Abstract
Objective
To assess the impact on absolute values and reproducibility of adding portal venous (PV) to arterial input functions in computed tomography perfusion (CTp) evaluations of liver tumors and normal liver.
Methods
Institutional review board approval and written informed consent were obtained; the study complied with HIPAA regulations. CTp source datasets, obtained from seven patients (containing 9 liver tumors) on two occasions, 2-7 days apart, were analyzed by deconvolution modeling using dual (“Liver” protocol, PV and aorta) and single (“Body” protocol, aorta only) vascular inputs. Identical tumor, normal liver, aortic and, where applicable, PV ROIs were used in corresponding analyses to generate tissue blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability (PS) values. Test-retest variability was assessed by within-patient coefficients of variation (wCV).
Results
For liver tumor and normal liver, median BF, BV and PS were significantly higher for the Liver protocol than Body protocol: 171.3-177.8 vs. 39.4-42.0 mL/min/100g, 17.2-18.7 vs. 3.1-4.2 mL/100g, 65.1-78.9 vs. 50.4- 66.1 mL/min/100g, respectively (all p<0.01). There were no differences in MTT between protocols. wCVs were lower for all parameters with the Liver protocol than Body protocol: BF, 7.5-11.2% vs 11.7-20.8%; BV, 10.1-14.4% vs. 16.6-30.1%; MTT, 4.2-5.5% vs. 10.4-12.9%; and PS, 7.3-12.1% vs. 12.6-20.3%, respectively.
Conclusion
Utilization of dual vascular input CTp liver analyses has substantial impact on absolute CTp parameter values and test-retest variability. Incorporation of the portal venous inputs may yield more precise results, however, it imposes substantial practical constraints on acquiring the necessary data.
INTRODUCTION
Computed tomography perfusion (CTp) is being increasingly explored in a number of oncologic body applications. Applications include evaluations of treatment response, prognostication, and characterization of tumors [1-4]. The attractions of CTp in these contexts include its noninvasive and quantitative capabilities.
The liver is an important organ for oncologic evaluations because it is a very common site for both primary and secondary tumors [5]. Hepatocellular carcinoma is one of the most common malignancies worldwide and is increasingly prevalent. Both primary and secondary tumors are being increasingly treated by targeted anti-vascular therapies [6, 7]. These agents are typically cytostatic rather than cytotoxic and therapeutic efficacy may be reflected more by changes in perfusion than by changes in size [8].
CTp evaluation of the liver however is challenging because of its intrinsic dual blood supply, from both the arterial and portal circulation. Not only is the necessary physiological modeling challenging, but the need to acquire both vascular inputs simultaneously imposes substantial constraints on image acquisition, the latter largely because of the limitations of z-axis coverage of currently available CT scanners.
The distributed parameter model and deconvolution is an approach to CTp analysis which allows quantitative characteristics of tissue perfusion, namely, blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability surface area product (PS) [9]. Software which handles dual vascular input deconvolution modeling is now commercially available (GE CT Perfusion 3, and higher versions, Milwaukee, WI, USA). Given that previous liver CTp studies using the distributed parameter model have utilized single vascular (arterial) inputs [10-13], it is important to gain an understanding of the impact that utilizing dual vascular inputs might have on that work, and indeed future work. Since quantification is a fundamental aspect of CTp, an evaluation of not only the absolute parameter values derived, but also their test-retest reproducibility is important.
Our objectives were to assess the effects on absolute values and reproducibility of utilizing dual vascular input (portal and arterial) compared to single (arterial) input functions in the analysis of CTp parameters in liver tumors and normal liver.
MATERIALS AND METHODS
Patients, Target Lesions, and CT Perfusion Scanning Technique
The prospective study that provided the patients for this study was approved by our institutional review board, and written informed consent had been obtained from all patients. The study complied with HIPAA regulations.
