Abstract
Background and Objectives
The hippocampus may be vulnerable to the effects of heavy alcohol use during adolescence, which is a time of continued neurodevelopment. However, differences in hippocampal volume may be due to risk factors such as a family history (FH) of alcoholism. We examined hippocampal volumes in youth with and without a FH of alcoholism prior to the initiation of alcohol use.
Methods
Participants were demographically matched adolescents (aged 12-14) with positive (n=15; FHP) and negative (n=15; FHN) FH of alcoholism. Each group consisted of 10 males and 5 females with minimal previous substance use. Manual hippocampal tracings were completed on high-resolution magnetic resonance images by reliable raters, and intracranial volumes were controlled in analyses.
Results
FH groups did not differ on memory or hippocampal volumes, but group × gender interactions (p<.05) indicated that FHP males had larger left hippocampi than FHN males. Females showed greater left versus right hippocampal asymmetry, while males showed larger right versus left asymmetry. For all adolescents, larger right hippocampal volumes predicted poorer delayed visual memory (p<.01).
Conclusion and Significance
Alcoholism risk factors, such as family history of alcoholism, may differentially influence adolescent hippocampal development for boys as compared to girls. Results suggest that FH does not account for prior findings of reduced left hippocampal volumes in heavy drinking youth. Findings are preliminary, but suggest that future studies examining the effects of alcohol use on the adolescent brain should consider the influence of FH, especially among boys.
Keywords: family history of alcoholism, alcohol, adolescence, hippocampus, memory, gender
Introduction
Problematic alcohol use in youth is prevalent, with up to 10% of late adolescents meeting alcohol use disorder (AUD) criteria (1). Identifying risk factors associated with heavy alcohol use may help determine which teens eventually develop alcohol-related problems. For example, individuals with a positive family history of AUD (FHP) have an increased risk for developing AUD relative to those with no such family history (FHN) (2).
FHP youth have shown cognitive decrements in multiple domains (3, 4) and neuroanatomical differences from FHN youth (e.g., smaller amygdalar volume) (5) suggesting that neurobiological mechanisms may underlie the development of AUD. An inherited neurodevelopmental lag (6) may explain the neurobiological differences and place FHP youth at risk for the development of AUD. Studying individuals prior to the initiation of substance use (i.e., early adolescence) can help determine whether these differences were present before significant alcohol use occurred. Further, because neuromaturation continues throughout adolescence (7), teens may be especially vulnerable to neurotoxic effects of heavy alcohol use. For example, adolescents with AUDs have demonstrated memory and other cognitive deficits (8).
The hippocampus, responsible for creating new memories (9), may be particularly vulnerable to the effects of heavy alcohol use (10). Among healthy adolescents, the hippocampus appears to increase in size during the teen years (11, 12), likely due to ongoing myelination (13). In addition to overall hippocampal size, the relative volume of the right (RH) versus the left hippocampus (LH) (i.e., symmetry) appears to be important. Thus far, normative studies of healthy youth suggest that RH>LH asymmetry is typical (11, 14).
Few studies have examined hippocampal volume in adolescents with AUD. DeBellis and colleagues (2000) found that youth with AUD had smaller RH and LH compared to demographically matched individuals (15). Nagel et al. (2005) confirmed smaller LH volumes among heavy alcohol using teens as compared to non-using controls (16). Medina et al. (2007) examined users of both marijuana+alcohol, in addition to heavy alcohol users and non-using controls (17). They found smaller LH among alcohol users compared to marijuana+alcohol users but not controls. Alcohol users also had greater RH>LH asymmetry compared to both other groups, and the degree of abnormality increased linearly with AUD symptoms. Whether these differences pre-dated heavy alcohol use was not possible to ascertain.
This study examined the possibility that the atypical hippocampal volumes shown in adolescent substance users were present prior to initiating alcohol use, and may be due to other factors related to the development of AUD, such as a FH of alcoholism. We compared 15 FHP and 15 FHN adolescents to determine whether hippocampal volumes and asymmetry varied by FH prior to the initiation of significant alcohol use. If normally developing hippocampi increase in size during adolescence (11, 12) and FHP youth experience a neurodevelopmental lag (6), then we would expect FHP youth to have smaller hippocampi than FHN teens. Previous findings of memory deficits (3, 4) and smaller amygdalar volume (5) among non-drinking FHP youth also support that non-drinking FHP adolescents will show smaller hippocampi or abnormal asymmetry relative to non-drinking FHN teens. In light of the literature highlighting sex differences in hippocampal size (11, 12), we also examined gender effects.
