Abstract
The increasing rate of obesity worldwide is predicted to be associated with a surge in diseases. Notably, obesity has been linked to approximately 20% of cancer cases in the United States; obesity is associated with both increased risk and worse outcomes after diagnosis. Altered levels of circulating factors are strongly implicated including insulin, insulin-like growth factor 1, leptin, adiponectin and interleukin-6 (IL-6). Additionally, increasing attention has focused on the consequences of local adipose inflammation. Inflammatory foci characterized by crown-like structures consisting of dead adipocytes encircled by macrophages occur in white adipose depots, including the breast tissue, of most overweight and obese women. Saturated fatty acids, released as a consequence of obesity-associated lipolysis, induce macrophage activation via Toll-like receptor 4, thereby stimulating NFκB signaling. This, in turn, activates transcription of proinflammatory genes including cyclooxygenase-2, IL-6, IL-1β, and tumor necrosis factor α. Elevated levels of proinflammatory mediators cause both local and systemic effects. Of particular relevance with regard to breast cancer is increased transcription of the CYP19 gene encoding aromatase, the rate-limiting enzyme for estrogen synthesis. Notably, this obesity-inflammation-aromatase axis provides a plausible explanation for increased rates of post-menopausal, hormone receptor-positive breast cancer associated with obesity and hence may offer targets for interventions to attenuate risk or improve prognosis. Potential approaches include weight reduction, exercise, and suppression of obesity-driven signaling pathways using pharmaceutical or dietary agents. A key future goal is to identify biomarkers that accurately report adipose inflammation, both for identification of at-risk individuals and to assess the efficacy of interventions.
Background
Using the conventional definition for obesity of body mass index (BMI) ≥30 kg/m2 [(weight in kg)/ (height in m)2], it was recently estimated that over 500 million adults worldwide are obese, and almost twice that number are overweight (defined as BMI 25.0–29.9) (1). Recent decades have witnessed a steady rise in both absolute numbers and the proportion of obese individuals. Because obesity is a key driver of diseases such as type II diabetes, cardiovascular disease and cancer, its rising incidence has profound clinical implications. Excessive adiposity is specifically associated with increased risk of multiple malignancies including non-Hodgkins lymphoma, esophageal adenocarcinoma, and cancers of the colon, liver, pancreas, gallbladder, kidney, uterine endometrium and breast (2, 3). For female breast cancer, differential associations have been identified according to menopausal status. There is clear evidence for increased breast cancer risk as a function of increasing BMI in post-menopausal women, but epidemiological analyses suggest a reduced overall risk of pre-menopausal breast cancer associated with obesity (2, 3). However, emerging data indicate a positive association between obesity and triple negative disease prior to menopause (4). Additionally, obesity is associated with worse prognosis after breast cancer diagnosis (5).
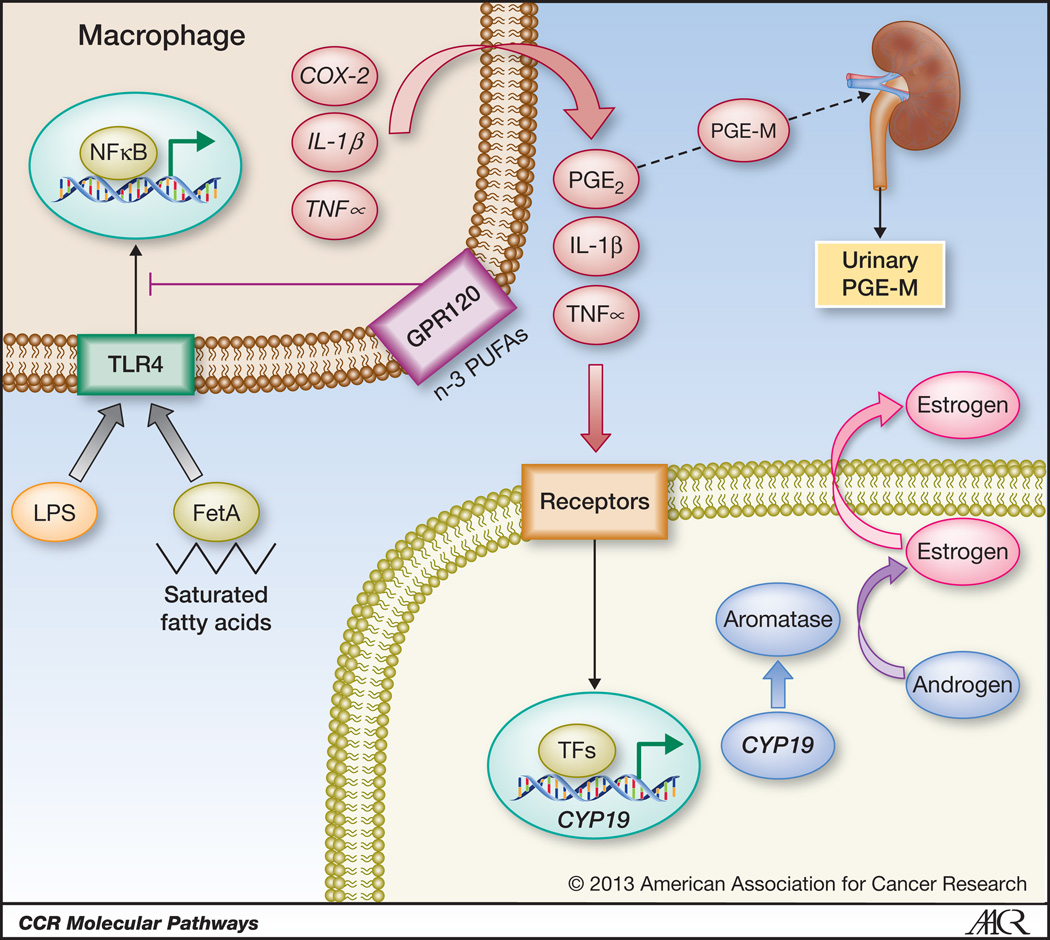
Multiple molecular changes arising as a consequence of increased body mass are likely to contribute to the increased incidence of neoplasia and worse outcomes in obese individuals. These include hyperinsulinemia, elevated insulin-like growth factor 1 (IGF-1) levels, adipokine imbalances, and increased cytokine and estrogen levels (6). Importantly, obesity is characterized not only by increased adipose burden but also by altered adipose biology. Specifically, white adipose tissues from obese individuals and murine obesity models exhibit inflammation, defined by infiltration of leukocytes, including macrophages, as well as CD8-positive T lymphocytes and mast cells (7). Adipose inflammation is increasingly recognized as a key component of obesity-associated diseases such as type II diabetes. Here we focus on the proneoplastic consequences of inflamed adipose, delineating paracrine interactions between adipocytes, macrophages and other cell types (Figure 1) which are likely to contribute to the elevated cancer incidence in general and worse overall outcomes associated with excess adiposity. These observations also have unique implications for estrogen-driven carcinogenesis.
