Abstract
Dietary isothiocyanates are a group of promising chemopreventive agents obtained primarily from cruciferous vegetables. Due to their potent chemopreventive and/or anti-cancer activities, there is a growing interest in assessing dietary isothiocyanate exposure and its impact on human health. Using the HPLC-based cyclocondensation assay, the current study measured total isothiocyanate yield from raw cruciferous vegetables. A total of 73 samples comprising nine types of cruciferous vegetables were analyzed. We observed a wide range of isothiocyanate content across the individual vegetables with an average level of 16.2 μmol/100g wet weight, ranging from 1.5 μmol in raw cauliflower to 61.3 μmol in raw mustard greens. The data represent the maximum amount of isothiocyanates released from the intake of raw cruciferous vegetables. Given that the vegetables assayed in this study include the most commonly consumed cruciferous vegetables in western diets, the data may be particularly useful in estimation of dietary isothiocyanate exposure in these populations. However, due to the variation observed within each vegetable, biomarkers such as urinary isothiocyanate level may be necessary for accurate estimation of individual exposure.
Keywords: Isothiocyanates, cruciferous vegetables, phytochemicals, glucosinolates, food composition, the United States
1. Introduction
Dietary isothiocyanates are phytochemicals primarily derived from cruciferous vegetables. With the shared active –N=C=S structure, these compounds have shown multifaceted chemopreventive activities against cancer, including modulation of phase 1 and phase 2 enzymes to block carcinogenesis, and induction of apoptosis and cell cycle progression to inhibit growth of malignant cells (Conaway, Yang, & Chung, 2002; Hecht, 1999; Nakamura, 2009; Navarro, Li, & Lampe, 2011; Singh & Singh, 2012; Thornalley, 2002; Zhang, Yao, & Li, 2006). The potent chemopreventive activities of dietary isothiocyanates have been established in multiple cancer models, including bladder, colon, liver, lung, mammary gland, and esophagus, in both in vitro and in vivo systems. Due to highly promising experimental evidence, there is a growing interest in the assessment of dietary isothiocyanate exposure in humans and examination of their potential role in chemoprevention of cancer. However, research into the relationships between isothiocyanates and cancer risk is hindered by the lack of food composition data for isothiocycanates in cruciferous vegetables.
Isothiocyanates are stored as glucosinolates in cruciferous vegetables. In plants, glucosinolates coexist with but are physically segregated from an endogenous enzyme myrosinase (thioglucoside glucohydrolase, EC 3.2.1.1). Upon vegetable cutting or chewing, myrosinase is released from the damaged plant cells to hydrolyze glucosinolates. It is noteworthy that human intestinal microflora also possess myrosinase activity, therefore, a portion of unhydrolyzed glucosinolates could be converted to isothiocyanates in the human gastrointestinal tract (Getahun & Chung, 1999; Shapiro, Fahey, Wade, Stephenson, & Talalay, 1998). Depending on the chemical structure of glucosinolates such as aliphatic, aromatic, or indole glucosinolates, coexisting factors in vegetables such as epithiospecifier protein (ESP), ascorbic acid or Fe2+, as well as environmental conditions such as pH value, end products of hydrolyzed glucosinolates are different, including isothiocyanates, indoles, nitriles, and thiocyanates (Burow, Markert, Gershenzon, & Wittstock, 2006; Fahey, Zalcmann, & Talalay, 2001; Verkerk, et al., 2009). A single plant can contain as many as 15 different glucosinolates (Verkerk, et al., 2009), rendering it difficult to characterize and quantify each individual glucosinolate. Importantly, it has been shown that ESP tilts myrosinase-catalyzed hydrolysis of glucosinolates toward nitriles at the cost of isothiocyanates, while the presence of ESP varies in vegetables with a strong activity in broccoli but not in mustard or horseradish (Cole, 1978; Kaoulla, MacLeod, & Gil, 1980; Matusheski, Juvik, & Jeffery, 2004; Matusheski, et al., 2006; Petroski & Tookey, 1982; Wittstock & Burow, 2007). Moreover, ESP has varied substrate specificity with a high efficiency on the hydrolysis of aliphatic glucosinolates compared to a small impact on aromatic glucosinolates (Cole, 1978; Kaoulla, et al., 1980; Matusheski, et al., 2004; Matusheski, et al., 2006; Petroski & Tookey, 1982; Wittstock & Burow, 2007). Therefore, although glucosinolate content in cruciferous vegetables has been reported in several studies (Agudo, et al., 2008; Kushad, et al., 1999; McNaughton & Marks, 2003; Verkerk, et al., 2009), estimation of total dietary glucosinolate intake does not represent total dietary isothiocyanate exposure in humans.
