Abstract
Objectives
Determine the bidirectional transfer and distribution of vancomycin and telavancin across the dually perfused term human placental lobule.
Study design
The technique of dually perfused placental lobule was used in its recirculating mode to determine the Maternal-to-Fetal (n=20) and Fetal-to-Maternal (n=18) transfer of each antibiotic which were co-perfused with their radioactive isotopes. The concentrations of drugs were determined by liquid scintillation spectrometry.
Results
In the Maternal-to-Fetal direction, the transfer of vancomycin (9.6 ± 4%) and telavancin (6.5 ± 2%) were low; however, telavancin retention by the perfused lobule was greater than that of vancomycin (p < 0.01). The normalized transplacental transfer of telavancin across the placental lobule in the F→M direction was higher than in the M→F direction (p < 0.01), suggesting the involvement of placental efflux transporters.
Conclusions
The ex vivo perfusion experiments revealed low transfer of vancomycin and telavancin to the fetal circuit.
Keywords: Methicillin-resistant S. aureus, pregnancy, telavancin, transplacental transfer, vancomycin
INTRODUCTION
Methicillin-resistant S. aureus (MRSA) is an emerging problem in women during pregnancy and the postpartum period. Between 2000 and 2004, the rate of MRSA infections in pregnant women increased 10-fold1. Postpartum infections due to MRSA are often serious and potentially life-threatening,2,3 and until recently, vancomycin has been the antibiotic of choice for treatment. However, the development of vancomycin-resistant strains of Staphylococcus aureus (VRSA) have compromised its use4,5,6 and led to the development of its derivative, telavancin.
Telavancin is a lipoglycopeptide antibiotic with improved activity against a wide range of gram-positive organisms with reduced susceptibility to vancomycin7,8,9,10. Telavancin has at least two mechanisms of action. The first, similar to vancomycin, is inhibition of late-stage peptidoglycan synthesis by binding to the D-ala-D-ala terminus of the peptidoglycan intermediate, thus preventing cross-linking of the cell wall. The second mechanism of action of telavancin increases the permeability of bacterial cell membranes11. Owing to telavancin’s multifunctional mechanism of action, it has enhanced (over vancomycin) bactericidal activity, activity against bacterial strains with reduced susceptibility to vancomycin, and lower frequency of resistance. However, due to the limited data on the adverse effects of telavancin to the fetus as well as its pharmacokinetics during pregnancy, it is designated as a Class B drug and is currently not approved by the FDA for treatment of pregnant patients. Therefore, additional preclinical and clinical information on its effectiveness and safety during pregnancy is required. The first step in obtaining this information is to determine the role of human placenta in the disposition of telavancin and, consequently, its concentration in the fetal circulation.
Vancomycin (1485 Da) and telavancin (1792 Da) have higher molecular weights than most medications. To the best of our knowledge, transplacental transfer of telavancin has not been reported. On the other hand, transplacental transfer of vancomycin has been investigated by utilizing the technique of dual perfusion of placental lobule (DPPL)12 and in vivo13,14. The transplacental passage of vancomycin was observed in women with amnionitis13 as well as in non-infected pregnant women14. The data obtained from in vivo investigations demonstrated that vancomycin readily crosses the infected (second and third trimesters) and non-infected (term) placentas and suggested that intrauterine exposure of the fetus to vancomycin is possible. However, these data did not agree with those obtained from ex vivo experiments indicating that vancomycin was not transferred across placenta. The observed difference between the in vivo and in vitro/ex vivo data was explained by the formation of a heparin-vancomycin complex in the perfusion medium that prevented its transfer from the maternal to fetal circuit12. To clarify this controversial data, it was imperative to determine the extent of transplacental transfer of vancomycin and telavancin as well as their retention by placental tissue.