Details of the patient inclusion criteria have been presented previously [14]. In brief, patients with solid liver lesions larger than 2.5 cm in longest axial diameter were eligible for participation, and underwent CTp scanning 2-7 days apart without any intervening therapy, on a 16-row multidetector CT scanner (LightSpeed, GE Healthcare, Waukesha, WI), between June 2004 and May 2005. Data acquisition started 5 seconds after intravenous injection of 50 mL of a nonionic contrast agent (ioversol [Optiray], 320 mg of iodine/100 mL; Mallinckrodt, Inc., St. Louis, MO) using an automatic injector (MCT/MCT Plus; Medrad, Pittsburgh, PA) and an injection rate of 7 mL/second. Images were reconstructed every half second. The CTp scans were obtained in two phases: Phase 1, 30s cine acquisition during a breath-hold, followed 15 seconds later by Phase 2, which consisted of six further short cine scans at 18 second intervals acquired during free breathing (the final Phase 2 acquisition commencing 135s after the start of the Phase 1 acquisition).
The patient cohort in this study was the same as previously reported (14). In that study we described the CTp acquisition protocol, and compared the CTp parameter values derived from two acquisition durations and two image registration techniques using dual input CTp analyses. The results of that work suggested that the least variability in CTp values was derived from utilizing the full duration of acquired data and applying the image registration techniques described below. In the current work, we compare the effects of applying a single input CTp analytical approach to the same patient cohort and same dataset that yielded the smallest CTp parameter variability when using the dual input approach.
CT Perfusion analysis
The images were analyzed using CTp software on a workstation (CT Perfusion 4 version 4.3.1, Advantage Windows 4.4; GE Healthcare, Waukesha, WI). The algorithm uses a distributed parameter physiological model and deconvolution. Analyses were undertaken using two algorithms of the vendor software: the Liver protocol, which utilizes a dual (arterial and portal) vascular input; and the Body protocol, which utilizes a single (arterial) vascular input. For both analyses, arterial inputs were obtained from regions of interest (ROI) in the aorta on the source images, and for the Liver Protocol analysis, ROIs were included from the portal vein.
Before the CT perfusion analyses were undertaken, the Phase 1 and Phase 2 images of each patient dataset were anatomically registered, as previously described [15]. Liver tumor ROIs and normal liver ROIs were drawn as previously described [14]. In brief, tumor ROIs were drawn freehand on all (four) CT levels in which the tumor was adequately visualized. Two liver tissue ROIs were drawn in normal liver where possible. All ROIs were saved to enable identical analyses to be undertaken with the two above protocols, the only difference being that the portal vein input function was not utilized in the Body protocol analysis.
Mean BF, BV, MTT and PS parameter values were obtained from all (four) CT levels for tumor and normal liver using each of the two CTp protocols above (Fig. 1). For the Liver protocol analysis, hepatic arterial fraction (HAF) was also obtained; this is the fraction of total hepatic blood flow (arterial and portal) which is of hepatic arterial origin.
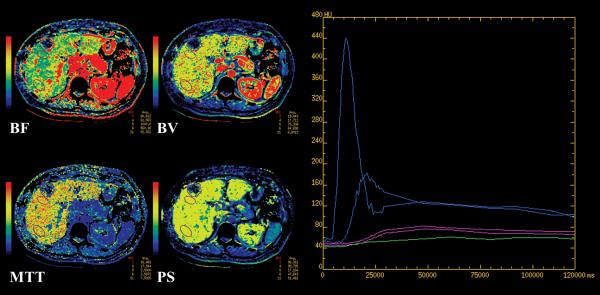
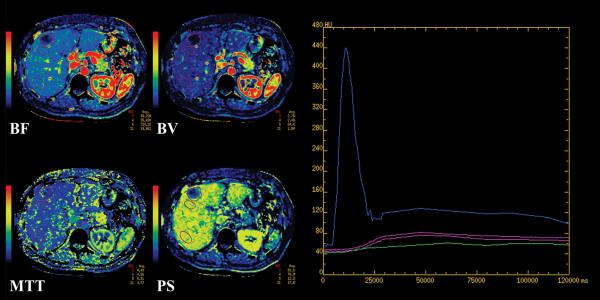
FIGURE 1.
CT perfusion maps for BF, BV, MTT and PS from a 60 year old woman with metastatic melanoma, using (a) Liver protocol and (b) Body protocol, with accompanying time attenuation curves.
Corresponding maps have the same window widths and levels. Purple outlines are tumor and normal liver ROIs. Curves in plots: blue, arterial and portal vein; purple, normal liver; green, tumor. The y-axis scale indicates CT density, in Hounsfield units, and the x-axis scale, time, in milliseconds.