To the best of our knowledge, only one study by Jiao et al. (Jiao, Yu, Hanker, Low, & Chung, 1998) has measured total isothiocyanate content in nine types of cruciferous vegetable, but primarily those commonly consumed in Asia, including choi sum, kai choi, kai lan, bok choi, wong nga pak, watercress. Although broccoli, cabbage, and cauliflower were included in the analysis, the total isothiocyanate contents were considerably different from the values obtained from those same vegetables in the United States (Shapiro, et al., 1998). The deviation could be attributed to methodological variation between the laboratories, however, the primary cause may be the different growing conditions and cultivation practices between the countries. Furthermore, Jiao et al. measured isothiocyanate content in cooked vegetables by adding exogenous myrosinase, which does not represent the natural hydrolysis process of glucosinolates in the vegetables (Jiao, Yu, Hanker, Low, & Chung, 1998). The values obtained from cooked vegetables may also underestimate the isothiocyanate yield, as cooking can lead to loss of glucosinolates, inactivation of myrosinase, and destruction of heat-labile isothiocyanates (Getahun & Chung, 1999; Shapiro, et al., 1998). The current study was designed to measure total amount of isothiocyanates released naturally from raw cruciferous vegetables that are frequently consumed in Western diets, with the purpose of providing food composition data to public and research society for estimation of dietary isothicoyanate exposure.
2. Materials and methods
2.1 Materials
1,2-Benzenedithiol was purchased from Aldrich, purified by vacuum distillation, and stored in small aliquots at −20°C. Sulforaphane was purchased from LKT Laboratories. Allyl isothiocyanate, myrosinase, and all other chemicals were reagent grade and were purchased from Sigma-Aldrich.
2.2. Sample preparation
A total of 73 samples of nine commonly consumed cruciferous vegetables, including broccoli, cabbage, cauliflower, Brussels sprout, kale, collard green, mustard green, and turnip green, were purchased from local grocery stores in the Buffalo, New York, metropolitan area. Each store obtains vegetables either from local farms or from outside of the western New York region such as California, Virginia, or Hawaii. To increase generalizability of our samples, each type of vegetable was purchased from at least three different stores and on at least three different dates spanning a period of six months (June to November). Each sample was weighed and homogenized into a fine paste with deionized water (1:3, weight:volume) in a Waring blender. The mixture was centrifuged at 300 × g for 3 minutes at 4 °C to remove insoluble materials. The supernatants were stored at −70 °C until analysis of isothiocyanate. All vegetables were processed on the day of purchase.
2.3. Cyclocondensation assay
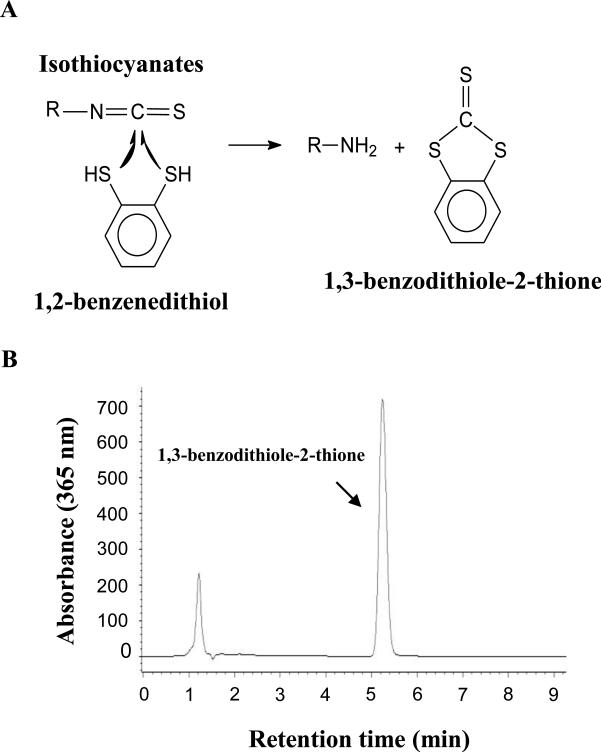
The isothiocyanate level was measured by the high-performance liquid chromatography (HPLC)-based cyclocondensation assay using 1,2-benzenedithiol as the detection reagent (Tang, Zhang, et al., 2006; Zhang, Wade, Prestera, & Talalay, 1996). The cyclocondensation assay relies on the reaction of the carbon atom of the –N=C=S group of isothiocyanate molecule with thiol groups of 1,2-benzenedithiol to form a five membered 1,3-benzodithiole-2-thione and the corresponding amine (Figure 1a). Using a simple reverse phase HPLC with an isocratic mobile phase of 80% methanol and 20% water at a flow rate of 2 mL/min, 1,3-benzodithiole-2-thione is eluted at 5-6 min (Figure 1b). Due to the presence of the characteristic –N=C=S structure, naturally occurring isothiocyanates (all aliphatic and aromatic isothiocyanates) react quantitatively with an excess of 1,2-benzenedithiol. Therefore, the HPLC-based cyclocondensation assay provides a generic analytical method for measurement of total isothiocyanates, but is not able to differentiate each individual isothiocyanates (Zhang, 2012).
