Abstract
Early phase clinical trials targeting the programmed death receptor-1/ligand-1 (PD-1/PD-L1) pathway to overcome tumor-mediated immunosuppression have reported promising results for a variety of cancers. This pathway appears to play an important role in the failure of immune reactivity to malignant plasma cells in multiple myeloma patients, as the tumor cells express relatively high levels of PD-L1 and T cells show increased PD-1 expression. In the current study, we demonstrate that PD-1/PD-L1 blockade with a PD-L1-specific antibody elicits rejection of a murine myeloma when combined with lymphodepleting irradiation. This particular combined approach by itself has not previously been shown to be efficacious in other tumor models. The anti-tumor effect of lymphodepletion/anti-PD-L1 therapy was most robust when tumor antigen-experienced T cells were present either through cell transfer or survival after non-myeloablative irradiation. In vivo depletion of CD4 or CD8 T cells completely eliminated anti-tumor efficacy of the lymphodepletion/anti-PD-L1 therapy, indicating that both T cell subsets are necessary for tumor rejection. Elimination of myeloma by T cells occurs relatively quickly as tumor cells in the bone marrow were nearly non-detectable by five days after the first anti-PD-L1 treatment, suggesting that anti-myeloma reactivity is primarily mediated by pre-activated T cells, rather than newly generated myeloma-reactive T cells. Anti-PD-L1 plus lymphodepletion failed to improve survival in two solid tumor models, but demonstrated significant efficacy in two hematologic malignancy models. In summary, our results support the clinical testing of lymphodepletion and PD-1/PD-L1 blockade as a novel approach for improving the survival of patients with multiple myeloma.
INTRODUCTION
Multiple myeloma (MM) is an incurable B-cell cancer arising from the monoclonal proliferation of malignant plasma cells. MM cells accumulate in the bone marrow (BM), secrete antibody, and cause progressive osteolytic bone disease and end-organ damage. Despite advances in treatment options, nearly all patients relapse and succumb to MM. Complicating the clinical management of relapsed MM are treatment-related toxicities and the frequent occurrence of drug-resistant tumor. Alternative treatment modalities to control or eradicate MM after relapse are an area of active research. Tumor immunotherapy, in particular, has exciting potential in MM as seen by clinical responses elicited by vaccination with cell-derived proteins (1).
Similar to other hematologic malignancies, MM establishes an immunosuppressive microenvironment that must be overcome for immunotherapy to be successful (2, 3). In studies that utilized a murine model of MM, 5T33, our lab recently showed that the programmed death-1 (PD-1)/PD-ligand-1 (PD-L1) pathway contributes to tumor-mediated suppression in vivo (4). PD-1 is a member of the immunoglobulin superfamily and is upregulated on activated T cells, B cells, NK and NKT cells, activated macrophages, and dendritic cells (5). PD-1 has two known ligands: PD-L1 (or B7-H1) and PD-L2 (or B7-DC); each with distinct cell and tissue expression patterns. PD-L2 expression is restricted to APCs and some tumors (6, 7), while PD-L1 is expressed on T and B cells, APCs, various parenchymal cells, and on a wide variety of hematologic and solid tumor cancers where its expression is generally a poor prognostic indicator (8-11). PD-L1 is rarely expressed on B cell malignancies (12), with MM the notable exception (4, 13). Although reports have shown that PD-L1 and PD-L2 can co-stimulate T cells in some conditions (14, 15), it is unknown if this effect is mediated through PD-1 or another receptor (16). The major effect of PD-1 ligation is inhibitory (17, 18), and PD-L expression by cancer cells impairs T-cell mediated anti-tumor immunity by inhibiting TCR signaling (19). Interestingly, PD-L1 also mediates T cell-suppression through interactions with CD80 (16). Because PD-L1 binds two receptors, anti-PD-L1 blockade inhibits two inhibitory pathways on T cells. Anti-PD-1 blockade, on the other hand, inhibits two ligands but only one pathway. It is unknown whether blocking PD-1 or PD-L1 would result in better anti-tumor immunity as the relative contributions of PD-L1:PD-1 and PD-L1:CD80 inhibition are unclear. Antibody-based immunotherapies designed to block the immune inhibitory effects of the PD-1/PD-L pathway have shown remarkable promise in recently reported clinical studies (20, 21).