Methods
Participants
Participants were selected from a neurocognitive study of youth at risk for substance problems (16). This sample (N=30) included matched adolescents (aged 12-14) with (FHP: n=15) and without (FHN: n=15) a FH of alcoholism. Each group consisted of 10 males and 5 females, and participants were typically from upper middle to upper class families. Adolescents had minimal or no previous substance use at study intake (Table 1).
Table 1.
Demographic, substance use, cognitive function, and hippocampal morphometry information according to family history (FH) group
| FHN (n = 15) M (SD) or % |
FHP (n = 15) M (SD) or % |
|
|---|---|---|
| Demographics | ||
| Age | 13.6 (0.9) | 13.5 (0.9) |
| % Female | 33.3% | 33.3% |
| % Caucasian | 100% | 100% |
| Family history density score*** | 0.0 (0.0) | 0.8 (0.4) |
| Pubertal development score a | ||
| Girls | 15.5 (1.9) | 13.8 (3.2) |
| Boys | 10.7 (1.7) | 11.3 (2.6) |
| Hollingshead Parental SES b | 22.5 (12.7) | 29.9 (12.8) |
| Annual parental salary ($ thousands) | 108.0 (47.9) | 101.3 (45.3) |
| CBCL Externalizing T-score c | 44.9 (6.6) | 45.8 (8.4) |
| Beck Depression Inventory Total d | 1.6 (2.1) | 2.1 (2.2) |
| Spielberger State Anxiety Total e | 27.5 (6.2) | 28.9 (8.7) |
| Baseline Substance Use | ||
| Lifetime uses of alcohol | 0.3 (0.8) | 0.5 (1.6) |
| Largest # drinks on one occasion, last 3 mo | 0.1 (0.3) | 0.2 (0.8) |
| Lifetime uses of cigarettes | 0.0 (0.0) | 0.0 (0.0) |
| Lifetime uses of marijuana | 0.0 (0.0) | 0.1 (0.4) |
| Follow-up Substance Use f | ||
| Average drinks per month, last 3 mo | 5.9 (10.3) | 18.6 (52.4) |
| Largest # drinks on one occasion (lifetime)** | 3.9 (4.0) | 11.6 (7.9) |
| Lifetime drinking days* | 38.2 (69.4) | 84.1 (76.0) |
| Lifetime occasions of drug use** | 7.8 (15.8) | 149.2 (281.3) |
| Cognitive Function | ||
| WASI IQ standard score | 114.0 (11.9) | 111.5 (9.4) |
| CVLT-C 1st trial z-score | 0.2 (0.9) | 0.7 (1.0) |
| CVLT-C Total recall T-score | 51.9 (6.7) | 55.1 (8.7) |
| CVLT-C Long-Delay Free Recall z-score | 0.3 (0.9) | 0.2 (0.9) |
| CVLT-C Discriminability z-score | 0.2 (0.5) | 0.4 (0.5) |
| ROCF Copy accuracy | 27.8 (3.2) | 27.8 (3.8) |
| ROCF Delayed recall accuracy | 16.4 (5.8) | 16.7 (4.6) |
| ROCF % retention | 58.9 (20.8) | 60.4 (15.3) |
| Hippocampal Morphometry | ||
| Intracranial volume (ICV) (cc3) | 1603.7 (136.5) | 1612.4 (142.5) |
| Left hippocampal volume (LH) volume (cc3) | 3.2 (0.5) | 3.4 (0.4) |
| Right hippocampal volume (LH) volume (cc3) | 3.3 (0.4) | 3.3 (0.4) |
| Hippocampal asymmetry (RH − LH)/(RH + | 0.0111 (0.0517) | −0.0117 (0.0451) |
Pubertal Development Scale (Peterson et al., 1988).
Higher scores represent lower SES (Hollingshead, 1965).
Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001).
Beck Depression Inventory (Beck, 1978).
Spielberger State Trait Anxiety Inventory (Spielberger et al.,1970).