Statistical Analysis
Summary statistics of the CTp parameters BF, BV, MTT, and PS were provided in the form of medians and ranges by method of analysis. We assumed that all CT parameters follow log-normal distributions, so they were transformed to the logarithmic scale for subsequent variance component analyses. A variance component analysis was performed to estimate the between- and within-patient variation for each parameter in each CTp analytical protocol. After each variance component analysis, the within-patient coefficient of variation (wCV) was calculated using the formula wCV = (ewSD – 1), in which the within-patient standard deviation (wSD) is the square root of the within-patient variation. The 95% confidence interval (CI) of the wCV was calculated on the basis of the CI of the wSD [16].
Comparisons between Body and Liver protocols with respect to CTp parameters were made by the paired t-test on the logarithmic scale. Statistical analysis was performed with SAS version 9 (SAS Institute, Cary, NC), and data plotting, with S-Plus 7 software (Insightful Inc., Seattle, WA).
RESULTS
Patients and Target Lesions
In total, 10 patients were enrolled. Two patients were excluded because of technical errors in CTp acquisition in one of their pairs of visits. One patient was excluded because a portal vein input could not be delineated. Two of the 7 remaining patients had two lesions each, resulting in 9 lesions with evaluable imaging data.
The median age of the 7 patients (4 men and 3 women) was 58 years (range, 47.9–72.2 years). The primary tumors were lung (2), melanoma (2), neuroendocrine (2) and sarcoma (1). The median interval between scan and re-scan visits was 2 days (range, 2–7 days).
The median longitudinal diameter of the tumors was 4.7 cm (range, 2.8–5.6 cm), and the median size of the tumor ROIs was 1468 mm2 (range, 575-2520 mm2). The median size of the normal liver ROIs was 450 mm2 (range, 153-595 mm2). The median size of the aortic and portal vein ROIs were 16.3 mm2 (range, 9.8-75.4 mm2) and 19.9 mm2 (range, 10.8-46.1 mm2), respectively.
CTp absolute values: Body vs Liver protocols
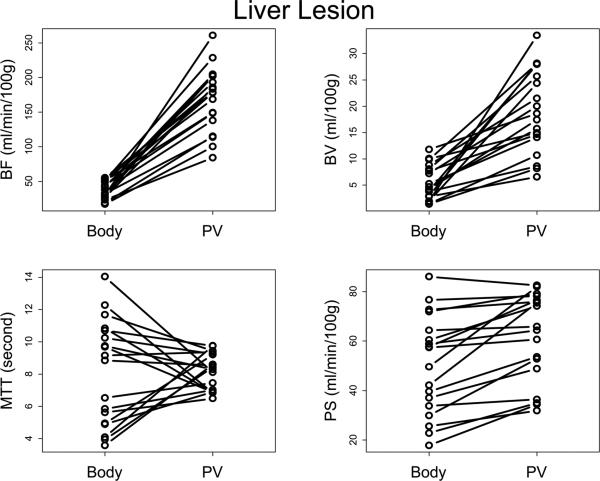
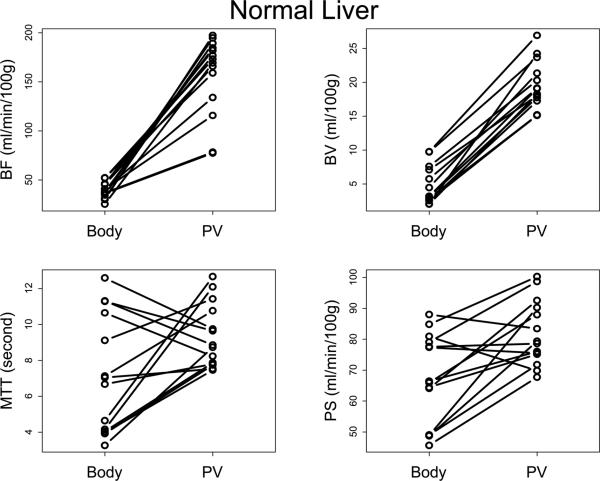
A summary of results is presented in Table 1. BF values for liver tumors and normal liver were significantly and invariably lower with the Body protocol compared to the Liver protocol (Figure 2, p<0.01), with median BF values approximately a quarter (23-24%) of those utilizing the Liver protocol: median BF values were 39.4-42.0 mL/min/100g compared to 171.3-177.8 mL/min/100g, respectively.
TABLE 1.