Therefore, the aim of this investigation was to determine the bidirectional transfer of vancomycin and telavancin and the distribution of these drugs in placental tissue using the dually perfused placental lobule. This technique retains the anatomic and functional integrity of placental tissue and has been validated for determining the bidirectional transfer, distribution, and metabolism of numerous drugs.
MATERIALS AND METHODS
Chemicals
[3H]-vancomycin (specific activity, 4.2 Ci/mmol) and [3H]-telavancin (specific activity, 16.3 Ci/mmol) were purchased from Vitrax Co (Placentia, CA). HPLC grade methanol, acetonitrile, sodium acetate and hydrocloric acid were obtained from Fisher Scientific (New Jersey). Telavancin was obtained from UTMB pharmacy as Vibativ™ (marketed by Astellas Pharma US, Inc.). Paclitaxel [o-benzamido-3H] (38 Ci/mmol) was purchased from Moravek Biochemicals, Inc. (Brea, CA). All other chemicals including radioactive [14C]-antipyrine (specific activity, 6.5 mCi/mmol), vancomycin hydrochloride were purchased from Sigma-Aldrich (St. Louis, Missouri).
Clinical material
Placentas from uncomplicated term (37 – 42 weeks) pregnancies (n=38) were obtained immediately following vaginal or abdominal deliveries at the Labor and Delivery Ward of the John Sealy Hospital at the University of Texas Medical Branch, Galveston, Texas, according to a protocol approved by the Institutional Review Board. Any evidence of maternal infection, systemic disease, and drug or alcohol abuse during pregnancy excluded the placenta from this investigation.
Dual Perfusion of Human Placental Lobule (DPPL)
The technique of DPPL was used as described in detail in reports from our laboratory15 and originally by Miller et al16. Briefly, each placenta was examined for tears and 2 chorionic vessels (one artery and one vein) supplying a single intact peripheral cotyledon were cannulated with 3F and 5F umbilical catheters, respectively. The cotyledon was trimmed and placed in the perfusion chamber with the maternal surface upward. The intervillous space on the maternal side was perfused by 2 catheters piercing the basal plate. The flow rate of the medium in the fetal and maternal circuits was 2.8 and 12 mL/min, respectively. The perfusion medium was made of tissue culture medium M199 (Sigma, St. Louis, MO) supplemented with: Dextran 40 (7.5 g/L in the maternal and 30 g/L in the fetal reservoir), 25 IU/mL heparin, 40 mg/L gentamicin sulfate, 80 mg/L sulfamethoxazole, and 16 mg/L trimethoprim. The maternal perfusate was equilibrated with a gas mixture made of 95% O2, 5% CO2, and the fetal perfusate with a mixture of 95% N2, 5% CO2. Sodium bicarbonate was added to the maternal and fetal circuits to maintain the pH at 7.4 and 7.35, respectively. All experiments were carried out at a temperature of 37° C.
Each placenta was perfused for an initial control period of one hour to allow the tissue to stabilize to its new environment. Perfusion was terminated if one of the following occurred: fetal arterial pressure exceeded 50 mmHg, a volume loss in fetal circuit in excess of 2 mL/h, or a pO2 difference between fetal vein and artery less than 60 mmHg, indicating inadequate perfusion overlap between the two circuits.
Transplacental transfer and distribution of vancomycin and telavancin
Following the control period, the maternal and fetal perfusates were replaced with fresh medium. Human serum albumin was added to both the maternal and fetal circuits in its physiological concentrations of 30 mg/mL17. The non-ionizable, lipophilic marker compound antipyrine (AP) 20 μg/mL and its [14C]-isotope (1.5 μCi) were co-transfused with vancomycin or telavancin to account for interplacental variations and to normalize the transfer of antibiotics.
AP and one of the antibiotics were added to either the maternal or fetal reservoir accordingly to the transfer direction investigated, from the maternal to fetal (M→F) or fetal to maternal (F→M), respectively. The initial concentration of vancomycin and telavancin in the donor circuit was 25 μg/mL, which corresponds to the therapeutic level of the antibiotics in patients’ plasma.