Figure 1. Paracrine interactions between macrophages and other cell types establish an inflammatory milieu in obese breast adipose tissue, resulting in activation of estrogen receptor-α-dependent gene expression.
Saturated fatty acids, released from adipocytes as a result of obesity-associated lipolysis, complex with Fetuin A (FetA) and activate Toll-like receptor 4 (TLR4) signaling resulting in enhanced NFκB activity in macrophages. Additionally, bacterial endotoxin (lipopolysaccharide; LPS) entering systemic circulation as a consequence of obesity-induced impairment of gut mucosal integrity may also elicit NFκB activation through TLR4 ligation. NFκB induces expression of proinflammatory genes including COX-2, IL-1β and TNFα in macrophages. n-3 polyunsaturated fatty acids (PUFAs) signal via GPR120 and can suppress TLR4 signaling. Cytokines and COX-2-derived PGE2 activate transcription of the CYP19 gene encoding aromatase in neighboring cells, including preadipocytes, leading to elevated expression and activity of aromatase. Consequently, estrogen biosynthesis is enhanced, which manifests as increased expression of ER target genes, including the progesterone receptor. Systemic consequences of adipose inflammation include increased circulating levels of cytokines as well as accumulation of the PGE2 metabolite PGE-M in urine. Urinary PGE-M levels may therefore provide a valuable biomarker of obesity-related white adipose tissue inflammation.
Adipose tissue macrophages can comprise up to 40% of the cells in obese adipose tissue and represent a rich source of cytokines which are key mediators of the increased risk of insulin resistance associated with obesity (7). Histologically, this macrophage infiltration manifests as inflammatory foci known as crown-like structures (CLS) which consist of dead adipocytes encircled by macrophages (8–10). Initially identified in visceral and subcutaneous fat, these inflammatory foci were recently observed in breast white adipose tissue from obese women and mice, where they were called CLS-B, “B” denoting breast (11–13). Strikingly, CLS abundance generally increases as a function of body mass, both in breast and other adipose depots (8–12, 14). Consistent with these observations, gene expression analyses have identified selective enrichment of macrophage markers in breast tissue from obese women (14). It is important to emphasize that the relationship between CLS-B presence and obesity is not linear: some obese individuals lack excess (or any detectable) CLS, while a minority of lean ones exhibit this sign of adipose inflammation. Hence, from the standpoint of targeting the consequences of obesity-associated inflammation, BMI alone is not sufficient for the selection of at-risk patients.
Macrophages can be broadly divided into two functional classes, M1, also referred to as classically activated, and M2 or alternatively activated. Although oversimplified, this binary classification of macrophage polarity has proven useful in understanding the link between inflammation and cancer biology. M1 macrophages secrete proinflammatory mediators including prostaglandin (PG) E2, interleukin-1β (IL-1β), IL-6 and tumor necrosis factor α TNFα), whereas M2 macrophages, triggered by Th2 cytokines, are functionally distinct and associated with tissue remodeling and immunosuppression. Resident macrophages in lean adipose tend to be M2-polarized whereas it has been suggested that M1 macrophages play an important role in inflamed adipose tissue in the obese (15). The formation of CLS provides a functional structure for macrophage clearance of dead adipocytes, but also results in macrophage exposure to saturated fatty acids because of obesity-associated lipolysis (16)(17) (Figure 1). Saturated fatty acids can activate Toll-like receptor 4 (TLR4) at the macrophage cell surface and thereby induce NFκB signaling which leads to transcriptional activation of proinflammatory genes including cyclooxygenase-2 (COX-2), IL-1β and TNFα (Figure 1) (18, 19). Importantly, the liver secretory protein, fetuin A, was recently found to act as an adaptor protein between free fatty acids and TLR4, providing a link between free fatty acids, white adipose tissue inflammation and insulin resistance (20). Ingress of bacterial endotoxin (lipopolysaccharide; LPS) facilitated by obesity-associated defects in gut mucosal integrity has also been suggested to lead to activation of NFκB via TLR4 ligation (21, 22). Proinflammatory molecules including TNFα, IL-1β and IL-6 released from activated macrophages have both local and systemic actions, which are likely to contribute both to insulin resistance, and to the increased cancer risk and worse outcomes associated with obesity (2, 3). Thus, the morphologic entity denoted CLS likely corresponds to a functional unit that contributes to obesity-related disease via increasing both local and systemic exposure to inflammatory mediators.
Using both genetic and diet-induced obesity models, it has recently been demonstrated that NFκB signaling and expression of COX-2, TNFα, and IL-1β are all increased in both mammary glands and visceral fat from obese female mice (12). Increased NFκB signaling and cytokine expression is detected in the stromal-vascular fraction of the mammary gland, consistent with up-regulation occurring, at least in part, in macrophages. Similar effects were observed in the inflamed breast white adipose tissue of obese women (11, 13). Thus adipose inflammation, histological and molecular, occurs in breast tissue in both obese mice and women, validating the mouse as a model of the human disease (11, 12).
