide enhancing the electrocatalytic activity of Pt centers during electrooxidations [43,94-99]. Several reports have described the synthesis of Pt–WO3 catalysts, the characterization of these catalysts, and the application to the electrooxidation of methanol and CO. The activity of the system reflects the oxide morphology, composition, and degree of hydration [94,100]. A physical factor, the increase in surface area, rather than direct catalytic activity of WO3, has been implicated in the efficacy of this system [43,94,95]. Among other important issues is the ability of WO3 to intercalate hydrogen atoms into its matrix, thereby forming hydrogen tungsten oxide bronzes, HxWO3. Spillover of hydrogen from Pt sites into the oxide occurs readily (Fig. 4), and it is facilitated by fast diffusion of hydrogen within the oxide phase [43,94]; consequently, the dehydrogenation steps in the methanol oxidation reaction are facilitated, which results in a higher turnover on Pt sites. An improvement in the ability to remove the inhibiting CO intermediate from the Pt sites has been postulated and explained in terms of direct involvement of WO3 in the CO removal [43,94].
Fig. 4.
Idealized view of the formation of hydroxyl groups (on the metal oxide matrix, e.g. WO3) capable of inducing oxidation of the passivating CO adsorbates on Pt nanoparticles.
In addition to the enhancement effects during electrooxidation of alcohols, there are many other electrocatalytic reactions in which a promoting effect by WO3 is postulated. Representative examples are formic acid and hydrogen electrooxidation [42,101]. Palladium catalysts supported on WO3 were applied to the electrorooxidation of formic acid [42]. They were prepared by two independent techniques utilizing sodium tungstate and phosphotungstic acid. The study showed that both preparation techniques led to the fabrication of more effective electrocatalytic systems than Pd alone. Among the conclusions were that specific interactions between Pd and WO3, which can promote the direct oxidation of HCOOH to CO2, occurred that stabilization of Pd nanoparticles against their fusion was observed [42]. Regarding hydrogen electrooxidation, addition of tungsten oxide to catalytic systems containing Pt or Pd nanoparticles resulted in an increase of the overall specific surface area [101]. It is reasonable to expect that highly conductive and reactive nonstoichiometric hydrogen tungsten oxide bronzes, HxWO3, coexist with WO3 or WO2, and they promote oxidation of hydrogen at the reaction boundary formed with platinum or palladium nanoparticles.
In general, researchers working with tungsten oxide and related compounds seem to agree that the improvement of catalytic properties observed by inclusion of these materials in a given system can be related to formation of –OH groups on WO3 (Fig. 3) diminishing the CO poisoning effect by promoting its oxidative removal from the platinum surface [94,102]. The formation of surface hydroxyl groups is favored by the presence of H+, but it can also occur by adsorption of water. In this regard, Pt supported on WO3 shows a high tolerance to CO even at low potentials such as ca. 0.1 V (vs. RHE) [103]. For a detailed assessment of the mechanisms involved, it is important to note that tungsten oxide can exist at the electrocatalytic interface in various forms differing not only in degrees of hydration but also in their compositions: in addition to conventional stoichiometric oxides (WO3, WO2), the mixed-valent W(VI,V) nonstoichiometric hydrogen tungsten oxide bronzes (H0.18WO3 and H0.35WO3) co-exist with sub-stoichiometric W(VI,IV) oxide phases (WO3-y where 0<y<1) alone or in combination with different ions [104-106]. Moreover, the tungsten bronzes can form cubic, tetragonal and hexagonal structures [104-106]. It has been postulated that small ions, such as H+, Li+, and Na+ can be accommodated within the cubic configuration, whereas a hexagonal crystal network can also host bigger cations such as K+, Rb+, Cs+ and NH4+ [104-106].
Fig. 3.
Schematic diagram illustrating the ability of hydrogen molecules to undergo dissociation at Pt nanoparticles followed by “hydrogen spillover” and diffusion in tungsten oxide matrix to form substoichiometric hydrogen bronzes, HxWO3.
Zirconium(IV) oxide is believed to act as a catalytic component capable of enhancing the reactivity of a system by increasing the overall acidity at the reaction interface. Under operating conditions of high temperature fuel cells, ZrO2 is characterized by a high oxygen ion conductivity. This feature is also of importance to the development of oxygen sensors and oxygen storage materials [26,107]. Apart from its acidic properties, ZrO2 exhibits good stability in oxidizing or reducing atmospheres; overall, it is physicochemically stable.
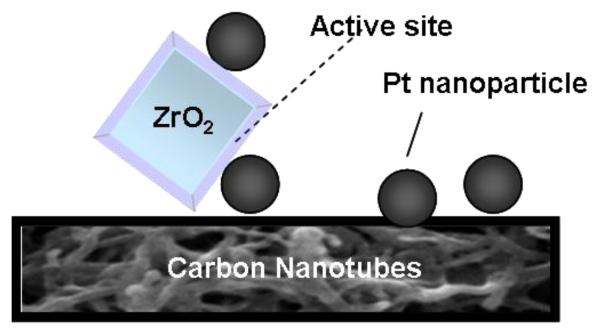
Zirconia can be applied as a support for catalysts or directly as a catalyst for several reactions, including hydration, dehydration, isomerization, oxidation, condensation, and conversion (e.g. of methanol) [108]. Carbon supported Pt-ZrO2 (1:1 molar ratio) was found to exhibit higher catalytic activity for ethanol oxidation than bare Pt/C. The investigation showed that the catalytic activity can be tuned during ethanol electrooxidation by changing the mole ratio of Pt to ZrO2 in the system [25]. The fact that oxidation of ethanol proceeds at more negative potentials at the system utilizing ZrO2 was explained in terms of the presence of oxygen vacancies within ZrO2. In turn, this favors the dissociation of H2O and thus the formation of –OH groups in addition to the transport of protons at the electrocatalytic interface. Under such conditions, oxidation of passivating –CO adsorbates (on Pt sites) to CO2 seems to be facilitated by –OH groups existing at the zirconia-containing interface (Fig. 5). Care must be exercised not to increase the content of ZrO2 relative to Pt above the optimum 1:1 ratio because of the limited electronic conductivity of zirconia [25]. A further increase in catalytic activity of Pt-ZrO2 was observed in direct ethanol and methanol FCs when the catalyst was supported on carbon nanotubes [109]. It is reasonable to expect that addition of carbon nanotubes (Fig. 6) increases overall conductivity at the electrocatalytic interface and permits better utilization of reactive sites are formed at the contact sites between Pt and ZrO2. In that study it also was postulated that the acidic properties of the zirconia-containing system (including proton mobility) and its hydrophilicity can be further enhanced by introducing SO42− into the structure. Under such conditions population of interfacial –OH sites was expected to increase. Indeed, both a decrease in charge transfer resistance and an enhancement of the reaction kinetics was observed [109].