Follow-up substance use was measured an average of 4.6 years after baseline assessment.
p<.05
p<.01
p<.001
Teens were recruited from local middle schools. Written informed consent and assent were obtained from parents and adolescents, respectively. Screening interviews assessed for substance use histories, psychiatric diagnoses, and FH of alcoholism. At least one parent was interviewed to corroborate adolescent reports. Participants were excluded for any current or past medical, physical, or psychiatric problems (16), including conduct disorder. The University of California San Diego Human Research Protections Program approved this study.
Measures
Family History of Substance Use Disorders
The Family History Assessment Module (FHAM) (18) assessed the lifetime AUD history in participants’ biological parents and grandparents. FH density was computed such that each parent with AUD history contributed 0.5 and each grandparent 0.25 (range=0-2).
Youth Substance Use
The Customary Drinking and Drug Use Record (CDDR) (19) assessed for lifetime and past 3-month alcohol, nicotine, and other drug use. Participants completed a structured phone screen each follow-up year to update substance use patterns.
Neuropsychological Testing
The Wechsler Abbreviated Scale of Intelligence (WASI) (20) generated a full-scale IQ estimate. The California Verbal Learning Test-Children’s version (CVLT-C) (21) assessed verbal learning and memory (total recall T-score; 1st trial, short- and long-delay free recall, discriminability z-scores). The Rey-Osterrieth Complex Figure Test (ROCF) copy and long delay recall (22) assessed visuospatial construction and memory (copy and delay accuracy raw scores, percent retention).
Procedures
A 1.5 Tesla General Electric Signa LX system acquired high-resolution MRI data. Structural images were collected sagittally using an inversion recovery prepared T1-weighted 3D fast spin echo sequence (TR = 2000 ms, TE = 16 ms, FOV = 240 mm, voxels 0.9375 × 0.9375 × 1.328 mm, 128 slices). A neuroradiologist examined all scans, and youth with abnormal anatomies were excluded from analyses (final sample N=30).
Intracranial volumes (ICVs) were obtained using a hybrid watershed and deformable surface semi-automated skull-stripping method (23) with editing. Two laboratory experts manually defined (i.e., traced) the hippocampal volumes onto the brain acquisitions using Analysis of Functional Images (AFNI). Tracers completed a similar number of brains in each group, were blind to participant characteristics, and were reliable (intraclass correlation coefficients = .95 for RH, .92 for LH). Because tracer was related to volume, all analyses controlled for tracer.
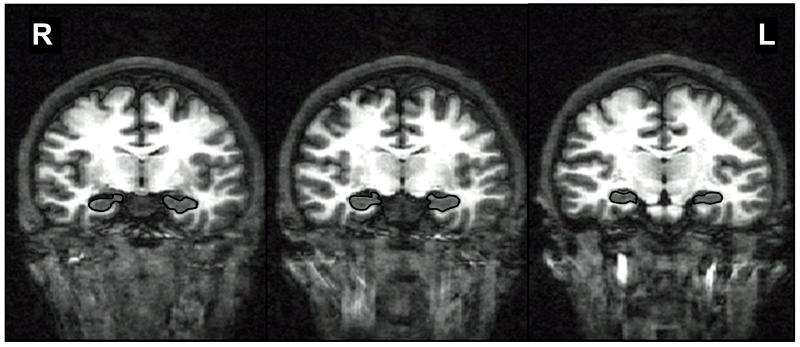
Hippocampal regions of interest were traced on contiguous coronal slices, perpendicular to the anterior commissure/posterior commissure plane, and included the dentate gyrus, subiculum, and cornu ammonis (Figure 1) (16). The stereotactic boundaries were determined as follows: anterior boundary = coronal slice through largest portion of mammillary bodies; superior/lateral boundary = temporal horn and alveus; inferior boundary = white matter of parahippocampal gyrus; medial boundary = ambient cistern; posterior boundary = where columns of the fornix are visible. Volumes were calculated by multiplying voxel counts by voxel dimensions. Due to individual variability in brain size (14), hippocampal volumes were examined as a ratio to intracranial volume (ICV). Hippocampal asymmetry was determined by subtracting LH from RH volume and dividing by their sum: (RH−LH/RH+LH) (17). Positive values denote RH>LH, and negative values indicate LH>RH hippocampal asymmetry.
Figure 1.
Examples of anterior, middle, and posterior hippocampal boundaries shown in coronal view of a T1-weighted magnetic resonance image of the brain; R=right; L=left.