Importantly, activation of estrogen receptor α (ERα)-dependent gene expression (e.g. progesterone receptor; PR) as a consequence of adipose inflammation has also been observed in both murine mammary fat pads and human breast. The relevance of this observation is increased by the knowledge that estrogen signaling is likely to be a key contributor to obesity-associated breast cancer (11–13). Approximately two-thirds of human breast carcinomas express ERα, and the importance of estrogen and estrogen receptor signaling in breast neoplasia is clearly illustrated by the protective effects of early menopause and oophorectomy, and by the utility of selective estrogen receptor modulators (SERMs) and aromatase inhibitors in the prevention and treatment of the disease (23, 24). Furthermore, estrogen metabolites have been proposed to have carcinogenic activity independent of ER signaling (25). Hence, the demonstration that there is increased expression of an estrogen-regulated gene suggests a critical role for inflammation – associated with obesity – in the pathogenesis of post-menopausal breast cancer.
These observations may resolve a longstanding clinical paradox, which is that increased rates of hormone-dependent breast cancer are seen in the decade after menopause when circulating estradiol is known to decrease and become non-cyclical. Perhaps the explanation is that local tissue concentrations of estrogen are actually increased in some patients because of obesity and localized inflammation. The primary site of estrogen biosynthesis in pre-menopausal women is the ovary but post climacteric, peripheral sources assume increased relative importance in estrogen synthesis. In particular, adipose tissue as well as breast cancer epithelium express the estrogen synthase aromatase, encoded by the CYP19 gene, and produce estrogen (26–30). Based on the importance of adipose as an estrogen source in post-menopausal women, it has long been assumed that increased adiposity is associated with elevated circulating estrogen, and indeed BMI is an established determinant of serum estradiol in post-menopausal women (31–33). However, recent studies identify increased aromatase expression in inflamed adipose tissue of obese women and mice due to up-regulation by proinflammatory mediators released, at least in part, from CLS-associated macrophages (11–13). CYP19 transcription can be induced by the interaction of PGE2, TNFα, and IL-1β with their cognate receptors (12, 34–39) (Figure 1). For example, PGE2 stimulates CYP19 transcription via a signaling cascade involving cAMP, protein kinase A (PKA), and phosphorylation of cAMP response element-binding protein (CREB) to induce aromatase (34–38, 40). Notably, a switch in CYP19 promoter usage occurs in breast cancers and cancer-proximal stromal tissue, involving selective utilization of cAMP-sensitive promoters (41–44). Positive correlations between COX and aromatase expression have been identified in human breast cancers (45–47), and evidence for a causal basis for these findings is provided by transgenic and knockout mouse studies (48). Thus, based on the observation of increased levels of PGE2, TNFα, and IL-1β in breast tissue from obese women and mice, with corresponding increases in the expression of aromatase and ERα target genes (11–13), it is likely that paracrine interactions between macrophages and other cell types establish an inflammatory milieu, resulting in elevated estrogen biosynthesis and signaling, as depicted in Figure 1. In support of this pathway, striking correlations are evident between the extent of inflammation in human breast tissue (i.e. CLS-B) and levels of components of the signaling pathway – COX-2 protein, PGE2, aromatase expression and activity, and PR protein levels (13). Additionally, correlations are observed between inflammation and cAMP levels and PKA activity (13), consistent with the ability of PGE2 to induce cAMP production (49).
In aggregate, these data establish that obesity can drive adipose inflammation leading to induction of aromatase and increased estrogen signaling in breast and other adipose depots. White adipose inflammation may also be associated with estrogen-independent activation of ERα signaling induced by covalent modification of ERα in response to elevated levels of growth factors or proinflammatory mediators. Delineation of the link between obesity-related white adipose tissue inflammation and induction of ERα-dependent gene expression provides mechanistic insight into the observed correlation between obesity and post-menopausal breast cancer risk, and may also explain the increasing proportion of ERα-positive breast cancer observed as a function of age (and despite cessation of ovarian estrogen production), given that aging is associated with inflammation (5). Notably, elevated cytokine levels may also impact carcinogenesis independent of estrogen biosynthesis. Multiple proneoplastic consequences of cytokine overproduction have been propounded (50). Similarly, COX/PG signaling is strongly implicated in neoplasia, and obesity-driven local prostanoid overproduction may drive tumorigenesis via pleiotropic mechanisms (51–53). Importantly, obesity-dependent increases in proinflammatory mediators not only exert proneoplastic effects locally, but also have systemic consequences, as discussed below.
Local versus Systemic Effects of White Adipose Tissue Inflammation
As detailed above, in-breast white adipose tissue inflammation is likely to help explain the link between obesity and post-menopausal, hormone-driven breast cancer. Importantly, multiple lines of evidence suggest that obesity-related changes in levels of circulating factors including insulin, IGF-1, adipokines (leptin, adiponectin) and proinflammatory mediators also play a significant role in the pathogenesis of breast cancer (54, 55). Increased circulating levels of TNFα and IL-6 are found in obese women, and have been associated with breast cancer development and progression (56–58). The relative importance of local white adipose tissue inflammation versus altered levels of circulating factors in the development and progression of breast cancer remains to be elucidated. It should be stressed, however, that the local and systemic effects of obesity are interrelated processes. If a woman has breast white adipose tissue inflammation, it is highly likely that inflammation will be present in other fat depots including within the abdomen. Obesity has already been shown to be associated with adipose inflammation in both mammary and visceral fat depots in murine models of obesity (12), and studies are underway in women to assess the relationship between breast white adipose tissue inflammation and inflammation in other fat depots.
