Abstract
The Caribbean tropical tree, Pimenta dioica has been used for a variety of human endeavors, such as in perfumery industry, food spice, as a natural pesticide, and in folk medicine. Discovered in Jamaica during the voyages of Christopher Columbus, the dried unripe berries of P. dioica also known as Allspice can be found in all continents with unique names in over 50 languages. Systematic investigation of aromatic constituents of Pimenta leaves and its unripe berries, Allspice, have resulted in discovery of many and novel aromatic compounds, mostly glycosides and polyphenols that show antibacterial, hypotensive, anti-neuralgic and analgesic properties. Recent studies have shown two of the known compounds isolated from Allspice, Eugenol and Gallic acid have selective antiproliferative and anti-tumor properties on human cancer cells and their animal models. New characterization of novel compounds such as Ericifolin from the aqueous extract of Allspice berries show potent anti-prostate cancer and anti-breast cancer properties that can be verified in vitro as well as in vivo. Considering its purity, mostly available as “organically grown” berries, availability at low cost, wide acceptance in culinary delights of many cultures world-wide, Allspice may have an additional space in most households, in their medicine cabinets.
Keywords: Allspice, cancer, diet and chemoprevention, eugenol, ericifolin, Pimenta dioica, quercetin
1. INTRODUCTION
The dried unripe berries of Pimenta Dioica [(L)Merr] are termed Allspice. It is also called Jamaican pepper, Pimenta or Newspice. A native plant from the Caribbean island Jamaica, P. dioica belongs to the family Myrtaceae. Unlike the common black peppercorns which are fruits from a tropical vine (Piper nigram, L) which is the native of South Asia, Pimenta is a typical Evergreen tree with a height of 22 ft. Occasionally the trees grow upto 43 feet tall with light gray bark and dark green leaves (4–8 cm long) Fig. (1). Small whitish flowers grow on the allspice tree in the summer that produces the berries. The berries are picked while still green and dried in the sun. The berries (Allspice) become brown when they’re dried and look like large peppercorns. Allspice was originally native to the tropical forests of South and Central America, southern Mexico and the West Indies. Allspice is grown commercially in Mexico, Honduras, Trinidad, Cuba and in Jamaica. Commercial Allspice is also obtained from the fruits of another related species, Pimenta racemosa (Myrtacea, L) mostly found in Central America. However, the fruits are larger and are known to be less aromatic. Jamaica is also the world’s largest producer of allspice and Jamaican allspice is renowned for being of exceptional quality because it contains a higher level of essential oils, which give it more flavor than Allspice grown in other Caribbean islands or in Central America [1–4]. Pimenta trees are dispersed around the world with Allspice spelled in over 50 languages from Arabic to Vietnamese.
Fig. 1.

Pimenta dioica (plant and seeds): All parts of P. dioica are used in medicine or cuisines. A: P. dioca tree about 10 years old. B. Bark of P. dioica; C. Fresh leaves of P. dioica; D. Allspice: Dried unripe seeds of P. dioica (source; Kingston, Jamaica).
Accounts of Allspice’s introduction in Europe go back to its discovery by Christopher Columbus on the Caribbean islands during his voyages to the New World. As is known in 15th century history, Columbus was seeking peppers and other common spices of the day, such as cinnamon and cloves, but he had actually never seen real pepper plants, so when he found Allspice in Jamaica in the beginning of 16th century, he gave it the name of Jamaican pepper and the genus name Pimenta, from the word “pimiento”, Spanish for peppercorn and dioica means, the male and female flowers grow on different plants (dioeciousplant). Allspice was introduced into and shipped to Europe in the 16th century. Later, the name “Allspice” was given by the British due to its strong aromatic flavor that resembles the combined aromas of cloves, pepper, cinnamon and nutmeg [1–4].
2. NON-MEDICINAL USE OF PIMENTA DIOICA
Whole or powdered Allspice is sold in the grocery stores throughout the world and is used in the cuisines of Middle East and Central America as well in European pastries. In Caribbean cuisines, Allspice is the most important spice and used extensively, it’s used in Jamaican jerk seasoning, in mole sauces and pickling. The meats that are seasoned with Allspice are then cooked over a P. dioica wood fire. Jamaicans also soak the berries in rum to make a special liqueur. In other countries Allspice is used mostly in baking desserts such as pumpkin pies, banana bread spice cake, bread pudding and gingerbread. In the British isles, Allspice is added in stews, sauces and for flavoring pickled vegetables.
The essential oil extracted from Allspice have typical aroma of a combination of pepper, nutmeg, clove and cinnamon. The scented oil from Allspice have been used in perfumery, candle making and in other cosmetic manufacturing. Similarly, scents extracted from fresh or dried leaves of Pimenta are also known to impart stimulating effect with the mildly spicy aroma. In recent years, in societies’ drive for natural alternatives to pesticides and fungicides, the extracts of Pimenta leaves have been used as food fumigant to preserve freshness and sterility of meat and poultry products [5, 6]. In similar areas where synthetic pesticides are used like the wood protection and plant disease treatments, Allspice essential oils also have been substituted as a natural alternate for pesticides and fungicide [7, 8].