Figure 1.
The HPLC-based cyclocondensation assay for measurement of isothiocyanates. A, reaction of isothiocyanates with 1,2-benzenedithiol; B, a typical chromatogram of a completed cyclocondensation reaction solution. (Adapted from Zhang 2012).
Briefly, in a 4 mL glass vial, each sample (up to 0.25 mL) was mixed with 0.25 mL of 100 mmol/L potassium phosphate buffer (pH 8.5), 0.5 mL of 10 mmol/L 1,2-benzenedithiol in methanol to form a 1.0 mL reaction mixture (adjusted with water). The reaction mixture was incubated for 2 hours at 65 °C, and was then cooled to room temperature. The mixture was centrifuged at a low speed to sediment insoluble materials. A 0.1 mL aliquot of the supernatant was injected into the HPLC system for analysis of isothiocyanate content. Each run included a blank containing only methanol, a control containing only 1,2-benzenedithiol, and a series of standards containing different amounts of pure isothiocyanates.
An Agillent 1100 series HPLC system equipped with a model G1313A autosampler, a model G1315B photodiode array detector, and an Agillent Chemostation chromatography data system was coupled to an analytical C18 reverse-phase column (Whatman Partisil 10 μm ODS-2, 4.5 × 250 mm) for the cyclocondensation assay. The HPLC mobile phase was methanol:water (80:20, v/v), at a flow rate of 1.75-2 mL/min. 1,3-Benzodithiole-2-thione, the cyclocondensation product of 1,2-benzenedithiol with isothiocyanate, was eluted between 5-6 minutes, and the eluent were monitored at 365 nm by the photodiode array detector. Each sample was eluted for a total of 10 minutes. With 1,3-benzodithiole eluted between 5-6 minutes, this 10-minute elusion will leave about 5-minute time interval to wash the column between sample injections. 1,3-Benzodithiole-2-thione (Zhang, et al., 1996) was dissolved in methanol and serially diluted to calibrate the system. This system has been used routinely in our laboratory with the detection limit of 5 pmol isothiocyanate (Munday, et al., 2008; Tang, Li, Song, & Zhang, 2006; Tang & Zhang, 2005; Tang, Zhang, et al., 2006).
2.4. Exogenous myrosinase treatment
To investigate whether conversion of glucosinolates to isothiocyantes is completed by endogenous myrosinase, three samples from each type of vegetables were randomly selected for treatment with exogenous myrosinase. Two aliquots of supernatant for each sample were mixed with or without myrosinase (1 unit per 0.1 mL vegetable supernatant). The mixtures were incubated at room temperature for 1 hour and then subjected to analysis of isothiocyanate. The values were compared between the two aliquots. The activity of exogenous myrosinase was confirmed in the laboratory using commercially available glucosinolates (results not shown).