It is easy to envision the importance of local white adipose tissue inflammation in the pathogenesis of breast cancer because the breast epithelium is surrounded by adipose tissue. Furthermore, visceral fat, which also exhibits obesity-associated inflammation, is contiguous with internal organs including the colon, pancreas and kidney, for which obesity is associated with increased cancer risk (2, 3). In contrast, for some other tumor types, systemic consequences of obesity-related white adipose tissue inflammation may outweigh local effects. For example, white adipose tissue inflammation contributes to insulin resistance and consequent hyperinsulinemia, which has been suggested to contribute to both the development and progression of tumors (59). The relative importance of circulating factors in altering the development and progression of cancer due to effects on epithelial versus stromal cells is uncertain and the subject of ongoing research. These effects do not necessarily relate to the specific changes in CYP19 expression and estrogen production discussed above, but collectively, these findings imply that developing interventions to attenuate obesity-related white adipose tissue inflammation or its consequences offers promise both as a risk reduction strategy and to improve the outcomes for a variety of malignancies including breast cancer. Of course, interrupting this systemic pathophysiology also promises to attenuate non-oncologic risks such as cardiovascular disease.
Clinical-Translational Advances
Identification of the obesity-inflammation axis depicted in Figure 1 provides a framework for designing rational interventions to reduce obesity-associated breast cancer risk and improve prognosis. Importantly, interventions that reduce breast white adipose tissue inflammation are also likely to have beneficial effects on other adipose depots and malignancies. Potential strategies can broadly be divided into three groups; those that aim to ameliorate adipose inflammation, those targeting key components of the dysregulated inflammatory signaling pathways, and those that seek to remediate the downstream consequences of adipose inflammation on tumor biology. Little is known about the potential utility of these different strategies to reduce the risk of obesity-related cancers or improve prognosis. Each of these potential strategies is considered below.
Reducing Adipose Inflammation
In terms of strategies to reduce obesity-related adipose inflammation, the most obvious candidate approach is weight loss, since attenuation of obesity is likely to be associated with amelioration of inflammation. Important proof-of-principle for this approach was provided by a clinical study in which weight loss achieved by feeding obese subjects a very low calorie diet for 28 days was associated with an improved inflammatory gene expression profile in subcutaneous fat, with effects most evident in the stromal vascular fraction (60). Furthermore, reductions in risk of recurrence were noted in a large randomized adjuvant treatment trial in which a low fat diet, associated with weight loss, was tested, although the subjects were not obese (61). Consistent data were obtained in a recent animal study of caloric restriction (CR). 30% CR for 7 or 14 weeks in a mouse diet-induced obesity (DIO) model arrested the weight gain induced by feeding a high fat diet, and was associated with a profound reduction in breast inflammation (i.e. CLS-B multiplicity), and with normalization of levels of proinflammatory mediators, aromatase and PR (62). Importantly, several clinical studies have established improvements in circulating biomarkers of inflammation associated with weight loss, dietary modification, and/or exercise (63–67). Together these datasets suggest the potential utility of altered energy balance as a strategy to resolve inflammation and potentially reduce the risk of breast cancer or improve outcomes in survivors. Based on this constellation of findings, a clinical trial to evaluate the efficacy of weight loss and exercise in improving the outcomes specifically of obese women with early stage breast cancer is needed (68). Nevertheless, concerns regarding the limited long-term success achieved clinically with diet and exercise in the at-risk population provide the impetus for parallel searches for effective pharmacological and/or surgical interventions.
Several weight loss drugs are currently available. Sympathomimetic drugs (e.g. phentermine) act as appetite suppressants. A combination formulation of phentermine with the anti-seizure medication topiramate was recently approved by the Food & Drug Administration. Lorcaserin, also approved in 2012, functions as an agonist for the 5-HT-2C receptor thereby increasing the sense of satiety, while the pancreatic lipase inhibitor orlistat reduces absorption of dietary fat. However, the extent of weight loss achieved with each of these drugs is modest and reversible upon drug cessation. To our knowledge, the potential utility of existing weight loss drugs for reducing obesity-related white adipose tissue inflammation or modulating the development or progression of cancer is uncertain. Thus, the potential of effective weight control agents to modulate obesity-related cancer risk or improve outcomes should be considered along with their more widely perceived benefits in terms of diabetes and cardiovascular risk.
The most effective treatment for significant and sustained weight loss is bariatric surgery, including Roux-en-Y gastric bypass and gastric banding. Gastric bypass surgery is frequently associated with complete remission of type II diabetes, although the rapid timeframe of improved insulin sensitivity frequently precedes significant weight loss (69, 70). Strikingly, reduced adipose macrophage density and decreased stromal vascular fraction expression of factors responsible for macrophage recruitment have been observed in subcutaneous white adipose tissue of patients 3 months after gastric bypass surgery (8). These findings suggest bariatric surgery as a potential route to achieve durable weight loss and resolution of inflammation. Notably, significant reductions in cancer incidence and mortality have been identified in gastric bypass patients compared to matched obese control subjects in both retrospective and prospective studies (71, 72). Reduced cancer incidence was observed in women but not in men (71).
Targeting Inflammatory Signaling
In addition to attempting to reduce adipose inflammation, targeting key components of the dysregulated inflammatory signaling pathways shown in Figure 1 may be beneficial. In obesity, both saturated fatty acids and endotoxin have been suggested to stimulate TLR4 signaling resulting in activation of NFκB (73). Increased circulating levels of endotoxin are believed to be a consequence of obesity-related increases in gut permeability and this may provide a signaling source that adds to or amplifies the effect of free saturated fatty acids released as a consequence of obesity-associated lipolysis (21, 22). In preclinical studies, modulating the gut microbiota can reverse high fat diet-induced metabolic disorders including fat mass gain, endotoxemia and adipose tissue inflammation (74). Possibly, therapies that modulate gut microbiota will reduce endotoxemia and thereby prevent this source of activation of TLR4 signaling, and secondarily suppress levels of inflammatory mediators in white adipose tissue depots including the breast. Targeting cross-talk between the host and gut microbiota to reduce obesity-related cancers is an exciting possibility that warrants investigation. In addition to attempting to reduce levels of TLR4 agonists, e.g., endotoxin, as a therapeutic strategy, we note that TLR4 antagonists have been developed. Although these agents possess anti-inflammatory effects (75), it is not known whether they can modulate obesity-related carcinogenesis.