3. MEDICINAL USE OF PIMENTA DIOICA
A. Pimenta Plant Products in Folk Medicine
All parts of P. dioica are used in Folk medicine, the kind of medicine developed over centuries by empirical and in oftentimes by anecdotal evidences and other approaches. In Caribbean, there is a long history of using Allspice berries for folk healing. Jamaicans also drink hot tea with Allspice for colds, dysmenorrhea (menstrual cramps) and dyspepsia (upset stomach). Costa Ricans are known to use Allspice to treat dyspepsia and diabetes. Guatemalans are known to apply crushed Allspice berries to bruises, sore joints and for myalgia (muscle ache). In Cuban medicine, Allspice along with other herbal mixtures is used to relieve indigestion. Not only used in the areas where Allspice originated from, its use has been incorporated in the Indian traditional medicine, the Ayurveda. Allspice use in Ayurveda, which has been developed over the last two millennia, has likely originated from European colonization and subsequent use of it in Portuguese and the English populace in India. Its use includes use to relieve respiratory congestion and assorted odontalgia (toothache). In Europe, anecdotal uses of Allspice extract for dyspepsia and as purgative exist. The use of Allspice in folk medicine was also followed by its incorporation in the British Pharmacopoeia of 1898, especially of pimento oil and pimento water. Oil of pimento, however, was deleted from the British Pharmacopeia of 1914. The British Pharmacopeia Codex supposedly still retained pimento water [9]. In modern herbal medicine, Allspice extract has been used for neuralgic pain. Allspice essential oil, when added to massage oils and baths, is known to promote circulation so as to relieve pain from muscle cramps and strains. Also, it is used for headache, to combat stress and depression and to overcome fatigue because of its comforting scent. Allspice blends well with ginger, lavender and other spices, making it diversified when it comes to the choices for aromatherapy.
The reason why Allspice is used for treating indigestion might be due to the abundance of the common polyphenol Eugenol in Allspice, which is known to stimulate digestive enzymes [10]. Furthermore, it has been shown that Eugenolhas analgesic effect in neuralgia; it is often used as anesthetic by dentists [10]. Eugenol could potentially contribute to the anti-inflammatory function associated with Allspice in traditional medicine. The widespread and diverse uses of Allspice, the leaves and barks of Pimenta have stimulated several studies in the last two decades on systematic investigations of the potential evidence-based medical use of Pimenta. Although, most uses of Allspice as folk medicine are inherited from ancestors among different cultures, more scientific studies have been carried out for a systematic assessment of its active components from leaves, bark and berries of Pimenta and to delineate potential medicinal values of chemicals enriched or isolated from P. dioica.
B. Potential Medicinal Components of P. dioica
Most of the chemical extractions of P. dioica have been reported for its leaves and berries. To date, the most common ingredients tested are polyphenols, lignins and terpenoids. Interestingly, none of the characterized compounds isolated is an alkaloid. (Table 1) lists the main well characterized compounds isolated to date from P. dioica. It should be noted however, other than the chemicals isolated from fresh leaves of P. dioica, many of the compounds reported to be in Allspice could potentially be from other known species that produce Allspice, P. racemosa, as commercial Allspice (both berries and powdered form) is a mix of berries from either species. The purpose of this review is to highlight compounds that have shown significant medicinal potential as validated by controlled studies involving either biochemical experiments or control animals studies. The biological properties exhibited by Allspice extracts can be loosely classified as oxygen scavenges (antioxidants), vasodilators (antihypertensive) and antiproliferative agents with potential for application in cancer chemoprevention and therapies.
Table 1.
Potential Pharmaceutical Products in Pimenta dioica
| Chemical Name | Chemical Group | Source | Yield | Biological Activities | References |
|---|---|---|---|---|---|
| Eugenol | Phenylpropene | Essential oil from berries and leaves | Berries 60–90% Leaves >90% | Antibacterial; Antifungal; Anti-inflammatory; Antioxidant; Anti-proliferative and apoptosis inducing; | [10, 30, 31, 33–41] |
| Quercetin | Flavonoids | Berries | N/A | Antiviral; Anti-inflammatory; Anti-cancer | [44–49, 53, 56] |
| Gallic Acid | Phenolic acid | Berries | N/A | Antiviral; Anti-inflammatory; Anti-cancer | [25–28, 58–62] |
| Ericifolin | Phenolic | Berries | Antibacterial; Anti-cancer | [14, 29] |
B.1. Antioxidant Activities
Oxygen free radicals like Reactive Oxygen Species (ROS), hydroxyl radical, as well as Reactive Nitrogen Species (RNS) are products of normal cellular metabolism, but their overproduction causes biological damage, the oxidative stress. Oxidative stress cause damage to DNA, proteins and lipids, and has been widely implicated in various pathological conditions involving cancer, neurological disorders, cardiovascular disease, diabetes, aging and other chronic and fatal diseases. Recently, there have been intensive researches about the antioxidant properties of natural dietary agents because of the free radical scavenging ability shown by the whole extract of plants and the components out of it. Examples of compounds derived from common dietary sources include resveratrol from grape seeds, green tea catecholamine such as (−)-epigallocatechin-3-gallate (EGCG) and the common, ascorbic acid. In addition, recently it has been shown that some commonly used herbs and spices are also rich in antioxidants, may even outperform those fruit and vegetables when compared in equivalent consumable quantities. Allspice is known for its antioxidant activities [11] and many compounds that have antioxidant activities have been isolated and characterized. The ethyl acetate extract of Allspice which contained polyphenols show strong antioxidant activities and free radical-scavenging activity against DPPH radical [12]. Many already known compounds are isolated from leaves and berries of Pimenta Dioica, with antioxidant properties, such as Eugenol [13], Quercetin [12], Gallic acid [14] and others. Some of the extensively characterized compounds will be discussed later in this article.