Perfusion Parameters and Variability: Body vs Liver protocol
| CTp Analytical Protocol | Tissue | Blood Flow | Blood Volume | Mean Transit Time | Permeability-Surface Area Product | Hepatic Arterial Fraction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR), mL/min/100g | wCV (95% CI), % | Median (IQR), mL/100g | wCV (95% CI), % | Median (IQR), seconds | wCV (95% CI), % | Median (IQR), mL/min/100g | wCV (95% CI), % | Median (IQR), unitless | wCV (95% CI), % | ||
| Body (arterial only) | Liver tumor | 42.0 (29.3-50.7) | 20.8 (10.7-31.8) | 4.2 (3.0-8.1) | 30.1 (15.2-46.9) | 9.0 (5.0-10.5) | 12.9 (6.7-19.4) | 50.4 (35.7-65.3) | 20.3 (10.5-31.0) | n/a | n/a |
| Normal Liver | 39.4 (34.8-45.6) | 11.7 (5.4-18.4) | 3.1 (2.5-7.1) | 16.6 (7.6-26.3) | 6.9 (4.0-10.6) | 10.4 (4.8-16.3) | 66.1 (49.0-79.1) | 12.6 (5.8-19.8) | n/a | n/a | |
| Liver (arterial + portal) | Liver tumor | 177.8 (148.5-192.4) | 11.2 (5.9-16.8) | 17.2 (13.4-25.7) | 14.4 (7.5-21.7) | 8.2 (7.2-9.2) | 5.5 (2.9-8.1) | 65.1 (45.5-77.3) | 12.1 (6.3-18.2) | 0.42 (0.34-0.54) | 21.9 (11.3-33.6) |
| Normal Liver | 171.3 (134.1-184.6) | 7.5 (3.5-11.6) | 18.7 (17.2-21.3) | 10.1 (4.7-15.8) | 8.8 (7.8-10.8) | 4.2 (2.0-6.5) | 78.9 (75.1-90.3) | 7.3 (3.4-11.4) | 0.17 (0.07-0.49) | 33.1 (14.6-54.6) | |
Note.—IQR = inter-quartile range; wCV = within-patient coefficient of variation; 95% CI = 95 percent confidence interval.
n/a = not applicable. Bottom two rows, as in reference 14.
FIGURE 2.
Plots of Body protocol vs Liver protocol for (a) liver tumor and (b) normal liver, by parameter.
Body=Body protocol; PV=Liver protocol.
Similarly, BV values for liver tumors and normal liver were significantly and invariably lower with the Body protocol compared to the Liver protocol (Figure 2, p<0.01), with median BV values approximately a fifth (17-24%) of those utilizing the Liver protocol: median BV values were 3.1-5.2 mL/100g compared to 17.2-18.7 mL/100g, respectively.
PS values were significantly, although not consistently, lower with the Body protocol compared to the Liver protocol (Figure 2, p<0.01), with median PS values approximately 77-84% of those when using the Liver protocol: median PS values were 50.4-66.1 mL/min/100g compared to 65.1-78.9 mL/min/100g, respectively.
There was no consistent pattern to the differences in MTT values between the two analytical protocols (Figure 2, p>0.02).
Our results did not support a linear correlation in the CTp values derived from the Liver versus Body protocols, with R2<0.82 for all four CTp parameters.
CTp reproducibility: Body vs Liver protocols
Test-retest variability in CTp parameter values for liver tumor and normal liver were consistently lower when using the Liver protocol compared to the Body protocol, with wCVs for BF, BV, MTT and PS of: BF, 7.5-11.2% vs 11.7-20.8%; BV, 10.1-14.4% vs. 16.6-30.1%; MTT, 4.2-5.5% vs. 10.4-12.9%; and PS, 7.3-12.1% vs. 12.6-20.3%, respectively (Table 1).
Hepatic arterial fraction
Using the liver protocol, median HAF values for normal liver and liver tumors were 17% and 42%, and corresponding wCVs were 33.1% and 21.9%, respectively (Table 1).
DISCUSSION
This work has been undertaken to assess the impact on CTp parameter values of utilizing a dual input approach in the analysis of perfusion in the liver, compared to a single arterial input, for which the necessary data and analysis is much easier to accomplish. Our results indicate that CTp values are markedly affected by the analytical algorithms that are utilized, specifically, Liver (dual vascular) versus Body (single vascular) protocols. Of note, tumor and normal liver BF, BV and PS values when utilizing the Body protocol were significantly lower than when using the Liver protocol: BF and BV values were consistently lower, approximately one quarter to one fifth of the values, and PS was generally lower. There was no consistent pattern for MTT values.