Fig. 5.
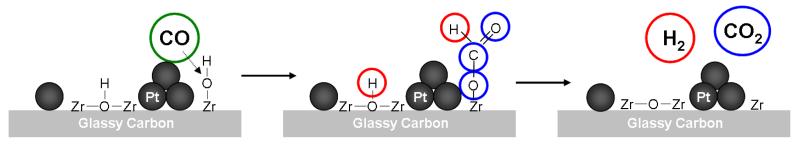
Involvement of -OH groups existing on zirconia in the oxidation of CO residues to CO2.
Fig. 6.
Formation of active sites at the interface formed by carbon nanotube supported metal oxide (e.g. ZrO2) with noble metal nanoparticles (e.g. Pt). Multi-walled carbon nanotubes facilitate distribution of electrons to poorly conducting metal oxide nanostructures.
ZrO2 was also employed in a mixture with WO3 [110,111]. At platinum dispersed within this system, the dynamics of hydrogen adsorption on Pt sites was believed to be improved, and an increase in total activity toward an isomerization reaction was observed [110,111]. At this stage it is unclear to what extend and how WO3 and ZrO2 components interact and activate each other. It is reasonable to expect that the adsorption process comprised dissociation of H2 on Pt sites to form hydrogen atoms followed by “spillover” and “diffusion” of hydrogen atoms onto the surface of the WO3/HxWO3 support. High population of hydroxyl groups originating from zirconia at the interface may facilitate formation of hydrogen bronzes and the hydrogen “spillover” effect.
Another example of a transition metal oxide that yields synergistic electrocatalysis upon interaction with Pt is V2O5 [25]. This oxide has been used extensively as a cathode in lithium ion batteries [112]. The V(IV)/V(III) redox couple has been employed in the construction of a redox type fuel cell [113]. V2O5 has been tested as anode for electrooxidation of toluene [114] and has served as a support for platinum to achieve oxidation of methanol [115]. It was shown that a carbon-supported composite of Pt and V2O5 exhibits higher electrocatalytic activity for the oxidation of methanol relative to simple carbon-supported Pt. The result can be rationalized in terms of the formation of an interface between Pt and V2O5 favoring “spillover” and oxidative removal of the CO intermediate [115]. It is reasonable to expect that V2O5 can facilitate oxidation of CO intermediates to CO2, thereby releasing the active sites on Pt for further electrochemical reaction [25,115]. In this respect, the situation resembles the generally accepted bifunctional mechanism developed for bimetallic PtRu [25,116] except vanadium oxo species can act in principle at lower potentials. It has been established that rapid oxidation and complete removal of CO adsorbates from Pt sites is crucial for improvement of the electrocatalytic efficiency of the oxidation process of not only methanol but practically all small organic molecules. It has been postulated that population of –OH adsorbates increases at lower potentials on Pt when it is contact withV2O5 [25]. The following reactions have been proposed to describe oxidation of adsorbed methanol (CH3OHad) at the electrocatalytic interface composed of Pt and V2O5, which supports the hypothesis that the presence of V2O5 favors oxidation of CO adsorbed on Pt (COad) to CO2, thus releasing active sites on Pt. On the basis of literature data [25] and general inorganic chemistry of transition metals, the following reactions can be proposed:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
In addition to vanadia and zirconia, TiO2 also has been widely studied as a catalyst or catalytic support for many photoelectrochemical and electrochemical oxidation reactions (e.g. of chlorinated compounds) [84]. Indeed, titanium dioxide is widely used in photoelectrocatalysis because of its exceptional physicochemical stability. It has been established that the morphology and particle size distribution of TiO2 catalysts can be controlled precisely using simple preparative methods. For example, nanoporous TiO2 films that contained so-called point defects introduced during anodization have been successfully utilized as supports for PtRu catalytic centers for the electrooxidation of methanol [84]. The observed electrocatalytic enhancement effect has been related to partial elimination of the poisoning effect through an increase of surface mobility of COad and OHad species, thereby increasing the rate of removal of the undesired CO intermediates [84].
