Abstract
IMPORTANCE
Mendelian forms of complex I deficiency are usually associated with fatal infantile encephalomyopathy. Application of “MitoExome” sequencing (deep sequencing of the entire mitochondrial genome and the coding exons of >1000 nuclear genes encoding the mitochondrial proteome) allowed us to reveal an unusual clinical variant of complex I deficiency due to a novel homozygous mutation in ACAD9. The patient had an infantile-onset but slowly progressive encephalomyopathy and responded favorably to riboflavin therapy.
OBSERVATION
A 13-year-old boy had exercise intolerance, weakness, and mild psychomotor delay. Muscle histochemistry showed mitochondrial proliferation, and biochemical analysis revealed severe complex I deficiency (15% of normal). The level of complex I holoprotein was reduced as determined by use of Western blot both in muscle (54%) and in fibroblasts (57%).
CONCLUSIONS AND RELEVANCE
The clinical presentation of complex I deficiency due ACAD9 mutations spans from fatal infantile encephalocardiomyopathy to mild encephalomyopathy. Our data support the notion that ACAD9 functions as a complex I assembly protein. ACAD9 is a flavin adenine dinucleotide–containing flavoprotein, and treatment with riboflavin is advisable.
Complex I or NADH: ubiquinone oxidoreductase (Enzyme Commission no. 1.6.5.3) is the largest of the 5 oxidative phosphorylation complexes that catalyze electron transfer from NADH along the respiratory chain, using the lipid-soluble ubiquinone as an electron acceptor.1 Complex I is composed of 45 structural subunits, 14 of which have catalytic functions and 31 of which probably contribute to the assembly, stabilization, and functional regulation of the complex.2 Complex I deficiency is the most common respiratory chain defect associated with early-onset fatal encephalomyopathy. Although many molecular defects have been described both in mitochondrial and nuclear structural subunits and in assembly factors, the molecular diagnosis remains unknown for many patients.3 MitoExome sequencing (deep sequencing of the entire mitochondrial genome and the coding exons of >1000 nuclear genes encoding the mitochondrial proteome) has proven extremely useful in identifying new genetic variants associated with infantile oxidative phosphorylation defects.4 Using MitoExome sequencing, we identified a novel pathogenic ACAD9 gene variant in a patient with infantile-onset and slowly progressive encephalomyopathy, thus enlarging the clinical spectrum of ACAD9-related disorders.
Report of a Case
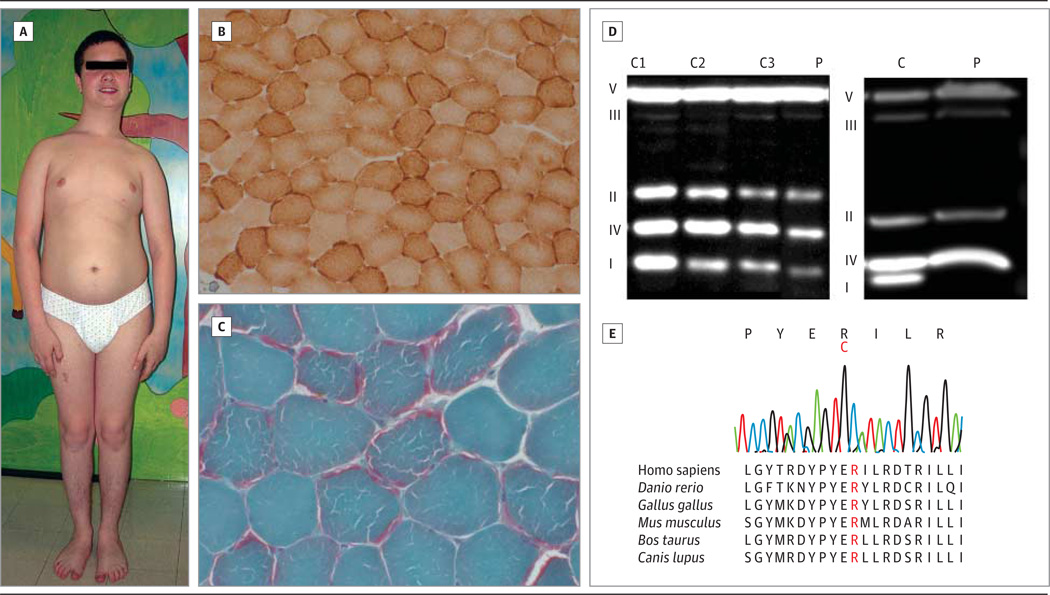
A 13-year-old boy was referred to us because of exercise intolerance, weakness, and muscle wasting. He was born after a normal pregnancy and delivery to nonconsanguineous Italian parents. Since the first year of life, he had difficulty eating and showed psychomotor delay. A neuromuscular evaluation revealed proximal muscle weakness, generalized hypotonia, ataxic gait, bradykinesia and bradylalia, scoliosis, and truncal obesity (Figure, A). His electromyogram was compatible with a myopathic process, and a muscle biopsy revealed mitochondrial proliferation (Figure, B and C). A cognitive assessment showed mild deficit (he had an IQ of 81). His cardiac function was normal, and there were no brain abnormalities detected during magnetic resonance imaging or magnetic resonance spectroscopy.