B.2. Extracts of Pimenta Leaves Have Hypotensive Effect
Known as folk remedy used for treatment of high blood pressure, obesity and digestion problem in Central America, as well as treatment for menstrual cramps and abdominal pain in Caribbean culture, cardiovascular effects of Pimenta dioica were investigated. Hypotensive action which is shown by decreased blood pressure level was confirmed by giving Sprague-Dawleyrats intravenous administration of aqueous extract of Pimenta Dioica [15]. Further, it has been reported that the P. dioica leaf extract has central nervous system (CNS) depressant effect at the same time. Analgesic and hypothermic effects were also observed with no significant changes in heart rate, body weight and no other abnormalities. Importantly, it is mentioned that aqueous fraction of Allspice generated greater hypotensive effect than the ethanol extract of identical doses. Another study using Spontaneously Hypertensive Rats (SHR) also showed the aqueous extract of Allspice, which outperforms than the ethanol counterpart, causes depression of the CNS in a dose-dependent manner, but it didn’t produce significant changes in blood pressure [16]. It is also noted that the hypotensive action was not mediated through cholinergic, alpha or beta adrenergic receptors and the extract may have vasorelaxing activity.
B.3. Menopause Treatment
Menopause symptoms affect about 70% of women approaching menopause with hormone replacement therapy (HRT) as the standard treatment. Interestingly, in south and Central America, women typically experience menopause earlier than the U.S. counterparts, however, they tend less to use HRT and they perceive the menopausal period quite positively. Epidemiology studies have shown that cultural and dietary influences may have an effect on the menopausal transition; women from Maya or Costa Rica have more plant-based dietary as well as use more herbal medicines in daily life. A study shown that the methanol extract of P. dioica acts as partial agonist/antagonists by enhancing estradiol-stimulated pS2 mRNA expression but reducing progesterone and PTGES mRNA expression with E2 [17]. Along with several other plants extracted studied, this might be one of the mechanisms by which herbal extracts used by South American women alleviates menopausal symptoms.
B.4. Cancer
Since chronic inflammation is implicated in many cancers including colorectal, breast and prostate cancer, consuming Allspice or other parts of P. dioica should provide a diet with strong antioxidant source. Consequently, there could be beneficial effect on cancer incidence and or mortality, at least from cancers that are directly implicated due to inflammation. However, few studies have been reported that estimates the type, quantity and potential implication of such a diet in countries that are known to use Allspice extensively. The epidemiological data on cancer incidence and mortality among countries in Caribbean basin is not low but high [18, 19]. On the other hand, cancer incidence in Mediterranean countries and in regions that consumes Mediterranean diet rich in Olive oil, green and red vegetable and lower amount of red meats has a lower incidence and mortality of these diseases. This discrepancy between Caribbean countries and Mediterranean countries may be due to many factors, discussion of which is out of the scope of this article. Briefly, there is limited scope in sequestering the contribution of a single dietary factor for changes in cancer incidence in a population. A diet rich in Allspice may also be rich in other antioxidants from sources such as citrus fruits, paprika, turmeric (a source of curcumin), green pepper (capsaicin), cinnamon and other source. Population in the Indian subcontinent has lower risk of colorectal, breast and prostate cancers [18, 20, 21]. However, whether this is due to diet or other factors is not clear at this time. On the other hand, limited implication of antioxidant rich Allspice or Pimenta products on cancer incidence in Caribbean countries may be attributed to the method and form of its consumption. Clearly, consumption of Allspice in beef jerky, Jamaican rum is unlikely to have cancer protection effect in population that extensively consumes these products. Red meat and charbroiled food are associated with increased clinical incidence of breast and prostate cancers [21–23].