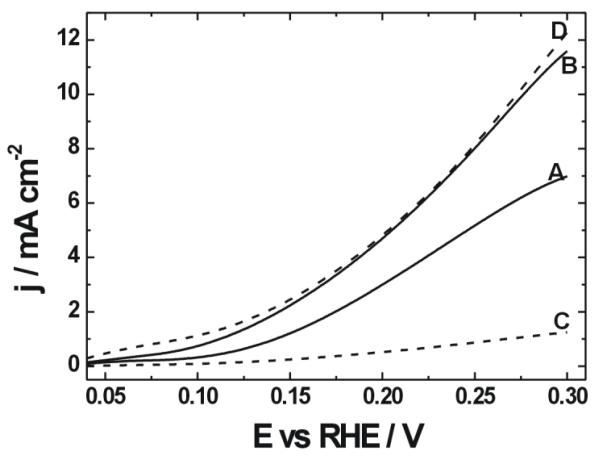
Unequivocal comparison of relative activities of metal oxide matrices is quite difficult on the basis of the available literature because of differences in experimental conditions, relative loadings of components etc. To address this problem, we have recently considered [117] a fairly simple model reaction, namely the oxidation of formic acid in acid medium, at Pd particles where kinetic limitations typically originate from some CO poisoning (Fig. 7). Both tungsten oxide and CNT-supported zirconia exhibited significant enhancement effects on the activity of Pd catalytic nanoparticles (at the same loading of 100 μg cm−2) on the oxidation of formic acid relative to bare Pd catalyst under voltammetric conditions at low potentials. Hydroxyl groups present on surfaces of ZrO2 and WO3 are capable of exhibiting exhibit specific interactions with the noble metal catalytic sites. But, in the investigated range of potentials, the semiconducting zirconia is much more poorly conducting than tungsten oxide capable of undergoing fast and reversible redox transitions to nonstoichiometric mixed valent tungsten oxide bronzes of the type HxWO3. It is apparent from the data in Fig. 2 that charge transport and electronic conductivity are important issues; in this regard, addition of CNTs to zirconia has improved the overall conductivity and has led to better distribution of electrons at the electrocatalytic interface; consequently, a significant enhancement effect has been observed (compare curves C and D). On the whole, the data emphasize the importance of meeting the so called triple-phase boundary requirements emphasizing the need for fast electron and ion displacements in addition to the availability of reactant at catalytically active sites.
Fig. 7.
Voltammetric monitoring (at low potentials) of the oxidation of 0.5 mol dm−3 formic acid in 0.5 mol dm−3 H2SO4 at the glassy carbon electrode containing the following nanoparticles [117]: Pd (A, solid line); WO3-supported Pd (B, solid line); ZrO2-supported Pd (C, dashed line) and Pd supported onto CNT-admixed ZrO2 (40w%) (D, dashed line). In all cases, Pd loading was 100 μg cm−2. Scan rate: 10 mV s−1.
8. Conclusions
Due to insufficient activity and the penchant to undergo poisoning by strong adsorption of reaction intermediates, there is a need to functionalize or modify existing electrocatalytic systems based on noble metal nanoparticles. Among typical approaches to enhance electrocatalytic processes for oxidation of organic fuels, reaction temperature is increased to remove largely passivating CO-type intermediates. Further, electrocatalytic nanoparticles are prepared as small as possible and are highly dispersed to produce systems of high surface areas. Enhancement of noble metal nanoparticles also can be achieved through the formation of bimetallic or trimetallic alloyed systems (e.g. PtRu, PtSn, PdAu, and PtSnRu) characterized by specific interactions between components, distinct electronic properties and often unique morphology. It is shown that certain inorganic oxides (e.g. WO3, MoO3, TiO2, ZrO2, V2O5, and CeO2) and polyoxometallates of molybdenum or tungsten influence supported metal centers in ways other than simple dispersion over electrode area. Evidence has been presented that the support can modify activity (presumably electronic nature) of catalytic metal nanoparticles thus affecting their chemisorptive and catalytic properties. Among useful characteristics of metal oxides and related systems are the following: they can generate –OH groups at low potentials that induce oxidation of passivating CO adsorbates (e.g. on Pt); they can potentially break C-H bonds (e.g. by hydrogen tungsten oxide bronzes); and they can possibly weaken C-C bonds during ethanol oxidation (e.g. through changes of the electronic properties of Pt). There have been numerous reports in this area, but further research is still necessary to elucidate exact enhancement mechanisms that are operative. To classify metal oxides with respect to their reactivity and ability to enhance activity of dispersed noble metal centers, systematic studies are needed with the aim of better description and understanding of the ability of oxides to switch between different valence states and to undergo outer-sphere or inner-sphere electron transfers in addition to other parameters that include morphology, porosity, stability, degree of crystallinity, nonstoichiometry, acidity, hydrophobicity or hydrophilicity [17]. Finally, although the focus of this review is on catalysts of direct interest to the development of fuel cells, the general mechanisms and therefore the cited research have implications to other areas of electrochemistry, including that of biological systems. In this regard, the role of adsorption in electron and oxygen transfer in oxidation of biological compounds has been discussed in relationship to the use of metal oxides, alone or in mixture, as catalysts [118].