This was not, and was not intended to be, a validation study. The dual input deconvolution algorithm has been validated in a study of liver tumors, albeit in an animal model [17]. However, some qualitative comparisons related to liver parenchyma can be made with prior clinical studies in human subjects. When considering BF, in a study of patients with hepatocellular carcinoma Sahani et al. and Zhu et al. [10, 11] have reported BF in background (cirrhotic) liver, using a single vascular (arterial) input, of 14.9 ± 2.8 ml/100g/min. In a study comparing patients with cirrhosis and controls, using dual vascular inputs, Li et al. [18] have reported BF in normal liver of 90.8 ± 23.8 ml/100g/min. These BF values for background liver obtained from single and dual vascular inputs appear to be broadly similar to our results for normal liver of approximately 40 ml/100g/min for single vascular vs 170 ml/100g/min for dual vascular inputs. The extent to which the observed differences might be due to different study populations, acquisition techniques, and/or single vs dual vascular inputs is difficult to unravel. Caution should be used in interpreting these results for “normal” liver, since the livers in most of these studies were not entirely normal; they were associated with some other disease process. From a physiological point of view, some validation studies have indicated normal liver BFs to be in the range 52.8-122.6 ml/min/100g [19].
A requirement to include the portal venous input in the modeling algorithm imposes considerable constraints and limitations on the data acquisition. The difficulties arise because of the need to include the region of the hepatic hilum in order to image the portal vein and/or its major branches, together with the relatively narrow z-axis coverage of most currently available CT scanners. The need to include a portal venous input in CTp evaluations of the liver are not necessarily limiting when setting out to investigate parenchymal liver disease, for example, studies utilizing dual vascular input data have been undertaken to investigate the severity of chronic liver disease, the degree of fibrosis in chronic hepatitis C, and the effects of chemotherapy on liver perfusion [20-22].
However, when the goal might be to evaluate focal liver lesions, the challenges of obtaining adequate portal venous inputs for analysis are more pronounced. The portal vein and proximal branches, the latter of a large enough size to enable adequate and reliable ROIs for input functions, may not be adequately visualized in axial sections where the intended tumor(s) for evaluation are located. Such locations include the superior portions of the liver, where there is substantial tissue and where inevitably a substantial number of tumors are located, and the inferior aspects of the liver. A further difficulty is that evaluable portions of the portal venous structures may be located in a predominantly axial plane, which consequently run the risk of partial volume averaging effects and movement completely out of the plane of imaging. These challenges are exacerbated if z-axis coverage is limited e.g. to 2-4 cm. Petralia et al. [12] indicated that they did not incorporate portal venous input in their study of treatment effects in hepatomas in the liver because “it was not possible to reliably place an ROI on it, or on its main branches, in all patients”. In a study of 28 patients with 36 focal liver tumors, 9 patients (13 tumors) did not have evaluable portal vein inputs, which represents about one third of patients/lesions [23]. In our study, one patient had to be excluded because a portal vein could not be visualized, and another would have been excluded because of the same difficulty had it not been excluded for other reasons.
The potential attrition of evaluable patients for liver perfusion studies due to difficulties in obtaining portal venous inputs would be a practical argument for consideration of utilizing just single (arterial/aortic) inputs, which are visible in essentially all patients. This might be an attractive option if there were a linear association in CTp parameter values between these two algorithmic approaches. Our data, however, did not support a linear association in CTp parameter values between Liver and Body protocols; this might be anticipated given the highly complex relationships between tissue perfusion and vascular input functions, the latter increased by considerations of dual inputs. Although resultant absolute CTp parameter values using simply a single arterial input might not reflect true liver perfusion, for the purposes of monitoring treatment responses or of longitudinal/serial evaluations, assessment of relative changes, notably percentage changes, in CTp values may still be of some value.