3. Results and discussion
The common name and nomenclature of the examined cruciferous vegetables as well as their total isothiocyanate yields are listed in Table 1. Depending on the availability from local grocery stores, 8 to 10 samples of each cruciferous vegetable were collected. A total of 73 samples comprising 9 different cruciferous vegetables were analyzed, with an average total isothiocyanate yield of 16.2 μmol/100g wet weight. A wide range (as much as 41-fold difference) of isothiocyanate yield was observed across the vegetables. Cauliflower had the lowest mean level of isothiocyanate yield (1.5 μmol/100g wet weight) and mustard greens had the highest mean level (61.3 μmol/100g wet weight).
Table 1.
Total isothiocyanate yield in commonly consumed cruciferous vegetables in USA.
| vegetables | Scientific name | N | ITC yield (μmol/100g wet weight) Mean and ranges |
|---|---|---|---|
| Broccoli | Brassica oleracea var. italica | 9 | 6.9 (2.6 – 18.1) |
| Cabbage | Brassica oleracea var. capitata | 10 | 31.7 (0.5 – 77.9) |
| Cauliflower | Brassica oleracea var. botrytis | 9 | 1.5 (0.7 – 2.7) |
| Brussels Sprout | Brassica oleracea var. gemmifera | 9 | 9.6 (0.6 – 21.1) |
| Kale | Brassica oleracea var. acephala | 9 | 3.7 (0.4 – 12.9) |
| Collard Green | Brassica oleracea var. viridis | 10 | 5.8 (0.1 – 28.5) |
| Mustard Green | Brassica juncea | 9 | 61.3 (0.4 – 137.9) |
| Turnip Green | Brassica rapa var. rapa | 8 | 9.0 (1.1 – 17.8) |
Vegetables were purchased from different local markets and processed in the same day. Total isothiocyanate yield from the vegetables was measured by the HPLC-based cyclocondensation
Variation in isothioycanate yield across vegetable type was also observed within each vegetable. For example, we observed up to 345-fold difference in isothiocyanate yield among nine samples of mustard green (ranging from 0.4 to 137.9 μmol/100g wet weight). In order to further delineate this source of variation, we compared total isothiocyanate yield among samples purchased from three different local markets and the samples purchased at three different time points spanning a 6-month period (Figure 2). The total isothiocyanate yield varied considerably across the markets, which may reflect differences in the source of vegetables of each market. Interestingly, the major vegetable source of store 2 is local farms, so one would presume that the time from harvest to purchase would be shorter, which could result in higher levels of isothiocyanates. However, the vegetables from store 2 did not produce higher yields of isothiocyanates overall compared with the vegetables from the other stores, indicating that freshness may not be the determining factor for isothiocyanate yield, although we do not know the number of days from harvest. Diverse results were also obtained from vegetables purchased at three different time points, indicating that seasonal differences also exist. Although only the results of broccoli, cabbage, and cauliflower are presented, similar results were obtained for all the other vegetables.
Figure 2.
Variation of total isothiocyanate yield from cruciferous vegetables across different local markets and time points. Each vegetable type was purchased from three different markets and at three different time points (at least two months apart). Values are mean ± SEM (n=3).
Variations in isothiocyanate yield within individual cruciferous vegetables may be attributed to environmental and genetic factors, which determine the content of glucosinolates and/or the ratio of isothiocyanate-producing glucosinolates to the other glucosinolates in cruciferous vegetables. Using broccoli as an example, Mithen et al. found that increasing soil sulfur and nitrogen content through fertilization significantly increases the amount of glucosinolates in the vegetable (Mithen, et al., 2003). Even under the same growing conditions, the content of total glucosinolates as well as the percentage of glucoraphanin (the predominant isothiocyanate-producing glucosinolate in broccoli) still varied across a variety of broccoli lines with different genetic backgrounds (Kushad, et al., 1999). Interestingly, harvest time and post-harvest condition also affect the glucosinolate content (Verkerk, et al., 2009). Fahey et al. reported that high amount of isothiocyanate-producing glucosinolates in broccoli was found in 3-day-old sprouts, and the content decreased precipitously during the growth of vegetables, with increase of other glucosinolates such as indole-producing glucosinolates (Fahey, Zhang, & Talalay, 1997). Mature broccoli has an approximately 15-fold lower amount of isothiocyanate-producing glucosinolates than 3-day-old broccoli sprouts. Cold transportation and storage of broccoli also causes a loss of glucosinolates up to 70-80% (Vallejo, Tomas-Barberan, & Garcia-Viguera, 2003).