Due to the relatively large number and content of aromatic and antioxidant compounds present in Allspice, several groups have attempted to isolate and characterize potential anti-tumor agents in Allspice and Pimenta leaves. The lack of fresh leaves and potential for alteration in content due to the soil conditions in places where Pimenta trees are grown, limited number of studies have been carried out on leaves versus that of Pimenta berries. Assessment of the anti-tumor activities of Pimenta leaf extract or Allspice extracts have used primarily in vitro cell proliferation assays on normal and tumor cells. It has been reported that polyphenols isolated from the methanol extract of P. dioica leaves remarkably inhibit the cell growth of Hep-G2 and HCT-116 cells with less effect on MCF-7 cells [24]. Further, studies have shown pro-proliferation effect on non-tumorigenic cells such as 1301 and RAW 264.7 cells [24]. However, there is indirect evidence that compounds that show antiproliferative activities are present in polyphenols isolated from Allspice. This include Eugenol, Quercetin, pedunculigan and Gallic acid, See Fig (2) all of which exhibit pro-apoptotic and antiproliferative activities on many established human tumor cell lines, including breast cancer cell lines, MCF-7 and MB231 [25, 26], prostate cancer cell lines PC-3 and LNCaP [27, 28]. Most, if not all studies reported on antiproliferative activities of Pimenta extracts and purified compounds from Allspice have been in vitro, no studies to date has reported studies on animal models of human cancers.
Fig. 2.
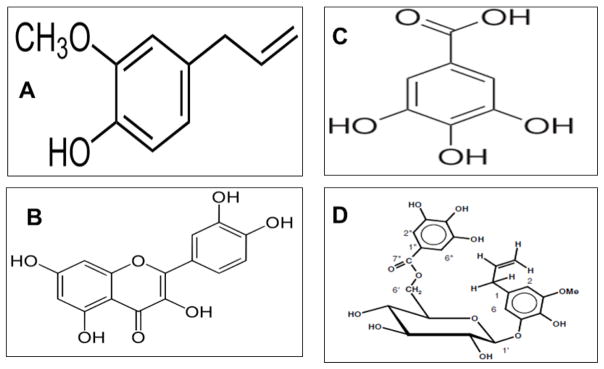
Structures of common phenolic compounds isolated from Pimenta dioica with anti-proliferative activities. A. Eugenol: 4-Allyl-2-methoxyphenol;B. Quercetin: 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one);C. Gallic acid: 3,4,5-trihydroxybenzoic acid; D. Ericifolin: Eugenol 5-0-b-(6′-O-galloylglucopyranoside).
Considering the limited testing of Pimenta products on antitumor activities, yet its abundance of a variety of poly-phenol and lignins, we undertook a series of studies on the Allspice. In an effort to mimic the route at which Allspice is consumed, we extracted powdered Allspice berries in water by boiling, and reconstituted lyophilized extract was tested against prostate, breast and pancreatic cancer cells. The details of the extraction and characterization of antiproliferative activity of Aqueous Allspice Extract (AAE) and its activity in tumor cells in vitro and antitumor activity in vivo is being published elsewhere (Ref: Shamaladevi N et al., Carcinogenesis, under consideration). Our characterization of AAE showed the presence of polyphenols at upto 35% of dry-weight of AAE and existence of unique antitumor compounds that are specific to prostate or breast cancers, both mutually exclusive. Activity based purification schema using batch processing, HPLC, mass-spectrometry and other techniques resulted in identification of one anti-proliferative compound, Eugenol 5-O-β-(6′-galloylglucopyranoside). This compound was previously identified and isolated from the leaves of Australian bottle brush tree (Melaleucaericifolia) and named Ericifolin [29]. Since both Melaleuca and Pimenta are from same family, Myrtacea, it is interesting that both produce a potent antiproliferative compound. The following section, we will briefly summarize other compounds that have been found in Pimenta although the major source is of some other tropical plant.
C. Potential Pharmaceutical Products in Pimenta Dioica
C.1. Eugenol
The most important component isolated from Allspice with strong antioxidant activity is Eugenol which composes 60–90% of the essential oil extracted from Allspice berries. The principal source of Eugenol however, is Clove oil. Eugenol is a phenyl propene and several studies have reported its pharmacological activities [10]. Historically, many natural products are used against microbial infections and many compounds have been found to play a role in the antibacterial activity. Likewise, it is shown that Eugenol inhibits the growth of many different pathogens [30–32] as well as has synergistic effect with known antibiotics [33]. Similarly, Eugenol has also been evaluated to show the antifungal activity against C. albicans thus shed a light on the combination therapy for treating Candidiasis [34]. Eugenol is also well known for its anti-inflammatory activity [35] and antioxidant effect [32, 36]. The anti-inflammatory effect was investigated on LPS induced activated macrophages, to which Eugenol has inhibitory effect on its COX-2 production as well as NF-KB pathway activation, which are characteristics for inflammation [35, 37, 38]. Radical-scavenging against DPPH assays were used to test the anti-oxidant effect of Eugenol and showed promising results [36].