Of interest, the test-retest variability in CTp values was lower when using the Liver protocol than when using the Body protocol. The test-retest reproducibility provides a measure of what observed changes in CTp values might constitute a significant change in a given patient, and it also provides data to allow design of adequately powered studies. It might be considered that the addition of the portal vein input function, with its inherent noise, might increase the overall uncertainties of the data and algorithmic processing. On the other hand, one might consider that the utilization of a more appropriate (dual input) algorithm for the liver, albeit introducing additional data with its own noise/variability, might inherently improve the overall estimation of parameters. It is also possible that hepatic perfusion truly varies, for example, for physiological and homeostatic reasons, and indeed that the ratio of arterial to portal flow may also vary. Our results indicated wCV for HAF of normal liver between test-retest visits of 33.1%. It is difficult to determine to what extent this might be due to true differences in HAF between visits or simply measurement error. It is possible, for example, that HAF may be genuinely quite variable (wCV, 33.1%), while overall liver BF may be relatively well maintained (wCV, 7.5%).
As noted above, tumor and normal liver BF and BV values utilizing the Body protocol were approximately one quarter to one fifth of those when utilizing the Liver protocol. The observed differences in CTp values between the two protocols may be largely due to the relative contributions to overall hepatic blood flow from the hepatic artery and portal vein, namely, approximately 20%:80%. In our study, the median hepatic arterial fraction for normal liver was 17%, which is thus in broad agreement. The hepatic arterial fraction for tumor was higher (42%), which is in concordance with the expectation that liver tumors receive a relatively greater arterial supply than their surrounding background liver [1]. Although dual input evaluations may yield more precise CTp values when assessing liver parenchyma, single vascular (arterial) input evaluations may be a reasonable practical compromise when setting out to assess liver tumors, particularly in the context of serial monitoring for perfusion changes.
It should be recognized that the distributed parameter model is one of a variety of other physiological models that attempt to describe hepatic perfusion, such as the dual-input single-compartment and the maximum slope models [24-26]. These face similar challenges in modeling hepatic perfusion because of the dual blood supply of the liver; for appropriate characterization of liver perfusion, they too require portal venous inputs. Of note, it has been shown that these 3 analytical methods and their associated CTp parameter results are not interchangeable [27].
We acknowledge and recognize several limitations in this work. First, the number of patients in our study was relatively small. Secondly, our z-axis cine scan range was limited to 20 mm. Regarding the reproducibility aspect of our study, we made a general assumption that the patients’ tumors did not change significantly within the scan–rescan interval of 2–7 days, and there were inevitably some subjective components in drawing of tumor ROIs and in defining the arterial and portal input functions between pairs of studies. However, at least in the evaluations of Liver vs Body protocols, we used identical ROIs and vascular input functions in these paired comparisons. Our work did not incorporate validation of the CTp values obtained; this would be a major undertaking and would require a separate study.
Although it might be desirable to utilize the additional vascular input of the portal vein in evaluation of liver perfusion parameters, there are practical issues that need to be considered. Some of the current practical difficulties may be partially alleviated by increasing the z-axis footprint of the cine scans, but this would be at the undesirable expense of an increase in radiation dose.
In conclusion, liver CTp parameters, in particular BF and BV, are markedly affected by whether the dual vascular supply of the liver is taken into consideration. Their test-retest reproducibility is also adversely affected by utilizing just single, rather than dual, input functions. Although including portal venous input into that of the arterial input in analysis of CTp parameters in the liver is likely more reliable, inclusion of the former imposes challenges on practical aspects of acquiring that data. It is possible that dual input modeling may not be mandatory in serial (baseline and follow-up) studies which set out to evaluate relative changes in CTp values, particularly for tumors. Further work is required to assess this arguably more pragmatic approach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Miles KA, Charnsangavej C, Lee FT, et al. Application of CT in the investigation of angiogenesis in oncology. Acad Radiol. 2000;7:840–850. doi: 10.1016/s1076-6332(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 2.Miles KA. Functional computed tomography in oncology. Eur J Cancer. 2002;38:2079–2084. doi: 10.1016/s0959-8049(02)00386-6. [DOI] [PubMed] [Google Scholar]