The perfusion system was used in its closed-closed configuration (re-circulation of the medium). The concentrations of the compounds were determined in 0.5 mL aliquots taken from the maternal and fetal arteries and veins at 0, 5, 10, 15, 30, 45, 60, 90, 120, 150, 180, 210 and 240 minutes. The amounts of [3H] and [14C] radioactivity in the maternal and fetal perfusates were determined simultaneously by liquid scintillation spectrometry using (1900TR; Packard Instruments, Inc, Shelton, CT). The concentration of vancomycin or telavancin in all samples was calculated after correcting for the specific activity as previously reported15.
At the end of each experiment, the perfused area of the tissue was dissected, weighed, and homogenized in a volume of saline equal to four times its weight. 1 mL of 1M NaOH was added to 1 mL of the homogenate and the samples were incubated for 12 hours at 60 °C in the dark to allow for luminescence decay. Scintillation cocktail was added to each sample and the concentration of each drug was determined.
Effect of telavancin on the uptake of [3H]-paclitaxel by the membrane vesicles
The method for preparing inside out vesicles (IOV) from placental brush border membranes is that originally described by Ushigome et al19 and modified in our laboratory as previously reported18. The effect of a range of telavancin concentrations (0–300 μM) on the uptake of [3H]-paclitaxel by IOV was determined in 140 μL of Uptake buffer (40 mM MOPs, 70 mM KCl, 10 mM MgCl2, pH 7.4) containing 40 μg of membrane protein, and 40 nM of [3H]-paclitaxel. The reaction was initiated by the addition of ATP, and AMP was used for control. The reaction was terminated after 1 min by the addition of 3 mL ice cold buffer followed by rapid filtration (Brandel Cell Harvester) to isolate the vesicles. The amount of [3H]-paclitaxel retained on the filter was determined by liquid scintillation analysis. Active uptake was calculated as the difference in the amount of [3H]-paclitaxel in the presence of ATP and AMP and expressed as pmol.mgprotein−1min−1. The IC50 was determined from the plots of the percent of the [3H]-paclitaxel uptake versus the log of telavancin concentration.
Data and statistical analysis
Statistical analyses were performed using GraphPad Prism © version 5.01. All reported values are expressed as mean ± S.D. Statistical significance of the differences observed between groups were calculated by the Mann-Whitney U-test and considered significant when P <0.05.
RESULTS
Bidirectional transfer of antipyrine
The bidirectional transfer of vancomycin and telavancin across term human placentas was normalized to the transfer of the highly diffusible marker compound antipyrine. The transfer of antipyrine co-perfused with vancomycin in the M→F and F→M directions did not differ from its transfer when co-perfused with telavancin. These data indicate that irrespective of direction, transplacental transfer of the two antibiotics was studied under the same experimental conditions, i.e the efficiency of the exchange between maternal and fetal circuits and vice versa was identical for vancomycin and telavancin.