Certain types of lipids possess anti-inflammatory activity. n-3 polyunsaturated fatty acids (PUFAs) including docosahexanoic acid (DHA) and eicosapentaenoic acid (EPA) can inhibit inflammation. Recently, the G-protein-coupled receptor GPR120 on macrophages was identified as a sensor for n-3 fatty acids, activating a β-arrestin/TAB1 signaling system that results in inhibition of TLR4 signaling (73) (Figure 1). n-3 fatty acids, constituents of fish oil, can also activate peroxisome proliferator-activated receptor γ (PPARγ) and block NFκB-mediated induction of proinflammatory mediators (76). Consistent with these effects, dietary n-3 PUFA administration suppresses adipose inflammation and hyperinsulinemia in mouse obesity models (77, 78). Epidemiological analyses of dietary n-3 PUFAs and human breast cancer incidence have yielded equivocal results (79). Intriguingly however, one recent report identified a protective effect of n-3 PUFA consumption selectively in obese women in a Mexican case-control study (80). Together these data suggest a potential application of n-3 PUFAs for reducing obesity-associated inflammation and consequent neoplastic risk.
The so-called calorie restriction mimetics are also potentially useful for blocking the activation of NFκB and suppressing inflammation. These agents are believed to favorably modulate metabolic and stress response pathways regulated by calorie restriction without actually lowering caloric intake. Not surprisingly, these compounds (e.g. resveratrol, rapamycin, metformin) target pathways involved in inflammation, growth factor signaling (especially insulin/IGF-1), oxidative stress and nutrient metabolism (81, 82). Resveratrol, a polyphenolic compound abundant in grapes and some berries, has pleiotropic activities including activation of sirtuin-1 (SIRT-1) and suppression of NFκB signaling, which likely contribute to its ability to attenuate high fat diet-induced adipose inflammation in mice (83).
Effects of the bacterially-derived immunosuppressant rapamycin are best understood in terms of its ability to inhibit mTOR complex 1 (mTORC1), a central regulator of cell growth responsible for integrating growth factor signaling, nutrient, energy and oxygen availability, and translating the net signal to provide the appropriate level of translational activity within the cell. mTOR inhibition attenuates insulin/IGF-1 signaling, a major dysregulated pathway in obesity. Furthermore, rapamycin-mediated mTOR inhibition can also block activation of NFκB signaling (84).
The biguanide metformin is currently generating considerable excitement as a potential anticancer drug (85, 86). Metformin is widely prescribed for the treatment of type II diabetes, and appears to increase whole-body insulin sensitivity by reducing hepatic gluconeogenesis and enhancing glucose uptake by skeletal muscle. Retrospective population studies have identified reduced cancer incidence associated with metformin use, although the anticancer effect has yet to be confirmed in prospective trials (85, 86). Nevertheless, several potential antineoplastic mechanisms have been ascribed to metformin ranging from systemic reductions in insulin signaling to local effects at the cancer cell level on key regulators of energy balance. Metformin directly affects mitochondrial electron transport and stimulates AMP-activated protein kinase (AMPK), including in human adipose tissue (87). There is emerging evidence that AMPK can suppress the activation of NFκB via its downstream mediators SIRT1, FoxO and PGC-1α (88). Metformin-mediated AMPK activation may also result in suppression of aromatase expression in breast adipose cells (89). Currently, metformin is being prospectively evaluated in early stage breast cancer patients in a randomized, placebo-controlled trial with disease-free survival as the primary endpoint (MA.32, NCT01101438). Result stratification according to BMI is expected to be informative with respect to understanding any selective benefit of metformin in obese patients.
White adipose tissue inflammation is associated with elevated levels of COX-2 and PGE2 (Figure 1). COX inhibitors, prototypic inhibitors of PGE2 synthesis, have been widely evaluated for cancer chemoprevention, although with little specific emphasis on obesity-associated disease. Protective effects have been documented for aspirin, conventional COX-inhibiting nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors in animal models, epidemiological analyses and clinical trials (51, 90). Importantly, these drugs can have pleiotropic actions in addition to COX inhibition including NFκB antagonism (91–96) and stimulation of AMPK (97, 98), which may be particularly important in the context of obesity. To date little clinical data exist addressing obesity or its associated inflammation as a modifier of, or sensitizer to, NSAID action in human neoplasia (99). Reductions in colon cancer risk associated with aspirin use were not found to be modified by BMI in two large-scale observational studies, the Health Professionals Follow-Up Study and the Nurses’ Health Study (100). In contrast, the Aspirin/Folate Polyp Prevention Study suggested that aspirin may be more effective in preventing colorectal adenomas in patients with higher BMI (101). These latter data suggest a selective protective effect of aspirin with respect to colon neoplasia in obese individuals, and offer a possible path forward for amelioration of elevated cancer risk in association with obesity through the use of aspirin or other COX inhibitors. Comparable data are not yet available for breast cancer. Of note however, some studies have identified correlations between the use of COX-inhibiting NSAIDs and reduced levels of serum estradiol in postmenopausal women (102, 103), consistent with the notion that PGE2 is a significant determinant of aromatase expression in vivo, and potentially indicative of utility in suppressing obesity-associated aromatase up-regulation. Testing this will require selection of the at-risk group using a validated biomarker for localized tissue inflammation. Elevated BMI alone is unlikely to be sufficient for the identification of patients who are most likely to benefit.