A structured phone screen conducted each year throughout the study obtained follow-up substance use information. The most recent follow-up time-point available for each participant was used (mean=4.6±1.0 years after baseline, range=3-6 years; mean=17.5±1.4 years-old at follow-up, range=15-20 years-old). The following variables summarized participants’ substance use at follow-up: (1) drinks per month in past three months, (2) largest number of drinks on one occasion in lifetime, and (3) lifetime substance use (lifetime occasions of alcohol and other drug use). Follow-up information was available for 97% of this sample (14 FHP; 15 FHN).
Statistical Analysis
Demographics, Cognitive Performance, & Substance Use
Fisher’s Exact Test compared categorical variables, and analysis of variance (ANOVA) compared groups on other demographics and neuropsychological performance. The non-parametric Mann-Whitney procedure examined group differences in substance use at follow-up.
Hippocampal Volume
Two-way analyses of covariance (ANCOVA) assessed group and gender differences in right (RH/ICV) and left (LH/ICV) hippocampal volume, covarying for tracer. A 2 (group and gender) × 2 (hippocampal hemisphere: RH/ICV and LH/ICV) repeated measures ANCOVA (covarying for tracer) assessed group and gender differences in hippocampal asymmetry.
Hippocampal Volume and Memory
Exploratory multiple regressions examined whether baseline hippocampal volumes predicted baseline memory performance. Block 1 entered tracer and Block 2 entered hippocampal volume (LH/ICV, RH/ICV, or asymmetry ratio [RH−LH/RH+LH]). Dependent variables were ROCF and CVLT-C scores.
Hippocampal Volume and Future Substance Use
Exploratory multiple regressions examined whether baseline hippocampal volumes predicted future alcohol or other substance use. Block 1 entered tracer and lifetime alcohol use at baseline, and Block 2 entered hippocampal volume (LH/ICV, RH/ICV, or asymmetry ratio). Dependent variables were follow-up alcohol and other substance use variables.
Results
Demographics, Substance Use, Cognition, and ICV
Groups were similar in demographic backgrounds, baseline IQ and memory scores, substance use at baseline, and ICV (Table 1). However, FHP had a higher familial density of alcoholism and more substance use approximately 4.6 years after baseline compared to FHN.
Left and Right Hippocampal Volumes
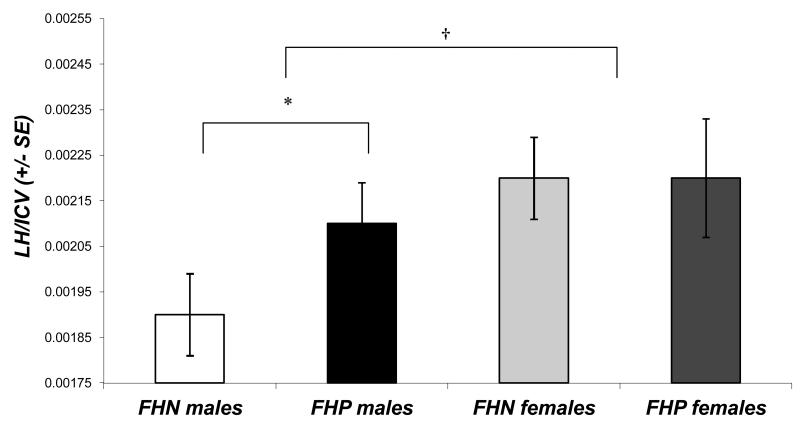
For LH/ICV, a trend [F(1,25)=4.11, p=.053] showed that males tended to have smaller LH than females. Further, a group-by-gender interaction [F(1,25)=5.25, p=.03] indicated that FHP males had larger LH than FHN males (Figure 2). For RH/ICV, no main effects or interactions were observed.
Figure 2.
Left hippocampal volume (expressed as a ratio to intracranial volume [LH/ICV]) in male and female youth with a positive (FHP) or negative family history of alcoholism (FHN). FHP males had larger left hippocampal volumes than FHN males (*p<.05), and overall, males tended to have smaller left hippocampal volumes than females (†p<.10). Error bars represent standard errors.