Maternal-to-Fetal transfer of vancomycin and telavancin
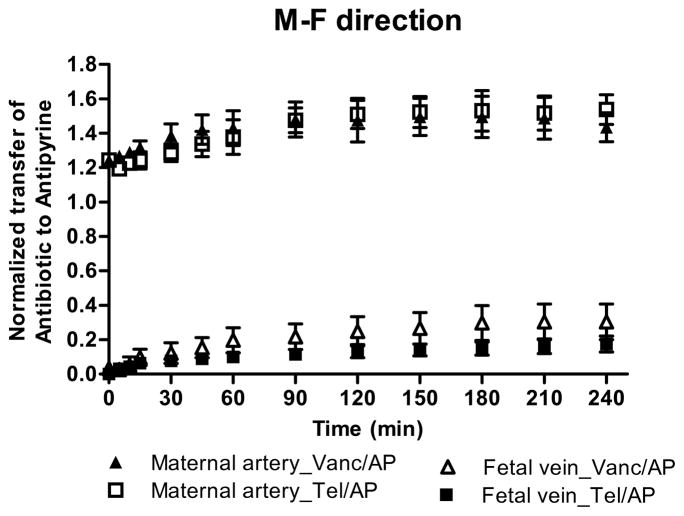
Both antibiotics crossed the placenta and appeared in the fetal circuit within the first five minutes of perfusion. The concentration of vancomycin in the fetal circuit at the end of the experiment reached 2.4 ± 0.9 μg/mL and accounted for 9.6 ± 4% of its initial concentration in the maternal circuit. However, under the same experimental conditions the transfer of telavancin to the fetal circuit was lower (1.6 ± 0.3 μg/mL, 6.5 ± 2% of its initial concentration). The lower transfer of telavancin to the fetal circuit was also confirmed after transfer of each antibiotic was normalized to that of the marker compound antipyrine (Figure 1A). The normalized transfer of telavancin to the fetal circuit 0.14 ± 0.04 was significantly lower than normalized transfer of vancomycin 0.23 ± 0.08 (p<0.01). Furthermore, at the end of the experimental period the retention of telavancin by the perfused lobule (11 ± 1 μg/g) was higher than the retention of vancomycin (7.5 ± 1 μg/g, p < 0.001).
Figure 1. A&B. Normalized transfer of vancomycin and telavancin across human placenta.
The M→F (A) transfer of vancomycin (n=10) and telavancin (n=10) and F→M (B) transfer of vancomycin (n=9) and telavancin (n=9) were normalized to the transfer of marker compound AP. In the M→F direction the normalized telavancin transfer to the fetal circuit was significantly lower than the normalized transfer of vancomycin, p<0.01. The difference between the normalized transfer of the antibiotics in the F→M direction did not reach statistical significance.
Taken together, these data indicate low transfer of vancomycin and telavancin to the fetal circuit and that the placental tissue retained 30% more of telavancin than vancomycin.
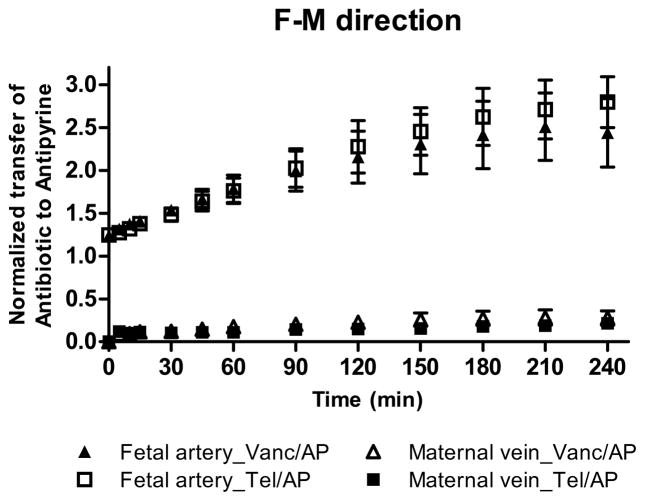
Fetal-to-Maternal transfer of vancomycin and telavancin
At the end of drug perfusion in the F→M direction, the concentration of vancomycin in the recipient maternal circuit was 1.4 ± 0.5 μg/mL and did not differ from the concentration of telavancin 1.2 ± 0.2 μg/mL. Moreover, the difference between the normalized transfer of vancomycin (0.21 ± 0.08) and telavancin (0.17 ± 0.03) was not significant (Figure 1B). On the other hand, at the end of the experimental period in the F→M direction, the retention of telavancin by the tissue (5.2 ± 1.2 μg/g) was significantly higher than that for vancomycin (3.4 ± 0.7 μg/g, p<0.01).