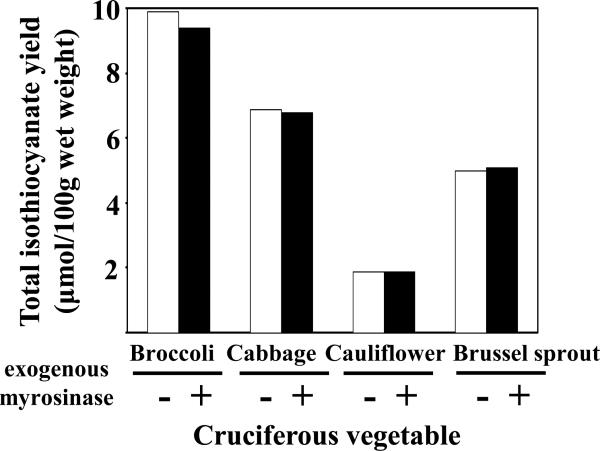
In order to examine the completeness of conversion from glucosinolates to isothiocyanates in the processed cruciferous vegetables, three samples from each type of cruciferous vegetable were randomly selected for treatment with exogenous myrosinase, and total isothiocyanate yield was compared between treated and untreated samples. The results of analyses of broccoli, cabbage, cauliflower, and Brussels sprout are presented in Figure 3. Isothiocyanate yields were essentially identical before and after treatment. Similar results were obtained for other cruciferous vegetables (results not shown). This finding is important as it illustrates not only the completeness of the conversion of glucosinolates to isothiocyanates in processed samples, but also indicates that the myrosinase content in cruciferous vegetable alone is sufficient for complete conversion of glucosinolates to isothiocyanates. It is noted that in certain cruciferous vegetables such as broccoli, the isothiocyanate yield from glucosinolates is determined by both myrosinase and ESP (Matusheski, et al., 2004). ESP interacts with the intermediate product of hydrolyzed glucosinolates and diverts isothiocyanate formation into nitrile. In well-controlled conditions, heating at a certain temperature (e.g. 60°C) destroys ESP but spares myrosinase, thus increasing isothiocyanate yield by 5 folds (Matusheski, et al., 2004). Therefore, although the values listed in Table 1 may represent the maximal isothiocyanate exposure in humans from intake of raw cruciferous vegetables, our reported values may not be the maximal isothiocyanate yield that can be produced by vegetables under ideal conditions.
Figure 3.
Total isothiocyanate yield from different cruciferous vegetables with or without additional treatment with exogenous myrosinase. Each vegetable was tested three times independently using samples from different sources. Due to the wide range of total isothiocyanate yield from different samples, only one set of representative results is presented. No statistically significant difference was observed in total isothiocyanate yield between myrosinase- treated and -untreated samples based on paired Student's t-test at α=0.05.
The purpose of the current study was to provide food composition data for estimation of dietary isothiocyanate exposure based on intake of cruciferous vegetables. However, the data need to be used with cautions.
First, the isothiocyanate levels observed in the current study represent the amount of isothiocyanates that could be obtained from intake of raw cruciferous vegetables. When vegetables are consumed in other forms, the yield of isothiocyanates would vary substantially depending on different cooking methods. McNaughton and Marks compared the glucosinate content in raw, cooked (unclassified including microwave, steam, stir fry, or boil), or boiled vegetables and found loss of glucosinolates in a range of 18.1 to 59.1%, with the highest loss by boiling (McNaughton & Marks, 2003). Moreover, cooking temperature and time also affect the release of isothiocyanates from glucosinolates due to the inactivation of myrosinase and ESP and destruction of heat-labile isothiocyanates (Matusheski, et al., 2004; Rungapamestry, Duncan, Fuller, & Ratcliffe, 2007). It has been reported that cooking reduces isothiocyanate exposure from cruciferous vegetables by 60% to 90% (Conaway, et al., 2000; Getahun & Chung, 1999; Rouzaud, Young, & Duncan, 2004; Shapiro, et al., 1998). Therefore, collection of detailed information on vegetable consumption form (cooked or raw) in epidemiology studies could significantly improve the accuracy of the estimation of dietary isothiocyanate exposure and help detect true associations between isothiocyanate intake and human disease.