More importantly, Eugenol has been shown to have antiproliferative effect and induce apoptosis in various cancer cell lines. On HeLa cells (cervical cancer), Eugenol showed selective cytotoxicity compared to normal mammalian cells. In addition, combination with an established chemotherapy drug, gemcitabine yields better growth inhibition effect with apoptosis induction [38]. ROS production dependent apoptosis was found in Eugenol treated human erythroleukemia model HL-60 cells [39]. The anti-proliferative effect of Eugenol as well as its isomer isoeugenol was also shown in melanoma cells using xenograft model [40] and epidermoid carcinoma A431 cells [41]. By using xenograft model, Eugenol was implicated in reducing tumor size and inhibition of metastasis, while cell cycle blockage seemed to be the mechanism in the A431 cells.
C.2. Quercetin
Quercetin is a dietary polyphenol which is found in many foods that are consumed daily, it is also isolated from Allspice berries, although in a very limited portion [12]. The pharmacological properties of Quercetin have been extensively documents. Briefly the results revealed the pluripotent activities of Quercetin such as antiviral, anti-inflammatory and anti-cancer effects. Quercetin has been reported to have antiviral effect against different types of virus [42], also shown protective effect for cardiovirus infected mice [43]. And the mechanisms for anti-inflammatory effect of Quercetin lie in down-regulation of NF-KB pathway [44], inhibition of inflammatory cytokines expression [45] and affecting inflammatory gene expression [46].
A large portion of the health benefit studies of Quercetin focus on its anti-cancer potentials and it showed that Quercetin works through various mechanisms. DNA damage rescue and preventions is believed to be the mechanism behind tumorigenesis prevention, and Quercetin was shown to prevent DNA damage as well as increase DNA repair at lower dosages [47]. However, Quercetin might have pro-oxidant effect which causes cellular damage when administered in high dosage [48]. Although the pro-oxidant effects are not welcomed for the healthy cells, it actually enabled Quercetin to work like cytotoxic drug against cancer cells under higher dose. For example, it is known that Quercetin induces apoptosis in many cancer cell lines when treated at 40–50μM or higher concentration [49–51]. Anti-proliferation as well as apoptosis induction happens in both p53 dependent and independent pathways [49, 51]. In addition to the classic apoptosis pathways, Quercetin seems to exert more ways to facilitate the induction of cancer cell death. One potential strategy is by modulating the expression of chaperone protein such as HSP70, HSP27 [52] and HSP90 [53]. One important issue about Quercetin is its absorption and metabolism; it was shown that both the glycoside type of Quercetin, i.e. the aglycon and the parental compounds can be absorbed at multiple sites in the human body. And the anti-oxidant effect was significant when higher dosage of Quercetin is administered in the animal, with which to reach a pharmacologically achievable serum concentration of Quercetin [54, 55].
Although Quercetin doesnot seem to be the major component of Allspice, Quercetin does perform a strong anti-oxidant effect [56]. Its potent ROS scavenging function which allows for reducing DNA damage may powerfully contribute to the potential anti-cancer effect of Allspice or Pimenta extracts as well as the potential benefit associated with their consumption.
C.3. Gallic Acid
Gallic acid (3,4,5-trihydroxybenzoic, GA), which belongs to the phenolic acid family, is distributed among a variety of plants, foods and remedies. Similarly, Gallic acid has been shown to have antiviral effect [57]. The anti-cancer effect of Gallic acid seems very general throughout many types of cancers. Towards prostate cancer, GA induces ROS-dependent apoptosis in LNCaP cells [28], inhibits invasion of human prostate PC3 cells by modulating MMP-2 and -9 [35], induces cell cycle arrest and apoptosis in DU145 cells [58] and is able to decrease tumor growth and micro vessel density in a xenograft model [59]. In addition, GA showed anti-cancer potential against both Estrogen Receptor (ER) positive MCF-7 cells as well as ER negative MB-231 models [25, 26]. Besides solid tumors, Gallic acid is also reported to be effective in inhibiting growth and inducing apoptosis in lymphoma cells [60] and inhibiting invasion of human melanoma cells [61]. The anti-oxidant property of Gallic acid is also beneficial in animal model of Parkinson’s disease [62].
C.4. Ericifolin
Ericifolin, Eugenol 5-O-galloylglucoside, was first isolated from leaves extract of Melaleuca ericifolia [29], whose oil showed antimicrobial and anti-inflammatory activities [63]. With very preliminary studies about Ericifolin, it was found out that it poses antibacterial activity [63]. We have purified Ericifolin from AAE that shows strong antiproliferative, pro-apoptotic, pro-autophagy and anti-androgen receptor activities. When given intraperitoneally, Ericifolin is recovered in sera and pharmacologically effective dose have been observed (unpublished results). Further studies are underway in the authors’ laboratory to fully illustrate its potential.