Obesity and Cancer Therapy
It seems likely that local white adipose tissue inflammation in the breast or related changes in circulating factors, e.g., increased insulin, will affect tumor biology and reduce the efficacy of treatment by multiple mechanisms. In addition to attempting to reduce adipose inflammation or targeting inflammatory signaling pathways, specific pathways and processes within tumors may need to be targeted in obese individuals. In one recent preclinical study, the IKKβ/mTOR/VEGF signaling pathway was activated by proinflammatory TNFα in the mammary tumors of obese mice (104). Interestingly, inhibition of this pathway with clinically available drugs including aspirin, rapamycin and bevacizumab reduced obesity-mediated tumorigenesis. Whether such an approach would work in women is unknown. AMPK activity is decreased in obesity and the metabolic syndrome (88). As mentioned above, there is extensive preclinical evidence that activation of AMPK may be a useful anti-tumor approach and this has led to a clinical trial to evaluate the utility of metformin in early stage breast cancer patients.
Delineation of the obesity-inflammation-aromatase axis may also refocus existing treatment strategies, based on a new appreciation of the increase in estrogen biosynthesis that is likely to occur in association with adipose inflammation (Figure 1). Strikingly, in the Phase III Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, proportionately worse outcomes were seen in obese vs. lean women treated with the aromatase inhibitor anastrozole but not with the SERM tamoxifen (105), suggesting that efficacy of aromatase inhibition and its relative advantage decreases with increasing weight (although it was never inferior to tamoxifen). This finding may be attributable to the increased expression of aromatase in inflamed adipose (Figure 1), and could suggest a need for increased dosing to achieve comparable efficacy in the setting of obesity. Alternatively, covalent modification of ERα in response to circulating factors such as insulin/IGF-1 could result in estrogen-independent ERα signaling, which would be predicted to be refractory to increased doses of an aromatase inhibitor. Weight reduction, exercise or pharmacological strategies that reduce adipose inflammation might improve the efficacy of aromatase inhibitors in this population. Clearly, we are only at the beginning of developing personalized evidence-based approaches that will be useful for the prevention and treatment of obesity-related cancers
Biomarker Development
Noninvasive biomarkers of white adipose tissue inflammation would be useful and will be needed to enable the identification of individuals who may be at increased risk of cancer. Biomarkers that accurately report on the presence of white adipose tissue inflammation would also be valuable for assessing the efficacy of therapeutic interventions that aim to attenuate inflammation. The need for biomarkers is highlighted by the observation that not all overweight and obese individuals exhibit adipose inflammation or related molecular changes while some lean individuals do (11).
The molecular pathway shown in Figure 1 suggests that quantifying levels of PGE2 could inform on the presence of white adipose tissue inflammation. Urinary PGE-M is a stable end metabolite of PGE2 that reflects systemic PGE2 levels (106). Notably, two recent reports found increased levels of PGE-M in the urine of obese women (107, 108). Interestingly, an association between high levels of urinary PGE-M and increased risk of post-menopausal breast cancer was observed in women who did not use NSAIDs regularly (107). Additional studies are needed to assess the utility of this biomarker of inflammation. Whether it will prove superior to other biomarkers of inflammation such as CRP is unknown. Studies of serum and plasma are underway in an effort to develop a biomarker signature that will report on the presence of white adipose tissue inflammation. An algorithm incorporating multiple blood-based biomarkers may prove useful for inflammation-associated risk evaluation in both obese and lean patients.
Conclusions and Future Directions
Chronic inflammation has been linked to the development of numerous epithelial malignancies (109). It seems likely, therefore, that the recent discovery of breast white adipose tissue inflammation with its associated molecular changes will prove important for understanding the increased risk of breast cancer among obese post-menopausal women. Nonetheless, additional studies are needed to evaluate the significance of CLS-B as a determinant of both breast cancer risk and prognosis. In addition to the macrophages found in CLS, obesity causes numerous other immunological changes that could also be highly relevant in the pathogenesis of breast cancer and warrant investigation (7). Another significant question concerns the relevance of white adipose tissue inflammation for other tumor types. Will CLS be present at other organ sites and be associated with either increased risk or poor prognosis? If so, it will be important to establish the relative importance of local versus systemic effects of white adipose tissue inflammation on tumor biology. Certainly, given the growing incidence of overweight and obesity, there is a pressing need to both better understand the molecular mechanisms underlying white adipose tissue inflammation and to develop effective interventions that can be tested and applied to the at-risk subpopulation(s). Currently, we know that obesity is associated with poor prognosis for numerous malignancies but we don’t know whether either improved energy balance or alternate (dietary, pharmacological) strategies that attenuate white adipose tissue inflammation or related molecular changes will improve outcomes. Prospective clinical trials are needed in cancer patients to address this important question. In the meantime, we already know that improved energy balance can be helpful in managing common obesity-related comorbidities such as type II diabetes and cardiovascular disease. Taken together, it seems reasonable for more attention and effort to be made to control energy balance in the obese cancer patient with the goal of improving outcome.
Acknowledgments
Grant Support: This work was supported by the National Institutes of Health (CA154481 to A.J. Dannenberg); Breast Cancer Research Foundation (to A.J. Dannenberg, C.A. Hudis); and the Botwinick-Wolfensohn Foundation (to A.J. Dannenberg in memory of Mr. and Mrs. Benjamin Botwinick).