Maternal-to-Fetal vs Fetal-to-Maternal transfer of vancomycin and telavancin
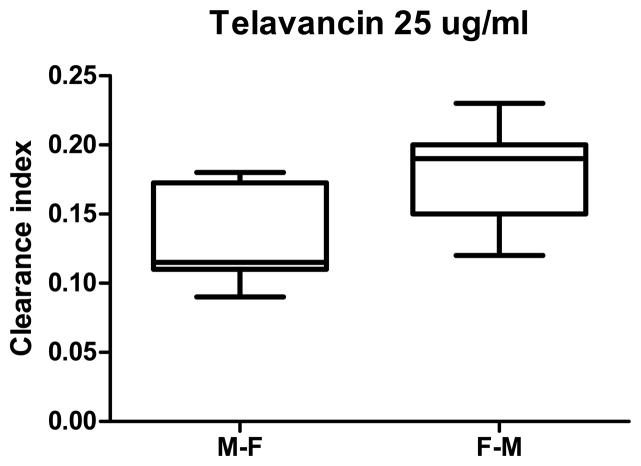
Although, the normalized transplacental transfer of vancomycin in F→M direction did not differ from its transfer in the M→F direction, telavancin transfer in the F→M direction was higher than in the M→F direction, p< 0.01 (Figure 2).
Figure 2. Maternal-to-Fetal and Fetal-to-Maternal transfer of telavancin.
The normalized transplacental transfer of telavancin across perfused human placental lobule in the F→M direction was higher than in the M→F direction, p< 0.01.
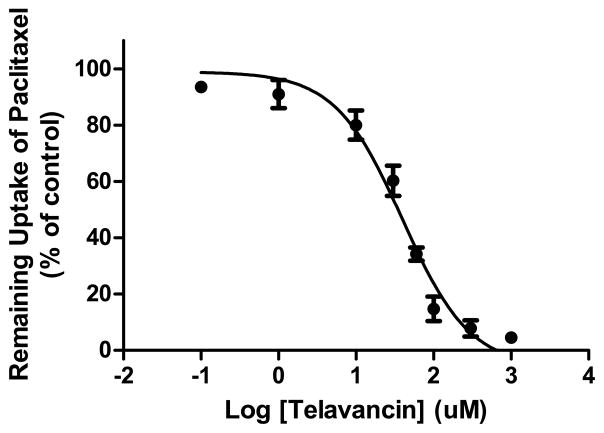
Furthermore, telavancin inhibited the ATP-dependent uptake of [3H]-paclitaxel (Figure 3). The IC50 value determined for telavancin inhibition of ATP-dependent uptake of [3H]-paclitaxel by IOV was 50 μM while vancomycin did not affect the uptake of [3H]-paclitaxel in the concentration range tested (100 to 1000 μM).
Figure 3. Inhibition of ATP-dependent transport of [3H]-paclitaxel in placental IOV by telavancin.
Telavancin inhibited ATP-dependent uptake of paclitaxel (40 nM) with the IC50 values of 50 μM. Each point represents the mean ± S.D. from 4 experiments in triplicate.
DISCUSSION
The aim of this investigation was to determine the bidirectional transfer of vancomycin and telavancin across the dually perfused term human placental lobule and their distribution between the tissue and the two circuits.
The bidirectional transfer (M→F and F→M) of vancomycin and telavancin was studied in the presence of the lipophilic and highly diffusible marker compound antipyrine (AP), which was co-perfused with each antibiotic. The transfer of AP across the placental lobule in the current investigation agrees with results previously reported from our laboratory15,20,21. In the ex vivo dual perfusion system, the extent of transfer depends on the placement of the maternal catheters opposite to the vessels on the fetal side of the lobule. In order to minimize the variability, the transfer of vancomycin and telavancin across term human placentas was normalized to the transfer of AP22. The effect of experimental conditions on the extent of the antibiotics’ transfer was minimal because the transfer of AP co-perfused with vancomycin was similar to the transfer of AP co-perfused with telavancin.