Acknowledgements
This work was supported by the Maestro Project 2012/04/A/ST4/00287 awarded by the National Science Center, Poland. Partial support was from the U.S. National Institutes of Health through grant R15GM087662-01 to JAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Watanabe M, Uchida H. Handbook of Fuel Cells - Fundamentals, Technology and Applications, Volume 5: Advances in Electocatalysis, Materials, Diagnostics and Durability. Wiley; 2009. Catalysts for the electro-oxidation of small molecules. [Google Scholar]
- [2].Maillant F, Pronkin S, Savinova ER. Handbook of Fuel Cells - Fundamentals, Technology and Applications, Volume 5: Advances in Electocatalysis, Materials, Diagnostics and Durability. Wiley; 2009. Influence of size on the electrocatalytic activities of supported metal nanoparticles in fuel cell – related reactions. [Google Scholar]
- [3].Campelo JM, Luna D, Luque R, Marinas JM, Romero AA. Sustainable preparation of supported metal nanoparticles and their application in catalysis. ChemSusChem. 2009;2:18. doi: 10.1002/cssc.200800227. [DOI] [PubMed] [Google Scholar]
- [4].Leger JM. Preparation and activity of mono or bimetallic nanoparticles in electrocatalysis. Electrochim. Acta. 2005;50:3123. [Google Scholar]
- [5].Singh AK, Xu Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem. 2013;5:652. [Google Scholar]
- [6].Zhou WP, Axnada S, White MG, Adzic RR, Hrbek J. Enhancement in ethanol electrooxidation by SnOx nanoislands grown on Pt(111): Effect of metal oxide-metal Interface sites. J. Phys. Chem. C. 2011;115:16467. [Google Scholar]
- [7].Sim K-S, Lim S-M, Kwen H-D, Choi S-H. Electrocatalytic activity for CO, MeOH, and EtOH oxidation on the surface of Pt-Ru nanoparticles supported by metal oxide. J. Nanomaterials. 2011:614983. [Google Scholar]
- [8].Lamy C, Lima A, LeRhun V, Delime F, Countanceau C, Leger J-M. Recent advances in the development of direct alcohol fuel cells (DAFC) J. Power Sources. 2002;105:283. [Google Scholar]
- [9].Lasch K, Hayn G, Jorissen L, Garche J, Besenhardt O. Mixed conducting catalyst support materials for the direct methanol fuel cell. J. Power Sources. 2002;105:305. [Google Scholar]
- [10].Macak JM, Barczuk PJ, Tsuchiya H, Nowakowska MZ, Ghicov A, Chojak M, Bauer S, Virtanen S, Kulesza PJ, Schmuki P. Self-organized nanotubular TiO2 matrix as support for dispersed Pt/Ru nanoparticles: Enhancement of the electrocatalytic oxidation of methanol. Electrochem. Commun. 2005;7:1417. [Google Scholar]
- [11].Napolskii KS, Barczuk PJ, Vassiliev SY, Veresov AG, Tsirlina GA, Kulesza PJ. Templating of electrodeposited platinum group metals as a tool to control catalytic activity. Electrochim. Acta. 2007;52:7910. [Google Scholar]
- [12].Napporn WT, Laborde H, Leger J-M, Lamy C. Electro-oxidation of C1 molecules at Pt-based catalysts highly dispersed into a polymer matrix: effect of the method of preparation. J. Electroanal. Chem. 1996;404:153. [Google Scholar]
- [13].Barczuk PJ, Lewera A, Miecznikowski K, Zurowski A, Kulesza PJ. Enhancement of catalytic activity of platinum-based nanoparticles towards electrooxidation of ethanol through interfacial modification with heteropolymolybdates. J. Power Sources. 2011;195:2507. [Google Scholar]
- [14].Zhou WJ, Zhou B, Li WZ, Zhou ZH, Song SQ, Sun GQ, Xin Q, Douvartzides S, Goula M, Tsiakaras P. Performance comparison of low-temperature direct alcohol fuel cells with different anode catalysts. J. Power Sources. 2004;126:16. [Google Scholar]
- [15].Trassati S. Oxide/aqueous solution interfaces, interplay of surface chemistry and electrocatalysis. Mat. Chem. Phys. 1987;16:157. [Google Scholar]
- [16].Trassati S. Electrolysis by oxides – attempt at unifying approach. J. Electroanal. Chem. 1980;111:125. [Google Scholar]
- [17].Trassati S. Interfacial electrochemistry of conductive oxides for electrocatalysis. In: Wieckowski A, editor. Interfacial Electrochemistry Theory, Experiment and Applications. Marcel Dekker; New York: 1999. pp. 769–792. [Google Scholar]
- [18].Koning U, Schultze JW. Bulk and surface states of reactive oxide films: an extended semiconductor model with Ti, Ni, and Fe as examples. In: Wieckowski A, editor. Interfacial Electrochemistry Theory, Experiment and Applications. Marcel Dekker; New York: 1999. pp. 649–672. [Google Scholar]
- [19].Neto AO, Dias RR, Tusi Marcelo M., Linardi M, Spinaće EV. Electro-oxidation of methanol and ethanol using PtRu/C, PtSn/C and PtSnRu/C electrocatalysts prepared by an alcohol-reduction process. J. Power Sources. 2007;166:87. [Google Scholar]
- [20].Jusys Z, Schmidt TJ, Dubau L, Lasch K, Jorissen L, Garche L, Behm RJ. Activity of PtRuMeOx (Me = W, Mo or V) catalysts towards methanol oxidation and their characterization. J. Power Sources. 2002;105:297. [Google Scholar]
- [21].Mann J, Yao N, Bocarsly AB. Characterization and analysis of new catalysts for a direct ethanol fuel cell. Langmuir. 2006;22:10432. doi: 10.1021/la061200c. [DOI] [PubMed] [Google Scholar]