Second, we presented here the total isothiocyanate content from raw cruciferous vegetables instead of individual isothiocyanate level. Although dietary isothiocyanates collectively show certain degree of chemopreventive and/or anti-cancer activities partly due to their shared –N=C=S structure, individual isothiocyanate with varied side chain may have different potencies as well as target different mechanisms (Jiao, et al., 1994; Navarro, et al., 2011; Singh & Singh, 2012; Wang, et al., 2011). By linking with the glucosinolate composition data, primary isothiocyanates from individual vegetables could be derived, which should be considered when assessing the impact of dietary isothiocyanates on different cancers or diseases.
Last, variation in isothiocyanate yield within each type of cruciferous vegetables attenuates the accuracy of the exposure estimation. The in vivo hydrolysis of ingested glucosinolates by microflora in the gastrointestinal tract adds another layer of complexity to accurately estimate dietary isothiocyanate exposure, as Kensler et al. reported a 44-fold inter-individual variability of in vivo hydrolysis of glucosinolates in an intervention trial (Kensler, et al., 2005). Therefore, if more accurate estimation of dietary isothiocyanates is required, internal biomarkers such as urinary isothiocyanate levels should be considered. For most epidemiological studies, individuals are ranked on dietary intake to examine the associations with the outcome of interest. The pattern of cruciferous vegetable intake is usually queried in a relatively long time period (e.g. usual dietary intake in a few years before diagnosis), and the frequency data are used to estimate the intake. These procedures would help balance the variations caused by each discrete intake. Indeed, using urinary isothiocyanate metabolites as biomarkers, Seow et al. found that estimation of dietary isothiocyanate intake was significantly correlated with urinary isothiocyanate level (Seow, et al., 1998).
4. Conclusion
There is a growing interest in understanding the role of dietary isothiocyanates in human health. However, lack of food composition data limits exposure assessment and impedes research progress. The current study provides food composition data for isothiocyanate yield from raw cruciferous vegetables, the major dietary source of isothiocyanates in human diets. Given that the type of vegetables examined in this study includes the most commonly consumed cruciferous vegetables in Western diet, the data may be particularly useful in estimation of dietary isothiocyanate exposure in this population.
AIF-1 is a highly conserved, inflammatory protein with multiple biologically active sites.
AIF-1 influences several key cellular and molecular events in inflammation activation.
AIF-1 may serve as a clinical marker of chronic and acute organ transplantation rejection.
AIF-1 is a central causal factor in the pathogenesis of autoimmune disorders and vasculopathy.
AIF-1 is tightly associated with central nervous system injury and cancer.
Acknowledgments
Grant support: The study was supported by National Cancer Institute (R25 CA114101; K07CA148888).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudo A, Ibanez R, Amiano P, Ardanaz E, Barricarte A, Berenguer A, Dolores Chirlaque M, Dorronsoro M, Jakszyn P, Larranaga N, Martinez C, Navarro C, Pera G, Quiros JR, Sanchez MJ, Tormo MJ, Gonzalez CA. Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. European Journal of Clinical Nutrition. 2008;62:324–331. doi: 10.1038/sj.ejcn.1602750. [DOI] [PubMed] [Google Scholar]
- Burow M, Markert J, Gershenzon J, Wittstock U. Comparative biochemical characterization of nitrile-forming proteins from plants and insects that alter myrosinase-catalysed hydrolysis of glucosinolates. Federation of European Biochemical Societies Journal. 2006;273:2432–2446. doi: 10.1111/j.1742-4658.2006.05252.x. [DOI] [PubMed] [Google Scholar]
- Cole RA. Epithiospecifier protein in turnip and changes in products of autolysis during ontogeny. Phytochemistry. 1978;17:1563–1565. [Google Scholar]
- Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung FL. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutrition and Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Current Drug Metabolism. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiology, Biomarkers and Prevention. 1999;8:447–451. [PubMed] [Google Scholar]
- Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. Journal of Nutrition. 1999;129:768S–774S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- Jiao D, Eklind KI, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Research. 1994;54:4327–4333. [PubMed] [Google Scholar]
- Jiao D, Yu MC, Hanker JH, Low SH, Chung FL. Total isothiocyanate contents in cooked vegetables frequently consumed in Singapore. Journal of Agricultural and Food Chemistry. 1998;46:1055–1058. [Google Scholar]