Hippocampal Asymmetry
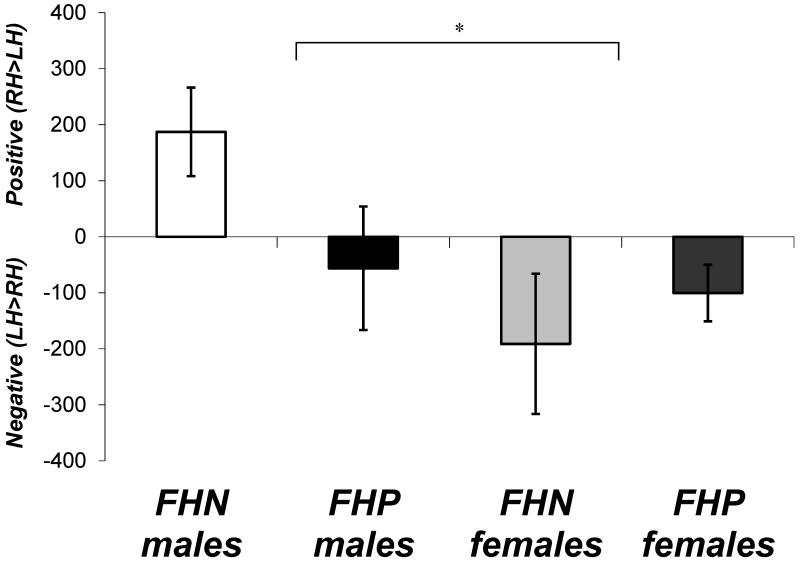
A main effect of hippocampal asymmetry [F(1,25)=4.77, p<.05] showed that, overall, participants had LH>RH. An asymmetry-by-gender interaction [F(1,25)=4.95, p<.05] suggested that, while females had LH>RH asymmetry, males tended to have slightly larger RH>LH (Figure 3). No other main effects or interactions were found.
Figure 3.
Hippocampal asymmetry in male and female youth with a positive (FHP) or negative family history of alcoholism (FHN). Female adolescents tended to have larger left (LH) than right hippocampi (RH) (*p<.05), while males tended to have larger RH than LH overall, primarily due to the FHN males. Positive values depict RH>LH, and negative values depict LH>RH. Error bars represent standard errors.
Hippocampal Volumes and Memory Function
After controlling for tracer, RH/ICV significantly predicted ROCF percent retention [F(2,27)=4.34, p<.05; R2Δ=14%; β=−.49, p<.05]. Having a larger RH/ICV was associated with poorer delayed visual memory. LH/ICV and hippocampal ratio did not predict memory scores.
Baseline Hippocampal Volumes and Future Substance Use
Baseline hippocampal volume did not predict the extent of substance use approximately 4.6 years later.
Discussion
Our hypothesis that FHP teens would show smaller hippocampi than FHN teens was not supported by these preliminary findings. Although males tended to have smaller LH than females, we found that FHP males had larger LH than FHN males. No difference in hippocampal asymmetry between FHP and FHN adolescents was found, but overall these young adolescents had larger LH versus RH asymmetry (LH>RH). This was primarily driven by the females, whereas the males demonstrated the opposite pattern (RH>LH).
Previously, smaller LH (15-17) and RH (15) volumes were reported among AUD adolescents relative to healthy youth or youth with comorbid marijuana+alcohol use. The present findings suggest that differences in hippocampal volume among youth with AUD may not predate alcohol use and, thus, may result from heavy alcohol use during adolescence. Our finding of overall LH>RH asymmetry is consistent with Medina et al. (2007), although males showed RH>LH, which is typically found in normative samples (11, 12). Differences in age range, developmental level, or psychiatric comorbidity and the considerable inter-individual variability in hippocampal volume may partially account for the disparities across studies.
Gender differences in development of the mesial temporal lobe, including the hippocampus, have previously been reported (11, 12). Presently, a gender difference in asymmetry (males: RH>LH; females: LH>RH) and a family history status by gender interaction in LH volume (FHN males<FHP males; overall: males<females) suggests an interaction between gender, family history status, and neurodevelopment. A similar pattern of asymmetry was detected in healthy male (RH>LH) and female (LH>RH) rats (24). Furthermore, inspection of the scatterplots reported in Giedd et al. (1996) revealed that males appeared to have relatively symmetrical RH versus LH volumes across development, whereas females appeared to develop more RH>LH asymmetry in older adolescence (12). Nevertheless, others did not detect gender differences in asymmetry (15). Still, gender effects appear important in brain development and should be further examined.