- 3.Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2003;76:220–231. doi: 10.1259/bjr/13564625. [DOI] [PubMed] [Google Scholar]
- 4.Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47:161–178. doi: 10.1016/j.rcl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Husband J, Reznek RH. Husband and Reznek's Imaging in Oncology. 3rd ed. Informa Healthcare; London: 2010. [Google Scholar]
- 6.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 7.Geva R, Prenen H, Topal B, et al. Biologic modulation of chemotherapy in patients with hepatic colorectal metastases: the role of anti-VEGF and anti-EGFR antibodies. J Surg Oncol. 2010;102:937–945. doi: 10.1002/jso.21760. [DOI] [PubMed] [Google Scholar]
- 8.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 9.St Lawrence KS, Lee TY. An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: II. Experimental validation. J Cereb Blood Flow Metab. 1998;18:1378–1385. doi: 10.1097/00004647-199812000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Sahani DV, Holalkere N-S, Mueller PR, et al. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 11.Zhu AX, Holalkere NS, Muzikansky A, et al. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–125. doi: 10.1634/theoncologist.2007-0174. [DOI] [PubMed] [Google Scholar]
- 12.Petralia G, Fazio N, Bonello L, et al. Perfusion computed tomography in patients with hepatocellular carcinoma treated with thalidomide: initial experience. J Comput Assist Tomogr. 2011;35:195–201. doi: 10.1097/RCT.0b013e31820ccf51. [DOI] [PubMed] [Google Scholar]
- 13.Ng CS, Charnsangavej C, Wei W, et al. Perfusion CT findings in patients with metastatic carcinoid tumors undergoing bevacizumab and interferon therapy. AJR. 2011;196:569–576. doi: 10.2214/AJR.10.4455. [DOI] [PubMed] [Google Scholar]
- 14.Ng CS, Chandler AG, Wei W, et al. Reproducibility of CT Perfusion Parameters in Liver Tumors and Normal Liver. Radiology. 2011;260:762–770. doi: 10.1148/radiol.11110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler A, Wei W, Herron DH, et al. Semiautomated motion correction of tumors in lung CT-perfusion studies. Acad Radiol. 2011;18:286–293. doi: 10.1016/j.acra.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313:106. doi: 10.1136/bmj.313.7049.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart EE, Chen X, Hadway J, et al. Hepatic perfusion in a tumor model using DCE CT: an accuracy and precision study. Phys Med Biol. 2008;53:4249–4267. doi: 10.1088/0031-9155/53/16/003. [DOI] [PubMed] [Google Scholar]
- 18.Li JP, Zhao DL, Jiang HJ, et al. Assessment of tumor vascularization with functional computed tomography perfusion imaging in patients with cirrhotic liver disease. Hepatobiliary Pancreat Dis Int. 2011;10:43–49. doi: 10.1016/s1499-3872(11)60006-4. [DOI] [PubMed] [Google Scholar]
- 19.Sherriff SB, Smart RC, Taylor I. Clinical study of liver blood flow in man measured by 133Xe clearance after portal vein injection. Gut. 1977;18:1027–1031. doi: 10.1136/gut.18.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Beers BE, Leconte I, Materne R, et al. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR. 2001;176:667–673. doi: 10.2214/ajr.176.3.1760667. [DOI] [PubMed] [Google Scholar]
- 21.Ronot M, Asselah T, Paradis V, et al. Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion CT. Radiology. 2010;256:135–142. doi: 10.1148/radiol.10091295. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Yuan ZG, Wang DQ, et al. Perfusion CT findings in liver of patients with tumor during chemotherapy. World J Gastroentero.l. 2010;16:3202–3205. doi: 10.3748/wjg.v16.i25.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsushima Y, Funabasama S, Aoki J, et al. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol. 2004;11:215–223. doi: 10.1016/s1076-6332(03)00578-6. [DOI] [PubMed] [Google Scholar]
- 24.Materne R, Van Beers BE, Smith AM, et al. Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond.) 2000;99:517–525. [PubMed] [Google Scholar]
- 25.Ippolito D, Sironi S, Pozzi M, et al. Hepatocellular carcinoma in cirrhotic liver disease: functional computed tomography with perfusion imaging in the assessment of tumor vascularization. Acad Radiol. 2008;15:919–927. doi: 10.1016/j.acra.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Meijerink MR, van Waesberghe JH, van Schaik C, et al. Perfusion CT and US of colorectal cancer liver metastases: a correlative study of two dynamic imaging modalities. Ultrasound Med Biol. 2010;36:1626–1636. doi: 10.1016/j.ultrasmedbio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Kanda T, Yoshikawa T, Ohno Y, et al. CT hepatic perfusion measurement: Comparison of three analytic methods. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.07.003. 10.1016/j.ejrad.2011.07.003. [DOI] [PubMed] [Google Scholar]