The data obtained in this investigation revealed that both antibiotics rapidly cross the perfused lobule and appear in the opposite circuit. However, in the M→F direction, the extent of the transfer of the high molecular weight antibiotics vancomycin (1485 Da, 9.6 ± 4% transfer) and telavancin (1792 Da, 6.5 ± 2% transfer) was low compared to the transfer of the marker compound AP, (molecular weight 188 Da, 44 ± 2% transfer). Furthermore, the more lipophilic nature of telavancin and its higher binding to proteins (93%) compared to vancomycin (50%)23 could explain the significantly higher (p < 0.01) retention of telavancin by the perfused lobule and its reduced transfer to the fetal circuit.
In the F→M direction (Figure 1B), the transfer of telavancin (5 ± 1% of its initial concentration in the fetal/donor circuit) to the maternal circuit did not differ from that for vancomycin (5 ± 2%). Furthermore, the normalized transfer of telavancin in the F→M direction was significantly higher than in the M→F direction (p < 0.01, Figure 2), suggesting that one or more placental efflux transporters could be involved in the transfer of telavancin across the placenta.
Telavancin was previously reported as an inhibitor of P-glycoprotein (P-gp)-mediated digoxin transport24. Data obtained in this investigation revealed that telavancin inhibited the ATP-dependent uptake of [3H]-paclitaxel (a well-characterized substrate of P-gp) by IOV prepared from placental brush border membranes. The IC50 value was 50 μM (Figure 3), which suggests that telavancin may be a substrate of placental P-gp. The involvement of other efflux transporters cannot be ruled out at this time and will require further investigations.
In summary, the data obtained in this investigation revealed low bidirectional transfer of vancomycin and telavancin across the dually perfused placental lobule. However, the interaction of telavancin with placental efflux transporters—together with its high binding to plasma proteins, especially albumin—might further decrease its transfer to the fetal circuit as compared to vancomycin.
Acknowledgments
The authors appreciate the support of the physicians and nurses of the Labor & Delivery Ward of the John Sealy Hospital, the teaching hospital at UTMB, Galveston, Texas, and the Perinatal Research Division of the Department of Obstetrics & Gynecology. The authors appreciate the Publication, Grant, & Media Support area of the Department of Obstetrics & Gynecology. This work was supported by the Obstetric Pharmacology Research Units (OPRU) network (NICHD U10 HD047891).
Footnotes
Disclosure: All authors do not have any conflict of interest
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laibl VR, Sheffield JS, Roberts S, McIntire DD, Trevino S, Wendel GD., Jr Clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus in pregnancy. Obstet Gynecol. 2005 Sep;106(3):461–465. doi: 10.1097/01.AOG.0000175142.79347.12. [DOI] [PubMed] [Google Scholar]
- 2.Stumpf PG, Flores M, Murillo J. Serious postpartum infection due to MRSA in an asymptomatic carrier: case report and review. Am J Perinatol. 2008 Aug;25(7):413–415. doi: 10.1055/s-2008-1075032. [DOI] [PubMed] [Google Scholar]
- 3.Sokolov KM, Kreye E, Miller LG, Choi C, Tang AW. Postpartum iliopsoas pyomyositis due to community-acquired methicillin-resistant Staphylococcus aureus. Obstet Gynecol. 2007 Aug;110(2 Pt 2):535–8. doi: 10.1097/01.AOG.0000269142.19323.88. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Staphylococcus aureus resistant to vancomycin. Center for Disease Control and Prevention. United State, 2002 MMWR Morb Mortal Wkly Rep. 2002;38:521–8. [Google Scholar]
- 6.Howden BP, Ward PB, Charles PG, et al. Treatment outcomes for serious infections caused by methicillin-resistant Stapylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis. 2004;38:4480–451. doi: 10.1086/381202. [DOI] [PubMed] [Google Scholar]