Footnotes
Conflicts of Interest: None
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the uk. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington DC: AICR; 2007. [Google Scholar]
- 3.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–314. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- 5.Iyengar NM, Morris PG, Hudis C, Dannenberg AJ. Obesity, inflammation, and breast cancer. In: Dannenberg AJ, Berger NA, editors. Obesity, inflammation and cancer. New York: Springer; 2013. pp. 181–217. [Google Scholar]
- 6.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 7.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 8.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 9.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of cox-2 and prostaglandin e2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, et al. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98:1173–1181. doi: 10.1210/jc.2012-2745. [DOI] [PubMed] [Google Scholar]
- 17.Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–E78. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, et al. Reciprocal modulation of toll-like receptor-4 signaling pathways involving myd88 and phosphatidylinositol 3-kinase/akt by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 19.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-a acts as an endogenous ligand of tlr4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 21.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, et al. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 22.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 23.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 24.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 25.Cavalieri EL, Rogan EG. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J Steroid Biochem Mol Biol. 2011;125:169–180. doi: 10.1016/j.jsbmb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, et al. Aromatase--a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 27.Bulun SE, Price TM, Aitken J, Mahendroo MS, Simpson ER. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome p450 transcripts using competitive polymerase chain reaction after reverse transcription. J Clin Endocrinol Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 28.Lipton A, Santen RJ, Santner SJ, Harvey HA, Sanders SI, Matthews YL. Prognostic value of breast cancer aromatase. Cancer. 1992;70:1951–1955. doi: 10.1002/1097-0142(19921001)70:7<1951::aid-cncr2820700723>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Miller WR, Anderson TJ, Jack WJ. Relationship between tumour aromatase activity, tumour characteristics and response to therapy. Journal of Steroid Biochemistry and Molecular Biology. 1990;37:1055–1059. doi: 10.1016/0960-0760(90)90465-w. [DOI] [PubMed] [Google Scholar]
- 30.Silva MC, Rowlands MG, Dowsett M, Gusterson B, McKinna JA, Fryatt I, et al. Intratumoral aromatase as a prognostic factor in human breast carcinoma. Cancer Research. 1989;49:2588–2591. [PubMed] [Google Scholar]
- 31.Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129:1120–1131. doi: 10.1093/oxfordjournals.aje.a115234. [DOI] [PubMed] [Google Scholar]
- 32.Kaye SA, Folsom AR, Soler JT, Prineas RJ, Potter JD. Associations of body mass and fat distribution with sex hormone concentrations in postmenopausal women. Int J Epidemiol. 1991;20:151–156. doi: 10.1093/ije/20.1.151. [DOI] [PubMed] [Google Scholar]
- 33.McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–1966. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brueggemeier RW, Richards JA, Joomprabutra S, Bhat AS, Whetstone JL. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J Steroid Biochem Mol Biol. 2001;79:75–84. doi: 10.1016/s0960-0760(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Purohit A, Ghilchik MW, Reed MJ. The regulation of aromatase activity in breast fibroblasts: The role of interleukin-6 and prostaglandin e2. Endocrine-Related Cancer. 1999;6:139–147. doi: 10.1677/erc.0.0060139. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by pge2 via cyclic amp leading to activation of promoter ii of the cyp19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 37.Subbaramaiah K, Hudis C, Chang SH, Hla T, Dannenberg AJ. Ep2 and ep4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a brca1 and p300 exchange. J Biol Chem. 2008;283:3433–3444. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- 38.Prosperi JR, Robertson FM. Cyclooxygenase-2 directly regulates gene expression of p450 cyp19 aromatase promoter regions pii pi.3 and pi.7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006;81:55–70. doi: 10.1016/j.prostaglandins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–69. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samarajeewa NU, Yang F, Docanto MM, Sakurai M, McNamara KM, Sasano H, et al. Hif-1alpha stimulates aromatase expression driven by prostaglandin e2 in breast adipose stroma. Breast Cancer Res. 2013;15:R30. doi: 10.1186/bcr3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome p450 (cyp19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab. 1996;81:3843–3849. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 42.Chen S, Itoh T, Wu K, Zhou D, Yang C. Transcriptional regulation of aromatase expression in human breast tissue. Journal of Steroid Biochemistry and Molecular Biology. 2002;83:93–99. doi: 10.1016/s0960-0760(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 43.Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome p450 gene expression in human adipose tissue. Journal of Biological Chemistry. 1993;268:19463–19470. [PubMed] [Google Scholar]
- 44.Zhou D, Chen S. Identification and characterization of a camp-responsive element in the region upstream from promoter 1.3 of the human aromatase gene. Arch Biochem Biophys. 1999;371:179–190. doi: 10.1006/abbi.1999.1454. [DOI] [PubMed] [Google Scholar]
- 45.Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, et al. Aromatase and cox-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001;79:41–47. doi: 10.1016/s0960-0760(01)00131-5. [DOI] [PubMed] [Google Scholar]
- 46.Brueggemeier RW, Quinn AL, Parrett ML, Joarder FS, Harris RE, Robertson FM. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett. 1999;140:27–35. doi: 10.1016/s0304-3835(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 47.Salhab M, Singh-Ranger G, Mokbel R, Jouhra F, Jiang WG, Mokbel K. Cyclooxygenase-2 mrna expression correlates with aromatase expression in human breast cancer. J Surg Oncol. 2007;96:424–428. doi: 10.1002/jso.20740. [DOI] [PubMed] [Google Scholar]
- 48.Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, et al. Her-2/neu status is a determinant of mammary aromatase activity in vivo: Evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 49.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 50.Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 51.Howe LR. Pharmacologic interventions with nsaids. In: Dannenberg AJ, Berger NA, editors. Obesity, inflammation and cancer. New York: Springer; 2013. pp. 257–303. [Google Scholar]
- 52.Howe LR, Subbaramaiah K, Brown AMC, Dannenberg AJ. Cyclooxygenase-2: A target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: A growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–213. doi: 10.1177/0300985809357753. [DOI] [PubMed] [Google Scholar]