- [22].Purgato FLS, Pronier S, Olivi P, Andrade A.R.d., Léger JM, Tremiliosi-Filho G, Kokoh KB. Direct ethanol fuel cell: Electrochemical performance at 90 °C on Pt and PtSn/C electrocatalysts. J. Power Sources. 2012;198:95. [Google Scholar]
- [23].Wang Z-B, Yin G-P, Shao Y-Y, Yang B-Q, Shi P-F, Feng P-X. Electrochemical impedance studies on carbon supported PtRuNi and PtRu anode catalysts in acid medium for direct methanol fuel cell. J. Power Sources. 2007;165:9. [Google Scholar]
- [24].Dsoke S, Kolary-Zurowska A, Zurowski A, Mignini P, Kulesza PJ, Marassi R. Rotating disk electrode study of Cs2.5H0.5PW12O40 as mesoporous support for Pt nanoparticles for PEM fuel cells electrodes. J. Power Sources. 2011;196:10591. [Google Scholar]
- [25].Maiyalagan T, Khan FN. Electrochemical oxidation of methanol on Pt/V2O5–C composite catalysts. Catal. Commun. 2009;10:433. [Google Scholar]
- [26].Bai Y, Wu J, Xi J, Wang J, Zhu W, Chen L, Qiu X. Electrochemical oxidation of ethanol on Pt–ZrO2/C catalyst. Electrochem. Commun. 2005;7:1087. [Google Scholar]
- [27].Das D, Samaddar PR, Sen PK, Das K. Oxidation of some aliphatic polyols on anodically deposited MnO2. J. Appl. Electrochem. 2008;38:743. [Google Scholar]
- [28].Giffin GA, Piga M, Lavina S, Navarra MA, D’Epifanio A, Scrosati B, Noto VD. Characterization of sulfated-zirconia/Nafion composite membranes for proton exchange membrane fuel cells. J. Power Sources. 2012;198:66. [Google Scholar]
- [29].Lewerenz HJ, Skorupska K, Munoz AG, Stempel T, Nüsse N, Lublow M, Vo Dinh T, Kulesza PJ. Micro- and nanotopographies for photoelectrochemical energy conversion. II: Photoelectrocatalysis – Classical and advanced systems. Electrochim. Acta. 2011;56:10726. [Google Scholar]
- [30].Tanaka S, Umeda M, Ojima H, Usui Y, Kimura O, Uchida I. Preparation and evaluation of a multi-component catalyst by using a co-sputtering system for anodic oxidation of ethanol. J. Power Sources. 2005;152:34. [Google Scholar]
- [31].Hamnett A. Mechanism of methanol electro-oxidation. In: Wieckowski A, editor. Interfacial Electrochemistry Theory, Experiment and Applications. Marcel Dekker; New York: 1999. pp. 843–884. [Google Scholar]
- [32].Jiang LH, Sun GQ, Sun SG, Liu JG, Tang SH, Li HQ, Zhou B, Xin Q. Structure and chemical composition of supported Pt–Sn electrocatalysts for ethanol oxidation. Electrochim. Acta. 2005;50:5384. [Google Scholar]
- [33].Grugur BN, Markovi NM, R PN., Jr. Electrochemical oxidation of carbon monoxide: from platinum single crystals to low temperature fuel cells catalysts. Part II. Electrooxidation of H2, CO and H2/CO mixtures on well characterized PtMo alloy. J. Serb. Chem. Soc. 2003;68:191. [Google Scholar]
- [34].Lima A, Countanceau C, Leger J-M, Lamy C. Investigation of ternary catalysts for methanol electrooxidation. J. Appl. Electrochem. 2001;31:379. [Google Scholar]
- [35].Hable CT, Wrighton MS. Electrocatalytic oxidation of methanol and ethanol: a comparison of platinum-tin and platinum-ruthenium catalyst particles in a conducting polyaniline matrix. Langmuir. 1993;9:3284. [Google Scholar]
- [36].Wang K, Gasteiger HA, Markovic NM, R PN., Jr. On the reaction pathway for methanol and carbon monoxide electrooxidation on Pt-Sn alloy versus Pt-Ru alloy surfaces. Electrochim. Acta. 1996;41:2587. [Google Scholar]
- [37].Golikand AN, Golab SM, Maragheh MG, Irannejad L. Electrocatalytic oxidation of methanol on (Pb) lead modified by Pt, Pt–Ru and Pt–Sn microparticles dispersed into poly(o-phenylenediamine) film. J. Power Sources. 2005;145:116. [Google Scholar]
- [38].Morimoto Y, Yeager EB. Comparison of methanol oxidations on Pt, Pt|Ru and Pt|Sn electrodes. J. Electroanal. Chem. 1998;444:95. [Google Scholar]
- [39].Song SQ, Zhou WJ, Zhou ZH, Jiang LH, Sun GQ, Xin Q, Leontidis V, Kontou S, Tsiakaras P. Direct ethanol PEM fuel cells: The case of platinum based anodes. International Journal of Hydrogen Energy. 2005;30:995. [Google Scholar]
- [40].Anjos DMD, Kokoh KB, Leger JM, Andrade ARD, Olivi P, Tremiliosi-Filho G. Electrocatalytic oxidation of ethanol on Pt-Mo bimetallic electrodes in acid medium. J. Appl. Electrochem. 2006;36:1391. [Google Scholar]
- [41].Chen XJ, Liu QL, Khor KA, Chan SH. High-performance (La,Sr)(Cr,Mn)O3/(Gd,Ce)O2–δ composite anode for direct oxidation of methane. J. Power Sources. 2007;165:34. [Google Scholar]
- [42].Feng L, Yan L, Cui Z, Liu C, Xing W. High activity of Pd–WO3/C catalyst as anodic catalyst for direct formic acid fuel cell. J. Power Sources. 2011;196:2469. [Google Scholar]
- [43].Shim J, Lee C-R, Lee H-K, Lee J-S, Cairns EJ. Electrochemical characteristics of Pt–WO3/C and Pt–TiO2/C electrocatalysts in a polymer electrolyte fuel cell. J. Power Sources. 2001;102:172. [Google Scholar]
- [44].Jiang L, Sun G, Zhou Z, Sun S, Wang Q, Yan S, Li H, Tian J, Guo J, Zhou B, Xin Q. Size-controllable synthesis of monodispersed SnO2 nanoparticles and application in electrocatalysts. J. Phys. Chem. B. 2005;109:8774. doi: 10.1021/jp050334g. [DOI] [PubMed] [Google Scholar]