SUMMARY AND CONCLUSIONS
The Jamaican pepper plant Pimenta dioica contains a cornucopia of medicinal compounds that have been exploited by native Caribbean population as well as medical systems of distant countries, such as India where it has a known entry in the Ayurveda system of medicine. We have presented a critical evaluation of its medicinal properties with special attention as a chemo-dietary prevention agent for chronic diseases and malignant cancers. Several compounds abundantly found in Allspice, namely Quercetin, Gallic acid and Ericifolin show both in vitro and in vivo antiproliferative and antitumor activities. Opportunities exist to identify several potential anticancer compounds from Allspice and test their bioavailability and mechanism of action on normal and tumor systems.
Acknowledgments
Lei Zhang is grateful to Dr. N. Shamaladevi for mentoring her in the art and science of natural products fractionation.
FUNDING SOURCES
NIH R01 AT003544, 1R01 CA 156776 and VA MERIT Award VA5312.01 and VA 5312.02 (All to BLL).
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.Bown D. The Royal Horticultural Society Encyclopedia of Herbs & their Uses. 3. London: Dorling Kindersley; 2002. [Google Scholar]
- 2.Sulistiarini D. Pimenta dioica (L.) Merrill. In: de Guzman CC, Siemonsma JS, editors. Plant Resources of South-East Asia No. 13 Spices. Leiden, the Netherlands: Backhuys Publishers; 1999. pp. 176–180. [Google Scholar]
- 3.Davidson A. The Oxford Companion to Food. 2. Oxford: Oxford University Press; 2006. [Google Scholar]
- 4.Vaughan JG, Geissler C. The New Oxford Book of Food Plants: a Guide to the Fruit, Vegetables, Herbs and Spices of the World. London: Oxford University Press; 1997. [Google Scholar]
- 5.Martinez-Velazquez M, Castillo-Herrera GA, Rosario-Cruz R, et al. Acaricidal effect and chemical composition of essential oils extracted from cuminumcyminum, pimenta dioica and ocimumbasilicum against the cattle tick rhipicephalus (boophilus) microplus (acari: Ixodidae) Parasitol Res. 2011;108:481–7. doi: 10.1007/s00436-010-2069-6. [DOI] [PubMed] [Google Scholar]
- 6.Zabka M, Pavela R, Slezakova L. Antifungal effect of pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind Crop Prod. 2009;30:250–3. [Google Scholar]
- 7.Park I, Kim J, Lee SG, Shin SC. Nematicidal activity of plant essential oils and components from ajowan (trachyspermumammi), allspice (pimenta dioica) and litsea (litseacubeba) essential oils against pine wood nematode (bursaphelenchusxylophilus) J Nematol. 2007;39:275–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Seo SM, Kim J, Lee SG, Shin CH, Shin SC, Park IK. Fumigant antitermitic activity of plant essential oils and components from ajowan (trachyspermumammi), allspice (pimenta dioica), caraway (carůmcarvi), dill (anethumgraveoiens), geranium (pelargonium graveoiens), and litsea (litseacubeba) oils against japanese termite (reticulitermessperatuskolbe) J Agric Food Chem. 2009;57:6596–602. doi: 10.1021/jf9015416. [DOI] [PubMed] [Google Scholar]
- 9.Natural Standard [Traditional Uses of Allspice] Somerville, MA: Natural Standard; [updated 2012 Oct 18; cited 2012 Sep 13]. Available from: http://www.naturalstandard.com/news/news201101040.asp. [Google Scholar]
- 10.Kamatou GP, Vermaak I, Viljoen AM. Eugenol—From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules. 2012;17:6953–81. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos A, Visozo A, Piloto J, Garc A, Rodr CA, Rivero R. Screening of antimutagenicity via antioxidant activity in Cuban medicinal plants. J Ethnopharmacol. 2003;87:241–6. doi: 10.1016/s0378-8741(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 12.Miyajima Y, Kikuzaki H, Hisamoto M, Nikatani N. Antioxidative polyphenols from berries of pimenta dioica. Bio Factors. 2004;21:301–3. doi: 10.1002/biof.5520220159. [DOI] [PubMed] [Google Scholar]
- 13.Padmakumari KP, Sasidharan I, Sreekumar MM. Composition and antioxidant activity of essential oil of pimento (pimenta dioica (L) merr.) from jamaica. Nat Prod Res. 2011;25:152–60. doi: 10.1080/14786419.2010.526606. [DOI] [PubMed] [Google Scholar]
- 14.Kikuzaki H, Sato A, Mayahara Y, Nakatani N. Galloylglucosides from Berries of Pimenta dioica. J Nat Prod. 2000;63:749–52. doi: 10.1021/np9906121. [DOI] [PubMed] [Google Scholar]
- 15.Suárez A, Ulate G, Ciccio JF. Cardiovascular effects of ethanolic and aqueous extracts of Pimenta dioica in Sprague-Dawley rats. J Ethnopharmacol. 1997;55:107–11. doi: 10.1016/s0378-8741(96)01485-7. [DOI] [PubMed] [Google Scholar]
- 16.Suárez Urhan A, Ulate Montero G, Ciccio JF. Effects of acute and subacute administration of Pimenta Dioica (Myrtaceae) exracts on normal and hypertensive albino rats. Rev Biol Trop. 2000;44–45:39–45. [PubMed] [Google Scholar]