Figure. Clinical, Histochemical, Biochemical, and Molecular Genetic Characteristics of the Patient.
The patient at 13 years of age shows scoliosis and truncal obesity (A); Gomori trichrome (B [original magnification ×20]) and cytochrome c oxidase (C [original magnification ×20]) histochemical stains show mitochondrial proliferation. Images of Western blot analysis of oxidative phosphorylation proteins (D) in the fibroblasts (left) and muscle (right) of the patient (P) and controls (C) are shown. I indicates complex; II, complex II; III, complex III; IV, complex IV; and V, complex V. The electropherogram of the patient’s DNA shows the variant (c.1240C>T) and evolutionary conservation of the mutated amino acid (E).
A metabolic workup showed increased levels of plasma lactic acid (10 mM; normal range, 0.63 mM–2.44 mM) and alanine (7.90 mg/dL; normal range, 1.84–4.44 mg/dL [to convert to micromoles per liter, multiply by 112.2]), an increased lactate to pyruvate ratio (67; normal range, 10–20), and an increased urinary lactic acid level (53.51 mg/mg creatinine; normal range, <0.28 mg/mg creatinine). He also had hypothyroidism (thyrotropin level, 5 mIU/L; normal level, 0.62 mIU/L), which required treatment with levothyroxine sodium, and an increased plasma free acylcarnitine level. Treatment with high-dose riboflavin (150 mg/d) improved his muscle strength and reduced his plasma lactate (2.75 mM) and alanine (5.63 mg/ dL) levels.
Respiratory chain enzyme activities showed severe complex I and moderate complex III deficiencies both in muscle and in fibroblasts (Table). The severe reduction of complex I was also documented by Western blot analysis of mitochondrial DNA–encoded respiratory chain components in muscle (54%) and fibroblasts (57%) (Figure, D). The patient’s DNA was subjected to next-generation exome sequencing using a mitochondrial gene library (“MitoExome”) as previously described.4–6 We identified a novel homozygous variant (c.1240C>T) in the ACAD9 gene (Figure, E) affecting a highly conserved amino acid (p.R414C) and confirmed that both parents were heterozygous. The mutation was predicted to be deleterious by use of the PolyPhen algorithm(http://genetics.bwh.harvard.edu/pph/). The patient is now 19 years old and has nonprogressive exercise intolerance and weakness.
Table.
Respiratory Chain Activities in Muscle and Fibroblastsa
| Complex | Muscleb | Fibroblastsc | ||
|---|---|---|---|---|
| Patient | Controls, Range | Patient | Controls, Mean (SD) |
|
| I | 0.22 | 0.67 (0.59) | ||
| I and III | 2 | 13–24 | 1.44 | 1.03 (0.31) |
| II and III | 26.2 | 15–28 | 1.25 | 0.66 (0.34) |
| III | 82 | 88–167 | ||
| IV | 332.2 | 120–220 | 0.54 | 0.59 (0.07) |
| V | 138.4 | 130–280 | ||
| II | 13.4 | 10.7–17.4 | 0.24 | 0.3 (0.12) |
| Citrate synthase | 242.3 | 80–210 | 0.34 | 0.24 (0.03) |
The numbers in bold are abnormal values.
Normalized to citrate synthase.
Normalized to milligram of protein.
Discussion
Mitochondrial complex I deficiency accounts for 35% of all oxidative phosphorylation defects, which are estimated to occur in 1 in every 5000 new borns.3 Complex I deficiency blocks the transport of electrons derived from oxidative catabolism of carbohydrates and fatty acids to coenzyme Q and further downstream to respiratory chain complexes III and IV.7 Thus, primary complex I deficiency can affect secondarily the activities of complex III and complex IV. The biochemical defect is usually associated with early-onset fatal encephalomyopathy. Molecular defects in the structural genes of the complex explain less the 40% of cases. In the past decade, an increasing number of mutations have been identified in non-structural genes with the powerful combined use of high throughput exome sequencing, homozygosity mapping, and proteomic analysis.3
Mutations in ACAD9 were first described in 2010 in 4 patients with early-onset and rapidly progressive hypertrophic cardiomyopathy, encephalopathy, and lactic acidosis, leading to death in childhood (46 days–12 years).7 A profound reduction in complex I activity and a milder reduction in complex IV were identified in the muscle, liver, and fibroblasts. The role of ACAD9 in the assembly or stability of complex I was suggested by studies in fibroblasts that showed a reduction in the level of complex I holoenzyme in blue-native gel electrophoresis; this reduction was completely reversed by transduction of wild-type ACAD9. Moreover, complex I activity was increased by supplementing a patient’s fibroblasts with high dose riboflavin, a known catalytic cofactor of ACADs.7 Recently, Gerards et al8 described a milder childhood-onset clinical form with myalgia and premature fatigue in a doubly consanguineous Dutch family. These patients had peculiar episodes of fatigue relieved by vomiting and difficulty with cognitive activities during these events, which lasted hours. Although there was no reduction in the level of complex I holoenzyme, Gerards et al8 proposed that ACAD9 might play a role in stabilizing the super complexes. Our case adds to the clinical heterogeneity of ACAD9 deficiency, which spans from infantile, rapidly progressive encephalomyopathy and hypertrophic cardiomyopathy to pure myopathy with exercise intolerance and lactic acidosis. Our patient had severe complex I and moderate complex III deficiencies in muscle and fibroblasts. Combined defects of complex I and complex III had been described in patients with mutations in the MTCYB gene,9 and studies of human and mouse cell lines carrying mutations in MTCYB demonstrated the interdependence of the 2 enzymes.10 We hypothesize that a reduction the number of electrons transported downstream to the complexes and a lack of complex I assembly can cause secondary complex III deficiency. However, we did not confirm in our patient the previously described complex IV deficiency.