- [45].Kim T, Kobayashi K, Takahashi M, Nagai M. Effect of Sn in Pt–Ru–Sn ternary catalysts for CO/H2 and methanol electrooxidation. Chem. Lett. 2005;34:798. [Google Scholar]
- [46].Kim T, Takahashi M, Nagai M, Kobayashi K. Methanol Electrooxidation on carbon-supported Pt3Ru2Sn ternary catalyst. Chem. Lett. 2004;33:478. [Google Scholar]
- [47].Rousseau S, Coutanceau C, Lamy C, Leger J-M. Direct ethanol fuel cell (DEFC): Electrical performances and reaction products distribution under operating conditions with different platinum-based anodes. J. Power Sources. 2006;158:18. [Google Scholar]
- [48].Zhu M, Sun G, Yan S, Li H, Xin Q. Preparation, structural characterization, and activity for ethanol oxidation of carbon-supported PtSnIn catalyst. Energy & Fuels. 2009;23:403. [Google Scholar]
- [49].Zoladek S, Rutkowska IA, Skorupska K, Palys B, Kulesza PJ. Fabrication of polyoxometallate-modified gold nanoparticles and their utilization as supports for dispersed platinum in electrocatalysis. Electrochim. Acta. 2011;56:10744. [Google Scholar]
- [50].Zhou SG, McIlwrath K, Jackson G, Eichhorn B. Enhanced CO tolerance for hydrogen activation in Au–Pt dendritic heteroaggregate nanostructures. J. Am. Chem. Soc. 2006;128:1780. doi: 10.1021/ja056924+. [DOI] [PubMed] [Google Scholar]
- [51].Lee JK, Lee J, Han J, Lim TH, Sung YE, Tak Y. Influence of Au contents of AuPt anode catalyst on the performance of direct formic acid fuel cell. Electrochim. Acta. 2008;53:3474. [Google Scholar]
- [52].Jiang J, Kucernak A. Nanostructured platinum as an electrocatalyst for the electrooxidation of formic acid. J. Electroanal. Chem. 2002;520:64. [Google Scholar]
- [53].Lamy C, Belgsir EM, Leger J-M. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC) J. Appl. Electrochem. 2001;31:799. [Google Scholar]
- [54].Zoladek S, Rutkowska IA, Kulesza PJ. Enhancement of activity of platinum towards oxidation of ethanol by supporting on titanium dioxide containing phosphomolybdate-modified gold nanoparticles. Applied Surface Science. 2011;257:8205. [Google Scholar]
- [55].Wang YQ, Wei ZD, Li L, Ji MB, Xu Y, Shen PK, Zhang J, Zhang H. Methanol electrochemical oxidation on Au/Pt electrode enhanced by phosphomolybdic acid. J. Phys. Chem. C. 2008;112:18672. [Google Scholar]
- [56].Wang S, Wang X, Jiang SP. Self-assembly of mixed Pt and Au nanoparticles on PDDA-functionalized graphene as effective electrocatalysts for formic acid oxidation of fuel cells. PCCP. 2011;13:6883. doi: 10.1039/c0cp02495c. [DOI] [PubMed] [Google Scholar]
- [57].Tian M, Malig M, Chen S, Chen A. Synthesis and electrochemical study of TiO2-supported PdAu nanoparticles. Electrochem. Commun. 2011;13:372. [Google Scholar]
- [58].Sadakane M, Steckhan E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998;98:219. doi: 10.1021/cr960403a. [DOI] [PubMed] [Google Scholar]
- [59].Herring AM. Inorganic–polymer composite membranes for proton exchange membrane fuel cells. Polymer Reviews. 2006;46:245. [Google Scholar]
- [60].Ferrell JR, III, Kuo MC, Turner AJ, Herring AM. Heteropoly acid as co-catalysts with platinum on the anode of a direct methanol fuel cell. In: Weidner J, Zaghib K, Dudney N, Minteer S, editors. Electrochemistry of Novel Electrode Materials for Energy Conversion and Storage: ECS Transactions 6, The Electrochemical Society. 2008. pp. 319–327. [Google Scholar]
- [61].Moffat JB. Metal-Oxygen Clusters. The Surface and Catalytic Properties of Heteropoly Oxometalates. Fundamental and Applied Catalysis, Kluwer Academic/Plenum Publishers; New York: 2001. [Google Scholar]
- [62].F JR, III, Kuo M-C, Turner JA, Herring AM. The use of the heteropoly acids, H3PMo12O40 and H3PW12O40, for the enhanced electrochemical oxidation of methanol for direct methanol fuel cells. Electrochim. Acta. 2008;53:4927. [Google Scholar]
- [63].Tanabe K, Misono M, Ono Y, Hottari H. New Solid Acids and Bases. Kodansha; Tokyo: 1989. [Google Scholar]
- [64].Kulesza PJ, Karnicka K, Miecznikowski K, Chojak M, Kolary A, Barczuk PJ, Tsirlina G, Czerwinski W. Network electrocatalytic films of conducting polymer-linked polyoxometallate-stabilized platinum nanoparticles. Electrochim. Acta. 2005;50:5155. [Google Scholar]
- [65].Zurowski A, Kolary-Zurowska A, Dsoke S, Barczuk PJ, Marassi R, Kulesza PJ. Activation of carbon-supported platinum nanoparticles by zeolite-type cesium salts of polyoxometallates of molybdenum and tungsten towards more efficient electrocatalytic oxidation of methanol and ethanol. J. Electroanal. Chem. 2010;649:238. [Google Scholar]
- [66].Borzenko MI, Chojak M, Kulesza PJ, Tsirlina GA, Petrii OA. Platinization assisted by Keggin-type heteropolytungstates. Electrochim. Acta. 2003;48:3797. [Google Scholar]
- [67].Cheng L, Cox JA. Preparation of multilayered nanocomposites of polyoxometalates and poly(amidoamine) dendrimers. Electrochem. Commun. 2001;3:285. [Google Scholar]
