Abstract
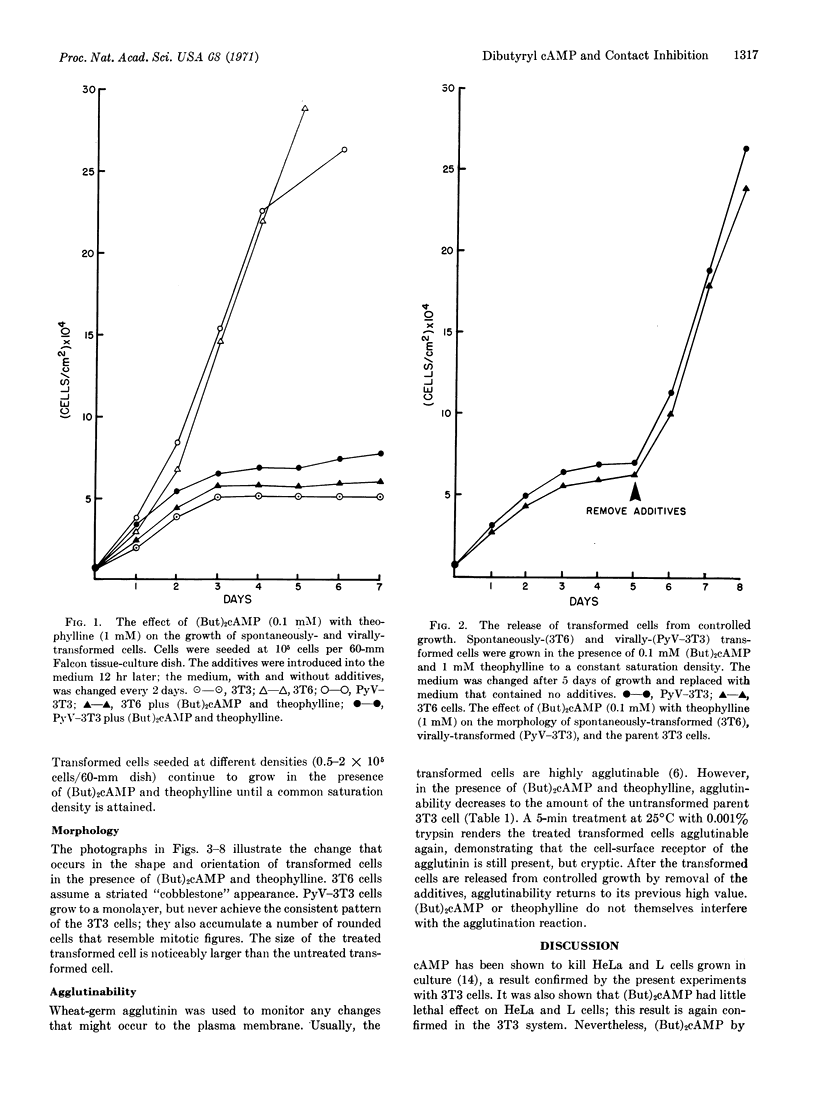
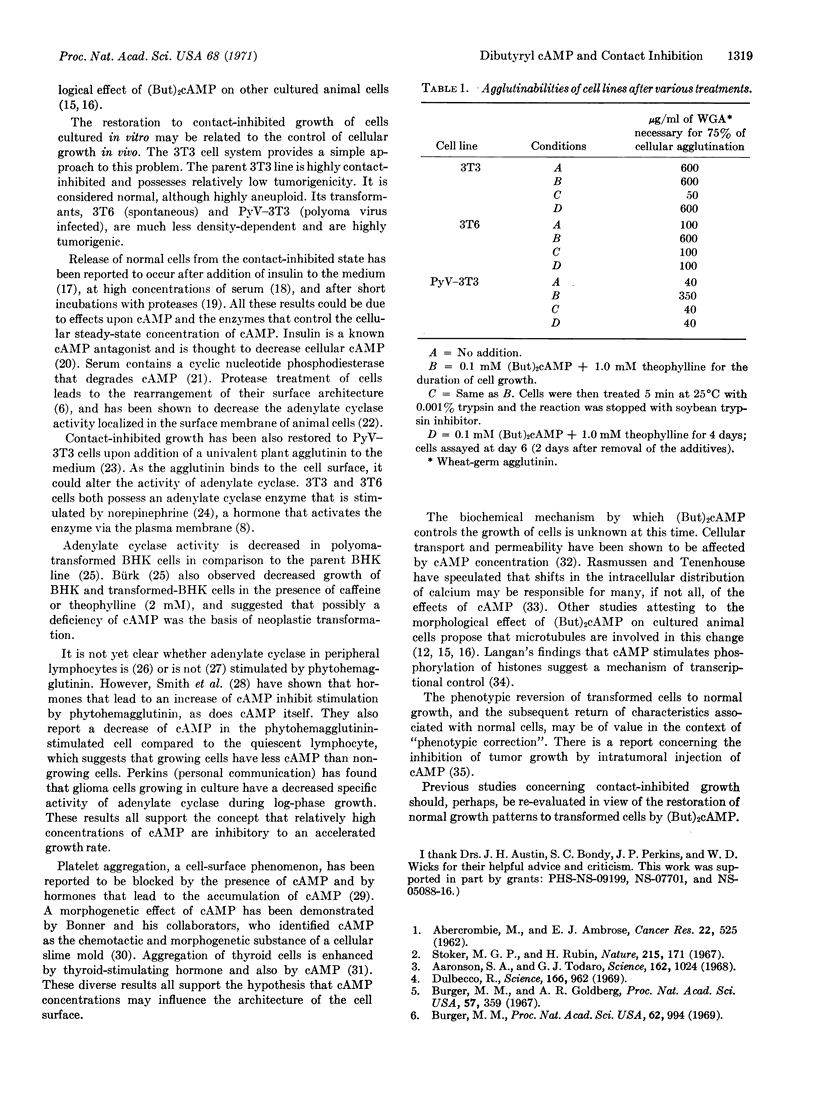
Spontaneously transformed and virally-transformed cells are restored to contact-inhibited growth by the addition of dibutyryl cyclic AMP to the nutrient medium. Theophylline, an inhibitor of the phosphodiesterase that degrades cyclic nucleotides, must also be present for maximal effect. Once the transformed cells reach a saturation density in the presence of the additives, release from the contact-inhibited state occurs upon removal of the dibutyryl cyclic AMP and theophylline from the medium. Agglutinability of the transformed cells by wheat-germ agglutinin (a monitor of architectural changes in the plasma membrane) is decreased by dibutyryl cyclic AMP-theophylline treatment, but increases again upon removal of the additives.
Keywords: theophylline, wheat-germ agglutinin, 3T3 fibroblasts, polyoma virus, plasma membrane
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., AMBROSE E. J. The surface properties of cancer cells: a review. Cancer Res. 1962 Jun;22:525–548. [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Bonner J. T. Induction of stalk cell differentiation by cyclic AMP in the cellular slime mold Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1970 Jan;65(1):110–113. doi: 10.1073/pnas.65.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Cell transformation by viruses. Science. 1969 Nov 21;166(3908):962–968. doi: 10.1126/science.166.3908.962. [DOI] [PubMed] [Google Scholar]
- Fayet G., Lissitzky S. Cyclic 3',5'-adenosine monophosphate-mediated follicular reorganization of isolated thyroid cells in culture. FEBS Lett. 1970 Dec;11(3):185–188. doi: 10.1016/0014-5793(70)80524-5. [DOI] [PubMed] [Google Scholar]