- 56.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: Fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 58.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson RD, Gallagher EJ, Cohen D, Tobin-Hess A, Alikhani N, Novosyadlyy R, et al. Hyperinsulinemia promotes metastasis to the lung in a mouse model of her2-mediated breast cancer. Endocr Relat Cancer. 2013;20:391–401. doi: 10.1530/ERC-12-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 61.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the women's intervention nutrition study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 62.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, et al. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2013;6:282–289. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 64.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. J Clin Oncol. 2012;30:2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 66.Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res. 2012;72:2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Rossum EF, Nicklas BJ, Dennis KE, Berman DM, Goldberg AP. Leptin responses to weight loss in postmenopausal women: Relationship to sex-hormone binding globulin and visceral obesity. Obes Res. 2000;8:29–35. doi: 10.1038/oby.2000.5. [DOI] [PubMed] [Google Scholar]
- 68.Ligibel JA, Goodwin PJ. New and renew: Building the case for weight loss in breast cancer. J Clin Oncol. 2012;30:2294–2296. doi: 10.1200/JCO.2012.42.5496. [DOI] [PubMed] [Google Scholar]
- 69.Ahn SM, Pomp A, Rubino F. Metabolic surgery for type 2 diabetes. Ann N Y Acad Sci. 2010;1212:E37–E45. doi: 10.1111/j.1749-6632.2011.05984.x. [DOI] [PubMed] [Google Scholar]
- 70.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am J Med. 2009;122:248–256. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 71.Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sjostrom L. Review of the key results from the swedish obese subjects (sos) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 73.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 74.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, et al. The tlr4 antagonist eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siriwardhana N, Kalupahana NS, Cekanova M, Lemieux M, Greer B, Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013;24:613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 77.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 78.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 79.Signori C, El-Bayoumy K, Russo J, Thompson HJ, Richie JP, Hartman TJ, et al. Chemoprevention of breast cancer by fish oil in preclinical models: Trials and tribulations. Cancer Res. 2011;71:6091–6096. doi: 10.1158/0008-5472.CAN-11-0977. [DOI] [PubMed] [Google Scholar]
- 80.Chajes V, Torres-Mejia G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, et al. Omega-3 and omega-6 polyunsaturated fatty acid intakes and the risk of breast cancer in mexican women: Impact of obesity status. Cancer Epidemiol Biomarkers Prev. 2012;21:319–326. doi: 10.1158/1055-9965.EPI-11-0896. [DOI] [PubMed] [Google Scholar]
- 81.Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: Lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- 82.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of nf-{kappa}b is controlled by mtor and raptor in association with ikk. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: Translational challenges. J Mol Endocrinol. 2012;48:R31–R43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 86.Pollak MN. Investigating metformin for cancer prevention and treatment: The end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 87.Boyle JG, Logan PJ, Jones GC, Small M, Sattar N, Connell JM, et al. Amp-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: A randomised glycaemia-controlled crossover study. Diabetologia. 2011;54:1799–1809. doi: 10.1007/s00125-011-2126-4. [DOI] [PubMed] [Google Scholar]
- 88.Salminen A, Hyttinen JM, Kaarniranta K. Amp-activated protein kinase inhibits nf-kappab signaling and inflammation: Impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown KA, Hunger NI, Docanto M, Simpson ER. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of amp-activated protein kinase. Breast Cancer Res Treat. 2010;123:591–596. doi: 10.1007/s10549-010-0834-y. [DOI] [PubMed] [Google Scholar]
- 90.Chan AT, Arber N, Burn J, Chia WK, Elwood P, Hull MA, et al. Aspirin in the chemoprevention of colorectal neoplasia: An overview. Cancer Prev Res (Phila) 2012;5:164–178. doi: 10.1158/1940-6207.CAPR-11-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kopp E, Ghosh S. Inhibition of nf-kappa b by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 92.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of nf-kappab activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the nf-kappab pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 94.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of i(kappa)b kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 95.Din FV, Stark LA, Dunlop MG. Aspirin-induced nuclear translocation of nfkappab and apoptosis in colorectal cancer is independent of p53 status and DNA mismatch repair proficiency. Br J Cancer. 2005;92:1137–1143. doi: 10.1038/sj.bjc.6602455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loveridge CJ, MacDonald AD, Thoms HC, Dunlop MG, Stark LA. The proapoptotic effects of sulindac, sulindac sulfone and indomethacin are mediated by nucleolar translocation of the rela(p65) subunit of nf-kappab. Oncogene. 2008;27:2648–2655. doi: 10.1038/sj.onc.1210891. [DOI] [PubMed] [Google Scholar]
- 97.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates amp-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin inhibits mtor signaling, activates amp-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–15. e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hudis CA, Subbaramaiah K, Morris PG, Dannenberg AJ. Breast cancer risk reduction: No pain, no gain? J Clin Oncol. 2012;30:3436–3438. doi: 10.1200/JCO.2012.44.8597. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X, Smith-Warner SA, Chan AT, Wu K, Spiegelman D, Fuchs CS, et al. Aspirin use body mass index, physical activity, plasma c-peptide, and colon cancer risk in us health professionals. Am J Epidemiol. 2011;174:459–467. doi: 10.1093/aje/kwr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim S, Baron JA, Mott LA, Burke CA, Church TR, McKeown-Eyssen GE, et al. Aspirin may be more effective in preventing colorectal adenomas in patients with higher bmi (united states) Cancer Causes Control. 2006;17:1299–1304. doi: 10.1007/s10552-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 102.Gates MA, Tworoger SS, Eliassen AH, Missmer SA, Hankinson SE. Analgesic use and sex steroid hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:1033–1041. doi: 10.1158/1055-9965.EPI-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hudson AG, Gierach GL, Modugno F, Simpson J, Wilson JW, Evans RW, et al. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:680–687. doi: 10.1158/1055-9965.EPI-07-2739. [DOI] [PubMed] [Google Scholar]
- 104.Chen CT, Du Y, Yamaguchi H, Hsu JM, Kuo HP, Hortobagyi GN, et al. Targeting the ikkbeta/mtor/vegf signaling pathway as a potential therapeutic strategy for obesity-related breast cancer. Mol Cancer Ther. 2012;11:2212–2221. doi: 10.1158/1535-7163.MCT-12-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sestak I, Distler W, Forbes JF, Dowsett M, Howell A, Cuzick J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the atac trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 106.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of pge2 by a liquid chromatographic/mass spectrometric assay: Determination of cyclooxygenase-specific pge2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 107.Kim S, Taylor JA, Milne GL, Sandler DP. Association between urinary prostaglandin E2 metabolite and breast cancer risk: A prospective, case-cohort study of postmenopausal women. Cancer Prev Res (Phila) 2013;6:511–518. doi: 10.1158/1940-6207.CAPR-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, et al. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer Prev Res (Phila) 2013;6:428–436. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]