- 17.Doyle BJ, Frasor J, Bellows LE, et al. Estrogenic effects of herbal medicines from Costa Rica used for the management of menopausal symptoms. Menopause. 2009;16:748–55. doi: 10.1097/gme.0b013e3181a4c76a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center MM, Jemal A, Lertet J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Gibson TN, Hanchard B, Waugh N, McNaughton D. Thirty-year trends in incidence and age-distribution of prostate cancer in Kingston and St Andrews, Jamaica, 1978–2007. West Indian Med J. 2011;60:9–12. [PubMed] [Google Scholar]
- 20.Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 21.Swaminathan R, Lucas E, Shankarnarayanan R. Cancer survival in Africa, Asia, the Caribbean and Central America: Database and attributes. IARC Sci Publ. 2011;162:23–31. [PubMed] [Google Scholar]
- 22.DeMarzo AM, Nakai Y, Nelson WG. Inflammation, atrophy and prostate carcinogenesis. Urol Oncol. 2007;25:398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Nakai Y, Nelson WG, DeMarzo AM. The dietary charred meat carcinogen 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007;67:1378–84. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- 24.Marzouk MSA, Moharram FA, Mohamed MA, Gamal-Eldeen AM, Aboutabl EA. Anticancer and antioxidant tannins from Pimenta dioica leaves. Z Naturforsch C. 2007;62:526–36. doi: 10.1515/znc-2007-7-811. [DOI] [PubMed] [Google Scholar]
- 25.García-Rivera D, Delgado R, Bougarne N, Haegeman G, Vanden-Berghe W. Gallic acid indanone and mangiferinxanthone are strong determinants of immunosuppressive anti-tumour effects of mangiferaindica L. bark in MDA-MB231 breast cancer cells. Cancer Lett. 2011;305:21–31. doi: 10.1016/j.canlet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Hsu JD, Kao SH, Ou TT, Chen YJ, Li YJ, Wang CJ. Gallic Acid Induces G2/M Phase Arrest of Breast Cancer Cell MCF-7 through Stabilization of p27 Kip1 Attributed to Disruption of p27 Kip1/Skp2 Complex. J Agric Food Chem. 2011;59:1996–2003. doi: 10.1021/jf103656v. [DOI] [PubMed] [Google Scholar]
- 27.Liu KC, Huang AC, Wu PP, et al. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and -9 signaling pathways. Oncol Rep. 2011;26:177–84. doi: 10.3892/or.2011.1264. [DOI] [PubMed] [Google Scholar]
- 28.Russell LH, Jr, Mazzio E, Badisa RB, et al. Autoxidation of Gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res. 2012;32:1595–602. [PMC free article] [PubMed] [Google Scholar]
- 29.Hussein SAM, Hashim ANM, El-sharawy RT, et al. Ericifolin: an eugenol 5-O-galloylglucoside and other phenolics from Melaleuca ericifolia. Phytochemistry. 2007;68:1464–70. doi: 10.1016/j.phytochem.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Ali SM, Khan AA, Ahmed I, et al. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob. 2005;4:20–24. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laekeman GM, Van Hoof L, Haemers A, Berghe DAV, Herman AG, Vlietinck AJ. Eugenol a valuable compound for in vitro experimental research and worthwhile for further in vivo investigation. Phytother Res. 1990;4:90–6. [Google Scholar]
- 32.Singh G, Maurya S, deLampasona MP, Catalan CAN. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45:1650–61. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Hemaiswarya S, Doble M. Synergistic interaction of Eugenol with antibiotics against gram negative bacteria. Phytomedicine. 2009;16:997–1005. doi: 10.1016/j.phymed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad A, Khan A, Khan LA, Manzoor N. In vitro synergy of Eugenol and methyleugenol with fluconazole against clinical candida isolates. J Med Microbiol. 2010;59:1178–84. doi: 10.1099/jmm.0.020693-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim SS, Oh OJ, Min HY, et al. Eugenol suppresses cyclooxy-genase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264. 7 cells. Life Sci. 2003;73:337–48. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 36.Ito M, Murakami K, Yoshino M. Antioxidant action of Eugenol compounds: Role of metal ion in the inhibition of lipid peroxidation. Food Chem Toxicol. 2005;43:461–66. doi: 10.1016/j.fct.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Bachiega TF, De Sousa JPB, Bastos JK, Sforcin JM. Clove and Eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J Pharm and Pharmacol. 2012;64:610–6. doi: 10.1111/j.2042-7158.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 38.Hussain A, Brahmbhatt K, Priyani A, Ahmed M, Rizvi TA, Sharma C. Eugenol enhances the chemotherapeutic potential of gemcitabine and induces anticarcinogenic and anti-inflammatory activity in human cervical cancer cells. Cancer Biother Radiopharm. 2011;26:519–27. doi: 10.1089/cbr.2010.0925. [DOI] [PubMed] [Google Scholar]