Another important question is whether hippocampal volume is related to future alcohol use patterns in this sample of non-drinking youth. Others reported associations between hippocampal volumes in heavy drinking youth and the age of onset, duration, and severity of AUD (15, 17). We found no associations between hippocampal volumes prior to alcohol use (mean=13.6 years-old) and the use of alcohol and other substances later in adolescence (mean=17.5 years-old). This exploratory finding further supports that differences in hippocampal volumes detected in youth with AUD were not present prior to significant alcohol use and may relate to the effects of heavy alcohol consumption during adolescence.
Finally, we found that smaller RH volumes were linked to better visual retention. This preliminary finding is consistent with a meta-analysis reporting a negative relationship between hippocampal volume and memory from childhood to young adulthood among healthy youth (25). Previously, Medina et al. (2007) reported that RH>LH asymmetry was associated with improved verbal learning among healthy late adolescents (17), and Foster et al. (1999) found that a smaller LH was related to improved verbal memory in healthy, older female adolescents (26). Neurodevelopmental stage differences may have contributed to variability between studies, but further research may clarify the relationship of memory with hippocampal size and asymmetry among youth with and without AUD.
The strengths of this study include well-matched groups with no psychiatric comorbidity and the examination of brain integrity prior to alcohol use. However, several limitations should be considered. First, by excluding youth with comorbid psychiatric disorders and history of significant substance use, this resulted in a high functioning sample that may not be representative of the general FHP population. Furthermore, because our sample was 100% Caucasian, findings should be replicated in other ethnic groups. Due to our small sample size we had relatively limited power for examining group and gender differences. Therefore, we regard these findings as preliminary.
In summary, this study found an interaction between family history of alcoholism, gender, and hippocampal size and asymmetry in adolescents with little or no previous alcohol exposure, particularly among males. This suggests that alcoholism risk factors, like FH, may influence adolescent hippocampal development differentially as a function of gender. Our results also suggest that FH does not explain prior findings of reduced LH volume or RH>LH asymmetry in heavy drinking youth. Future neurocognitive studies of substance use should consider the influence of FH, especially among boys.
Acknowledgements
This research was supported by grants R01 AA13419 (Tapert), 5 T32 AA013525 (Hanson; PI: Edward Riley), K08 NS52147 (Nagel), and F32 DA020206 (Medina) from the National Institutes of Health. Portions of this study were presented at the 2nd International Conference on Applications of Neuroimaging to Alcoholism in 2008. The authors would like to thank Veronique Boucquey, Sonja Eberson, Jesse Feng, Alejandra Infante, Sonia Lentz, Andria Norman, and Lindsay Squeglia for their contributions to this project.
References
- 1.Clark DB. The natural history of adolescent alcohol use disorders. Addiction. 2004;2:5–22. doi: 10.1111/j.1360-0443.2004.00851.x. [DOI] [PubMed] [Google Scholar]
- 2.Schuckit MA. Genetics and the risk for alcoholism. Journal of the American Medical Association. 1985;254:2614–2617. [PubMed] [Google Scholar]
- 3.Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14:31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- 4.Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: Putative risk factors, alcohol and other drug use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- 5.Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- 6.Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- 7.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 8.Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–83. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- 9.Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural Brain Research. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism: Cinical and Experimental Reseach. 1995;19(1):110–22. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Hagino H, Nohara S, Zhou S, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M. Male-specific volume expansion of the human hippocampus during adolescence. Cerebral Cortex. 2005;15:187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- 12.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. The Journal of Comparative Neurology. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51(6):477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6(4):551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 15.De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 16.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139(3):181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology & Teratology. 2007;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19(4):1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–38. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corp.; San Antonio, TX: 1999. [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober BA. Manual for the California Verbal Learning Test-Children’s Version. Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- 22.Osterrieth PA. Le test de copie d’une figure complexe. Archives of Psychology. 1944;30:206–356. [Google Scholar]
- 23.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Diamond MC, Johnson RE, Young D, Sukhwinder Singh S. Age-related morphologic differences in the rat cerebral cortex and hippocampus: Male-female; right-left. Experimental Neurology. 1983;81:1–13. doi: 10.1016/0014-4886(83)90153-x. [DOI] [PubMed] [Google Scholar]
- 25.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sunram SI, Cezayirli E, Roberts N. The hippocampus and delayed recall: Bigger is not necessarily better? Memory. 1999;7:715–732. doi: 10.1080/096582199387823. [DOI] [PubMed] [Google Scholar]