ACAD9 is a β-oxidation component, and the mechanism by which mutations in this pathway impair oxidative phosphorylation remains poorly understood. Our report confirms that ACAD9 has an important role to play in maintaining complex I activity and protein level, thus bolstering the role of ACAD9 as an assembly factor, although further studies are required to fully understand its function. Because ACAD9 is a flavin adenine dinucleotide–containing flavoprotein, it makes sense that patients would benefit from riboflavin supplementation. This was documented both by in vitro studies showing increased complex I activity in patients’ fibroblasts and by clinical experience in all reported patients. Therefore, ACAD9 deficiency is a potentially treatable mitochondrial disorder, and screening for the ACAD9 gene is indicated for all patient with isolated complex I deficiency.
Acknowledgment
Funding/Support: This work has been supported by the National Institutes of Health (grant HD032062), the Italian Congenital Metabolic Disorders Association, and the Marriott Mitochondrial Disorders Clinical Research Fund.
Footnotes
Author Contributions: Dr DiMauro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Garone, Mootha.
Acquisition of data: Garone, Donati, Sacchini, Garcia-Diaz, Bruno, Calvo, Mootha.
Analysis and interpretation of data: Garone, Garcia-Diaz, Calvo, Mootha, DiMauro.
Drafting of the manuscript: Garone, Donati, Sacchini.
Critical revision of the manuscript for important intellectual content: Garcia-Diaz, Bruno, Calvo, Mootha, DiMauro.
Statistical analysis: Garone, Garcia-Diaz, Mootha.
Obtained funding: Mootha.
Administrative, technical, and material support: Mootha.
Study supervision: Mootha, DiMauro.
Conflict of Interest Disclosures: Dr DiMauro receives compensation as a member of the editorial board of MedLink Neurology.
REFERENCES
- 1.Pagniez-Mammeri H, Loublier S, Legrand A, Bénit P, Rustin P, Slama A. Mitochondrial complex I deficiency of nuclear origin: I, structural genes. Mol GenetMetab. 2012;105(2):163–172. doi: 10.1016/j.ymgme.2011.11.188. [DOI] [PubMed] [Google Scholar]
- 2.DiMauro S, Garone C. Metabolic disorders of fetal life: glycogenoses and mitochondrial defects of themitochondrial respiratory chain. Semin Fetal Neonatal Med. 2011;16(4):181–189. doi: 10.1016/j.siny.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Nouws J, Nijtmans LG, Smeitink JA, Vogel RO. Assembly factors as a new class of disease genes for mitochondrial complex I deficiency: cause, pathology and treatment options. Brain. 2012;135(pt 1):12–22. doi: 10.1093/brain/awr261. [DOI] [PubMed] [Google Scholar]
- 4.Calvo SE, Compton AG, Hershman SG, et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci Transl Med. 2012;4(118):ra110. doi: 10.1126/scitranslmed.3003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagliarini DJ, Calvo SE, Chang B, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronchi D, Garone C, Bordoni A, et al. Next-generation sequencing reveals DGUOK mutations in adult patients with mitochondrial DNA multiple deletions. Brain. 2012;135(pt 11):3404–3415. doi: 10.1093/brain/aws258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haack TB, Danhauser K, Haberberger B, et al. Exome sequencing identifies ACAD9 mutations as a cause of complex I deficiency. Nat Genet. 2010;42(12):1131–1134. doi: 10.1038/ng.706. [DOI] [PubMed] [Google Scholar]
- 8.Gerards M, van den Bosch BJ, Danhauser K, et al. Riboflavin-responsive oxidative phosphorylation complex I deficiency caused by defective ACAD9: new function for an old gene. Brain. 2011;134(pt 1):210–219. doi: 10.1093/brain/awq273. [DOI] [PubMed] [Google Scholar]
- 9.Emmanuele V, Sotiriou E, Rios PG, et al. A novel mutation in the mitochondrial DNA cytochrome b gene (MTCYB) in a patient with mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes syndrome. J Child Neurol. 2013;28(2):236–242. doi: 10.1177/0883073812445787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acín-Pérez R, Bayona-Bafaluy MP, Fernández-Silva P, et al. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol Cell. 2004;13(6):805–815. doi: 10.1016/s1097-2765(04)00124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]