- 7.Leuthner KD, Cheung CM, Rybak MJ. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide nonsusceptible staphylococci and three vancomycin-resistant Stapylococcus aureus. J Antimicrob Chemother. 2006;58:338–343. doi: 10.1093/jac/dkl235. [DOI] [PubMed] [Google Scholar]
- 8.King A, Phillips I, Kaniga K. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration dependent antiinfective with multiple mechanism of action against gram-positive bacteria. J Antimicrob Chemother. 2004;53:797–803. doi: 10.1093/jac/dkh156. [DOI] [PubMed] [Google Scholar]
- 9.Saravolatz LD, Pawlak J, Johnson LB. Comparative activity of telavancin agains isolates of community-associated methicillin-resistant Stapylococcus aureus. J Antimicrob Chemother. 2007;60:406–409. doi: 10.1093/jac/dkm211. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein EJC, Citron DM, Merriam CV, Warren YA, Tyrrell KL, Fernandez HT. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents anaerobic gram-positive species and Corynebacterium spp. Antimicrob Agents Chemother. 2004;48:2149–2152. doi: 10.1128/AAC.48.6.2149-2152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Stapylococcus aureus. Antimicrob Chemother. 2005;29:1127–1134. doi: 10.1128/AAC.49.3.1127-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hnat MD, Gainer J, Bawdon RE, Wendel GD. Transplacental passage of vancomycin in the ex vivo human perfusion model. Infect Dis Obstet Gynecol. 2004;12:57–61. doi: 10.1080/10647440400009821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourget P, Fernandez H, Delouris C, Ribou F. Transplacental passage of vancomycin during the second trimester of pregnancy. Obstet Gynecol. 1991;78:908–911. [PubMed] [Google Scholar]
- 14.Laiprasert J, Klein K, Mueller BA, Pearlman MD. Transplacental passage of vancomycin in noninfected term pregnant women. Obstet & Gynecol. 2007;109:1105–1110. doi: 10.1097/01.AOG.0000260388.78339.b6. [DOI] [PubMed] [Google Scholar]
- 15.Nanovskaya T, Deshmukh S, Brooks M, Ahmed M. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 16.Miller R, Wier P, Maulik D, Di Sant’Agnese P. Human placenta in vitro: characterization during 12 hours of dual perfusion. Contrib Gynecol Obstet. 1985;13:77–84. [PubMed] [Google Scholar]
- 17.Krauer B, Dayer P, Anner R. Changes in serum albumin and alpha acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs. BJOG. 1984;91(9):875–881. doi: 10.1111/j.1471-0528.1984.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 18.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, Patrikeeva SL, Hankins GD, Ahmed MS. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010 Mar 15;79(6):921–5. doi: 10.1016/j.bcp.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ushigome F, Koyabu N, Satoh S, Tsukimori K, Nakano H, Nakamura T, Uchiumi T, Kuwano M, Ohtani H, Sawada Y. Kinetic analysis of P-glycoprotein-mediated transport by using normal human placental brush-border membrane vesicles. Pharm Res. 2003 Jan;20(1):38–44. doi: 10.1023/a:1022290523347. [DOI] [PubMed] [Google Scholar]
- 20.Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, Hankins GD, Ahmed MS. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006 Oct;195(4):1081–5. doi: 10.1016/j.ajog.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL, Hankins GD, Ahmed MS. Bidirectional transfer of methadone across human placenta. Biochem Pharmacol. 2005 Jan 1;69(1):187–97. doi: 10.1016/j.bcp.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Schneider H. Techniques: in vitro perfusion of human placenta. In: Sastry B, editor. Placental toxicology. Florida: CRC press; 1995. pp. 1–26. [Google Scholar]
- 23.Shaw JP, Seroogy J, Kaniga K, Higgins DL, Kitt M, Barriere S. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother. 2005 Jan;49(1):195–201. doi: 10.1128/AAC.49.1.195-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vibativ. Assessment report. European Medicines Agency; United Kingdom: 2011. Procedure No:EMEA/H/C/1240. [Google Scholar]