- Kaoulla N, MacLeod AJ, Gil V. Investigation of Brassica oleracea and Nasturtium officinale seeds for the presence of epithiospecifier protein. Phytochemistry. 1980;19:1053–1056. [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, Sun Y, Zhang QN, Zhang BC, Zhu YR, Qian GS, Carmella SG, Hecht SS, Benning L, Gange SJ, Groopman JD, Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. Journal of Agricultural and Food Chemistry. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- Matusheski NV, Juvik JA, Jeffery EH. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004;65:1273–1281. doi: 10.1016/j.phytochem.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Matusheski NV, Swarup R, Juvik JA, Mithen R, Bennett M, Jeffery EH. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. Journal of Agricultural and Food Chemistry. 2006;54:2069–2076. doi: 10.1021/jf0525277. [DOI] [PubMed] [Google Scholar]
- McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. British Journal of Nutrition. 2003;90:687–697. doi: 10.1079/bjn2003917. [DOI] [PubMed] [Google Scholar]
- Mithen R, Faulkner K, Magrath R, Rose P, Williamson G, Marquez J. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theoretical Applied Genetics. 2003;106:727–734. doi: 10.1007/s00122-002-1123-x. [DOI] [PubMed] [Google Scholar]
- Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, Zhang Y. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Research. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- Nakamura Y. Chemoprevention by isothiocyanates: molecular basis of apoptosis induction. Forum or Nutrition. 2009;61:170–181. doi: 10.1159/000212749. [DOI] [PubMed] [Google Scholar]
- Navarro SL, Li F, Lampe JW. Mechanisms of action of isothiocyanates in cancer chemoprevention: an update. Food & Function. 2011;2:579–587. doi: 10.1039/c1fo10114e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski RJ, Tookey HL. Interactions of thioglucoside glucohydrolase and epithiospecifier protein of cruciferous plants to form 1-cyanoepithioalkanes. Phytochemistry. 1982;21:3. [Google Scholar]
- Rouzaud G, Young SA, Duncan AJ. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiology, Biomarkers and Prevention. 2004;13:125–131. doi: 10.1158/1055-9965.epi-085-3. [DOI] [PubMed] [Google Scholar]
- Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proceedings of the Nutrition Society. 2007;66:69–81. doi: 10.1017/S0029665107005319. [DOI] [PubMed] [Google Scholar]
- Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiology, Biomarkers and Prevention. 1998;7:775–781. [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiology, Biomarkers and Prevention. 1998;7:1091–1100. [PubMed] [Google Scholar]
- Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Li G, Song L, Zhang Y. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anti-Cancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhang Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Molecular Cancer Therapeutics. 2005;4:1250–1259. doi: 10.1158/1535-7163.MCT-05-0041. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhang Y, Jobson HE, Li J, Stephenson KK, Wade KL, Fahey JW. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Molecular Cancer Therapeutics. 2006;5:935–944. doi: 10.1158/1535-7163.MCT-05-0476. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Isothiocyanates: mechanism of cancer chemopreventive action. Anti-Cancer Drugs. 2002;13:331–338. doi: 10.1097/00001813-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Vallejo F, Tomas-Barberan F, Garcia-Viguera C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. Journal of Agricultural and Food Chemistry. 2003;51:3029–3034. doi: 10.1021/jf021065j. [DOI] [PubMed] [Google Scholar]
- Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, De Schrijver R, Hansen M, Gerhauser C, Mithen R, Dekker M. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Molecular Nutrition & Food Research. 2009;53(Suppl 2):S219. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- Wang X, Di Pasqua AJ, Govind S, McCracken E, Hong C, Mi L, Mao Y, Wu JY, Tomita Y, Woodrick JC, Fine RL, Chung FL. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. Journal of Medicinal Chemistry. 2011;54:809–816. doi: 10.1021/jm101199t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U, Burow M. Tipping the scales--specifier proteins in glucosinolate hydrolysis. Interanational Union of Biochemistry and Molecular Biology Life. 2007;59:744–751. doi: 10.1080/15216540701736277. [DOI] [PubMed] [Google Scholar]
- Zhang Y. The 1,2-benzenedithiole-based cyclocondensation assay: a valuable tool for the measurement of chemopreventive isothiocyanates. Critical Reviews in Food Science and Nutrition. 2012;52:525–532. doi: 10.1080/10408398.2010.503288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Analytical Biochemistry. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proceedings of the Nutrition Society. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]