- 39.Yoo CB, Han KT, Cho KS, et al. Eugenol isolated from the essential oil of eugeniacaryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005;225:41–52. doi: 10.1016/j.canlet.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem. 2005;280:5812–9. doi: 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- 41.Kalmes M, Hennen J, Blomeke B. Eugenol and isoeugenol as anti-proliferative agents in skin cancer cells. Toxicol Lett. 2009;189:S100. [Google Scholar]
- 42.Kaul TN, Middleton E, Jr, Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol. 1985;15:71–9. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 43.Vrijsen R, Everaert L, Boeye A. Antiviral activity of flavones and potentiation by ascorbate. J Gen Virol. 1988;69:1749–51. doi: 10.1099/0022-1317-69-7-1749. [DOI] [PubMed] [Google Scholar]
- 44.Comalada M, Camuesco D, Sierra S, et al. In vivo quercitrin anti-inflammatory effect involves release of Quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur J Immunol. 2005;35:584–92. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 45.Min Y, Choi C, Bark H, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56:210–215. doi: 10.1007/s00011-007-6172-9. [DOI] [PubMed] [Google Scholar]
- 46.Boesch-Saadatmandi C, Wagner AE, Wolffram S, Rimbach G. Effect of Quercetin on inflammatory gene expression in mice liver in vivo - role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol Res. 2012;65:523–30. doi: 10.1016/j.phrs.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Min K, Ebeler SE. Quercetin inhibits hydrogen peroxide-induced DNA damage and enhances DNA repair in caco-2 cells. Food Chem Toxicol. 2009;47:2716–22. doi: 10.1016/j.fct.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Kim GN, Jang HD. Protective Mechanism of Quercetin and Rutin Using Glutathione Metabolism on H 2 O 2 -induced Oxidative Stress in HepG2 Cells. Ann N Y Acad Sci. 2009;1171:530–7. doi: 10.1111/j.1749-6632.2009.04690.x. [DOI] [PubMed] [Google Scholar]
- 49.Tan J, Wang B, Zhu L. Regulation of survivin and bcl-2 in HepG2 cell apoptosis induced by Quercetin. Chem Biodivers. 2009;6:1101–10. doi: 10.1002/cbdv.200800141. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–53. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G 2/M arrest and apoptosis. Toxicol In Vitro. 2009;23:797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Wang RE, Kao JL, Hilliard CA, et al. Inhibition of heat shock induction of heat shock protein 70 and enhancement of heat shock protein 27 phosphorylation by quercetin derivatives. J Med Chem. 2009;52:1912–21. doi: 10.1021/jm801445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aalinkeel R, Bindukumar B, Reynolds JL, et al. The dietary bioflavonoid, Quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate. 2008;68:1773–89. doi: 10.1002/pros.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos MR, Rodriguez-Gomez MJ, Justino GC, Charro N, Florenicio MH, Mira L. Influence of the metabolic profile on the in vivo antioxidant activity of Quercetin under a low dosage oral regimen in rats. Br J Pharmacol. 2008;153:370–4. doi: 10.1038/bjp.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Justino GC, Santos MR, Canario S, Borges C, Florencio MH, Mira L. Plasma Quercetin metabolites: structure-antioxidant activity relationships. Arch Biochem Biophys. 2004;432:109–21. doi: 10.1016/j.abb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Boots AW, Haenen GRMM, Bast A. Health effects of Quercetin: From antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Lo C, Lai TY, Yang JS, et al. Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and ras. Melanoma Res. 2011;21:267–73. doi: 10.1097/CMR.0b013e3283414444. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal C, Tyagi A, Agarwal R. Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther. 2006;5:3294–302. doi: 10.1158/1535-7163.MCT-06-0483. [DOI] [PubMed] [Google Scholar]
- 59.Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009;26:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim NS, Hwang BS, Jeong SI, et al. Gallic Acid Inhibits Cell Viability and Induces Apoptosis in Human Monocytic Cell Line U937. J Med Food. 2011;14:240–46. doi: 10.1089/jmf.2010.1160. [DOI] [PubMed] [Google Scholar]
- 61.Kratz JM, Andrighetti-Fröhner CR, Kolling DJ, et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentylgallate. Mem Inst Oswaldo Cruz. 2008;103:437–42. doi: 10.1590/s0074-02762008000500005. [DOI] [PubMed] [Google Scholar]
- 62.Sameri MJ, Sarkaki A, Farbood Y, Mansouri SMT. Motor disorders and impaired electrical power of pallidal EEG improved by Gallic acid in animal model of parkinson’s disease. Pak J Biol Sci. 2011;14:1109–16. doi: 10.3923/pjbs.2011.1109.1116. [DOI] [PubMed] [Google Scholar]
- 63.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) Oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