- Gericke D., Chandra P. Inhibition of tumor growth by nucleoside cyclic 3'-5'-monophosphates. Hoppe Seylers Z Physiol Chem. 1969 Nov;350(11):1469–1471. doi: 10.1515/bchm2.1969.350.2.1469. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Exton J. H., Butcher R. W., Sutherland E. W., Park C. R. Role of adenosine 3',5'-monophosphate in the effects of insulin and anti-insulin serum on liver metabolism. J Biol Chem. 1968 Mar 10;243(5):1031–1038. [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Makman M. H. Adenyl cyclase of cultured mammalian cells: activation by catecholamines. Science. 1970 Dec 25;170(3965):1421–1423. doi: 10.1126/science.170.3965.1421. [DOI] [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Effect of phytohemagglutinin and prostaglandins on cyclic AMP synthesis in rat lymph node lymphocytes. Biochim Biophys Acta. 1970 Aug 14;215(2):291–296. doi: 10.1016/0304-4165(70)90027-9. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Ryan W. L., Heidrick M. L. Inhibition of cell growth in vitro by adenosine 3',5'-monophosphate. Science. 1968 Dec 27;162(3861):1484–1485. doi: 10.1126/science.162.3861.1484. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., OYE I., BUTCHER R. W. THE ACTION OF EPINEPHRINE AND THE ROLE OF THE ADENYL CYCLASE SYSTEM IN HORMONE ACTION. Recent Prog Horm Res. 1965;21:623–646. [PubMed] [Google Scholar]
- Schröder J., Plagemann P. G. Growth of Novikoff rat hepatoma cells in suspension culture in the presence of adenosine 3',5'-cyclic monophosphate. J Natl Cancer Inst. 1971 Feb;46(2):423–429. [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. Cellular membranes and tumor behavior: a new hypothesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):868–874. doi: 10.1073/pnas.61.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. M., Shulman N. R. Adenyl cyclase activity in human platelets. Biochem Biophys Res Commun. 1969 Apr 29;35(2):265–272. doi: 10.1016/0006-291x(69)90277-0. [DOI] [PubMed] [Google Scholar]