- [68].Kulesza PJ, Chojak M, Karnicka K, Miecznikowski K, Palys B, Lewera A, Wieckowski A. Network films composed of conducting polymer-linked and polyoxometallate-stabilized platinum nanoparticles. Chem. Mater. 2004;16:4128. [Google Scholar]
- [69].Tess ME, Cox JA. Electrocatalysis with a dirhodium-substituted polyoxometalate anchored in a xerogel-based composite: Application to the oxidation of methionine and cystine at physiological pH. Electroanalysis. 1998;10:1237. [Google Scholar]
- [70].Corma A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 1995;95:559. [Google Scholar]
- [71].Tanabe K, Holderich WF. Industrial application of solid acid–base catalysts. Appl. Catal. A. 1999;181:399. [Google Scholar]
- [72].Li G, Ding Y, Wang J, Wang X, Suo J. New progress of Keggin and Wells–Dawson type polyoxometalates catalyze acid and oxidative reactions. J. Mol. Catal. A. 2007;262:67. [Google Scholar]
- [73].Himeno NIS. A voltammetric study on the formation of V(V)- and V(IV)-substituted molybdophosphate(V) complexes in aqueous solution. J. Electroanal. Chem. 1998;451:203. [Google Scholar]
- [74].Kulesza PJ, Matczak M, Wolkiewicz A, Grzybowska B, Galkowski M, Malik MA, Wieckowski A. Properties of conducting polymer based composite film containing dispersed Pt nanoparticles towards oxidation of methanol. Electrochim. Acta. 1999;44:2131. [Google Scholar]
- [75].Zhizhina EG, Kuznetsova LI, Maksimovskaya RI, Pavlova SN, Matveev KI. Oxidation of CO to CO2 by heteropolyacids in the presence of palladium. J. Mol. Catal. A. 1986;38:345. [Google Scholar]
- [76].Mittal VO, Kunz HR, Fenton JM. Membrane degradation mechanisms in PEMFCs. J. Electrochem. Society. 2007;154:B652. [Google Scholar]
- [77].Song IK, Barteau MA. Redox properties of Keggin-type heteropolyacid (HPA) catalysts: effect of counter-cation, heteroatom, and polyatom substitution. J. Mol. Catal. A. 2004;212:229. [Google Scholar]
- [78].Xu C, Shen PK, Ji X, Zeng R, Liu Y. Enhanced activity for ethanol electrooxidation on Pt–MgO/C catalysts. Electrochem. Commun. 2005;7:1305. [Google Scholar]
- [79].Das D, Sen PK, Das K. Carbohydrate electro-oxidation on chemically prepared MnO2. J. Electroanal. Chem. 2007;611:19. [Google Scholar]
- [80].Maiyalagan T, Khan FN. Electrochemical oxidation of methanol on Pt/V2O5–C composite catalysts. Cat. Commun. 2009;10:433. [Google Scholar]
- [81].Shen PK, Xu C. Alcohol oxidation on nanocrystalline oxide Pd/C promoted electrocatalysts. Electrochem. Commun. 2006;8:184. [Google Scholar]
- [82].Shim J, Lee C-R, Lee J-S, Cairns EJ. Electrochemical characteristics of Pt–WO3/C and Pt–TiO2/C electrocatalysts in a polymer electrolyte fuel cell. J. Power Sources. 2001;102:172. [Google Scholar]
- [83].Xu C, Shen PK. Novel Pt/CeO2/C catalysts for electrooxidation of alcohols in alkaline media. Chem. Commun. 2004:2238. doi: 10.1039/b408589b. [DOI] [PubMed] [Google Scholar]
- [84].Hepel M, Kumarihamy I, Zhong CJ. Nanoporous TiO2-supported bimetallic catalysts for methanol oxidation in acidic media. Electrochem. Commun. 2006;8:1439. [Google Scholar]
- [85].Zalc JM, Sokolovskii V, Loffle DG. Are Noble metal-based water–gas shift catalysts practical for automotive fuel processing? J. Catal. 2002;206:169. [Google Scholar]
- [86].Murray EP, Tsai T, Barnett SA. A direct-methane fuel cell with a ceria-based anode. Nature. 1999;400:649. [Google Scholar]
- [87].Epling WS, Campbell LE, Yezerets A, Currier NW, Parks JE. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. 2004;46:163. [Google Scholar]
- [88].Gandhi HS, Graham GW, McCabe RW. Automotive exhaust catalysis. J. Catal. 2003;216:433. [Google Scholar]
- [89].Pieta IS, García-Diéguez M, Herrera MC, Larrubia MA, Alemany LJ. In situ DRIFT–TRM study of simultaneous NOx and soot removal over Pt–Ba and Pt–K NSR catalysts. J. Catal. 2010;270:256. [Google Scholar]
- [90].Ou DR, Mori T, Fugane K, Togasaki H, Ye F, Drennan J. Stability of ceria supports in Pt–CeOx/C catalysts. J. Phys. Chem. C. 2011;115:19239. [Google Scholar]
- [91].Zhang J, Wang Y, Fachini ER, Cabrera CR. Electrochemically codeposited platinum/molybdenum oxide electrode for catalytic oxidation of methanol in acid s7olution. Electrochem. Solid State Lett. 1999;2:437. [Google Scholar]
- [92].Song C, Khanfar M, Pickup PG. Mo oxide modified catalysts for direct methanol, formaldehyde and formic acid fuel cells. J. Appl. Electrochem. 2006;36:339. [Google Scholar]
- [93].Jaksic JM, Vracar L, Neophytides SG, Zafeiratos S, Papakonstantinou G, Krstajic NV, Jaksic MM. Structural effects on kinetic properties for hydrogen electrode reactions and CO tolerance along Mo–Pt phase diagram. Surf. Sci. 2005;598:156. [Google Scholar]
- [94].Jayaraman S, Jaramillo TF, Baeck S-H, McFarland EW. Synthesis and characterization of Pt-WO3 as methanol oxidation catalysts for fuel cells. J. Phys. Chem. B. 2005;109:22958. doi: 10.1021/jp053053h. [DOI] [PubMed] [Google Scholar]
- [95].Barczuk PJ, Miecznikowski K, Kulesza PJ. Enhancement of the oxidation of methyl formate at multifunctional electrocatalyst composed of Pt/Pd and Pt/Ru nanoparticles. J. Electroanal. Chem. 2007;600:80. [Google Scholar]
- [96].Chojak M, Mascetti M, Wlodarczyk R, Marassi R, Karnicka K, Miecznikowski K, Kulesza PJ. Oxidation of methanol at the network film of polyoxometallate-linked ruthenium-stabilized platinum nanoparticles. J. Solid State Electrochem. 2004;8:854. [Google Scholar]
- [97].Dembinska B, Kulesza PJ. Multi-walled carbon nanotube-supported tungsten oxide-containing multifunctional hybrid electrocatalytic system for oxygen reduction in acid medium. Electrochim. Acta. 2009;54:4682. [Google Scholar]
- [98].Miecznikowski K, Kulesza PJ. Activation of dispersed PtSn/C nanoparticles by tungsten oxide matrix towards more efficient oxidation of ethanol. J. Power Sources. 2011;196:2595. [Google Scholar]
- [99].Salmaoui S, Sediri F, Gharbi N, Perruchot C, Aeiyach S, Rutkowska IA, Kulesza PJ, Jouini M. Hexagonal nanorods of tungsten trioxide: Synthesis, structure, electrochemical properties and activity as supporting material in electrocatalysis. Appl. Surf. Sci. 2011;257:8223. [Google Scholar]
- [100].Barczuk PJ, Tsuchiya H, Macak JM, Schmuki P, Szymanska D, Makowski O, Miecznikowski K, Kulesza PJ. Enhancement of the electrocatalytic oxidation of methanol at Pt/Ru nanoparticles immobilized in different WO3 matrices. Electrochem. Solid State Lett. 2006;9:E13. [Google Scholar]
- [101].Yatsimirskii VK, Lesnyak VV, Gut IN, Boldyreva OY. Catalytic activity of WO3 and MoO3 with Pt and Pd additives in oxidation of hydrogen. Theor. Exp. Chem. 2005;41:329. [Google Scholar]
- [102].Jin H, Zhu J, Li JHY, Zhang Y, Huang X, Ding K, Chen W. Structural and electronic properties of tungsten trioxides: from cluster to solid surface. Theor. Chem. Accounts. 2011;130:103. [Google Scholar]
- [103].Micoud F, Maillard F, Bonnefont A, Job N, Chatenet M. The role of the support in COads monolayer electrooxidation on Pt nanoparticles: Pt/WOx vs. Pt/C. PCCP. 2010;12:1182. doi: 10.1039/b915244j. [DOI] [PubMed] [Google Scholar]
- [104].Kulesza PJ, Faulkner LR. Electrochemical preparation of electrodes modified with non-stoichiometric mixed-valent tungsten(VI,V) oxides. J. Electroanal. Chem. 1988;248:305. [Google Scholar]
- [105].Kulesza PJ, Faulkner LR. Electrodeposition and characterization of three-dimensional tungsten(VI,V)-oxide films containing spherical Pt microparticles. J. Electrochem. Society. 1989;136:707. [Google Scholar]
- [106].Shim J, Lee CR, Lee HK, Lee JS, Cairns EJ. Electrochemical characteristics of Pt–WO3/C and Pt–TiO2/C electrocatalysts in a polymer electrolyte fuel cell. J. Power Sources. 2001;102:172. [Google Scholar]
- [107].Deshmane VG, Adewuyi YG. Synthesis of thermally stable, high surface area, nanocrystalline mesoporous tetragonal zirconium dioxide (ZrO2): Effects of different process parameters. Micro. Meso. Mater. 2012;148:88. [Google Scholar]
- [108].Yadav GD, Nair JJ. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Micro. Meso. Mater. 1999;33:1. [Google Scholar]
- [109].Song H, Qiu X, Li F. Promotion of carbon nanotube-supported Pt catalyst for methanol and ethanol electro-oxidation by ZrO2 in acidic media. Appl. Cat. A. 2009;364:1. [Google Scholar]
- [110].Barton DG, Shtein M, Wilson RD, Soled SL, Iglesia E. Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J. Phys. Chem. B. 1999;103:630. [Google Scholar]
- [111].Triwahyono S, Jalil AA, Hattori H. Study of hydrogen adsorption on Pt/WO3-ZrO2 through Pt sites. J. Nat. Gas Chem. 2007;16:252. [Google Scholar]
- [112].Wang Y, Cao GZ. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 2008;20:2251. [Google Scholar]
- [113].Larsson R, Folkesson B. On the kinetics of the oxidation of formic acid by vanadium(IV) in aqueous solution. Inorg. Chim. Acta. 1989;162:75. [Google Scholar]
- [114].D’Elia LF, Rincon L, Ortiz R. Test of vanadium pentoxide as anode for the electrooxidation of toluene: A theoretical approach of the electrode process. Electrochim. Acta. 2004;50:217. [Google Scholar]
- [115].Zhang KF, Guo DJ, Liu JLX, Li HL, Su ZH. Vanadium oxide nanotubes as the support of Pd catalysts for methanol oxidation in alkaline solution. J. Power Sources. 2006;162:1077. [Google Scholar]
- [116].Roth C, Benker N, Theissmann R, Nichols RJ, Schiffrin DJ. Bifunctional electrocatalysis in Pt–Ru nanoparticle systems. Langmuir. 2008;24:2191. doi: 10.1021/la7015929. [DOI] [PubMed] [Google Scholar]
- [117].Klak K, Marks D, Wadas A, Piatek M, Lotowska W, Zoladek S, Rutkowska IA, Kulesza PJ. Nanostructured hybrid electrocatalytic materials for ethanol oxidation: Activation of Pt-Ru centers through modification with selected metal oxides. ECS Transactions. 2013;45:13. [Google Scholar]
- [118].Popovic ND, Cox JA, Johnson DC. A mathematical model for anodic oxygen-transfer reactions at Bi(V)-doped PbO2-film electrodes. J. Electroanal. Chem. 1998;456:203. [Google Scholar]