In the J558L murine model of MM, PD-L1 blockade monotherapy delayed tumor growth but did not result in cure (22). Our lab previously showed that the 5T33 murine MM highly expresses PD-L1 and that T cells from 5T33-bearing mice have increased PD-1 expression and an exhausted phenotype (4). In that study, a multifaceted immunotherapy approach consisting of a tumor cell-based vaccine administered after hematopoietic stem cell (HSC) and T cell transfer was unsuccessful at treating established 5T33 myeloma. However, the addition of a PD-L1-specific blocking antibody significantly improved immunotherapy efficacy and completely eliminated disease in approximately 40% of treated animals.
In the current study, we sought to further explore the use of PD-L1/PD-1 blockade in anti-myeloma immunotherapy. We hypothesized that immune effector cells undergo robust proliferation in the radiation-induced lymphopenic environment and that anti-PD-L1 mAb treatment during the expansion phase overcomes PD-L1/PD-1-mediated tumor immunosuppression leading to successful tumor eradication. A pilot study showed increased survival when myeloma-bearing mice were given sublethal, non-myeloablative total body irradiation and anti-PD-L1 mAb. These results were repeated in a larger series of experiments, and we found that the anti-myeloma response in this setting required both CD4 and CD8 T cells. In addition, the immune response was most efficacious when tumor antigen-experienced T cells were present during the lymphopenia-induced homeostatic proliferation phase. These results indicate that lymphodepletion and PD-L1/PD-1 blockade could be a relatively simplistic therapeutic approach to treating myeloma.
MATERIALS AND METHODS
Mice
All mice were housed in the Medical College of Wisconsin Biomedical Resource Center, an AAALAC-accredited facility. C57BL/KaLwRij (KaLwRij), KaLwRij x C57BL/6.SJL (F1) mice, and B6.129S6-Rag2tm1FwaTg(TcraTcrb)1100Mjb (OT-1) were bred in house. Balb/cJ and C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine). All animal work was reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Tumor cells
The 5T33 murine MM cell line was derived from myeloma that spontaneously arose in a C57Bl/KaLwRij mouse (23, 24). 5T33GFP, a 5T33 line that stably expresses emerald green fluorescent protein (GFP), was created using the EmGFP control vector from the BLOCK-iT HiPerform Lentiviral Pol II miR RNAi Expression System with EmGFP (Invitrogen, Carlsbad, CA). No differences were noted in the tumorigenicity or survival of mice inoculated with either 5T33 or 5T33GFP. For experiments, 5T33 or 5T33GFP cells were thawed from a large frozen stock and cultured in RPMI 1640 + 10% fetal bovine serum for no longer than 2 weeks prior to inoculation of mice. AGN2a murine neuroblastoma is an aggressive Neuro-2a variant, derived in our laboratory as previously described (25). B16F10 murine melanoma cells were provided by Dr. Samuel Hwang at the Medical College of Wisconsin. Mice were inoculated with tumor as follows: 2×106 5T33 or 5T33GFP cells intravenously (i.v.); 1×105 B16F10 or AGN2a cells s.c.; 1×106 A20, EL4 or C1498 cells i.v. 5T33 and 5T33GFP-bearing mice were considered moribund and euthanized when they developed paraparesis or paraplegia. Occasionally, 5T33-inoculated mice developed tumor masses or lesions and were euthanized when the size of the mass or lesion exceeded 250 mm2; other symptoms of advanced 5T33 included splenomegaly, hepatomegaly, or neurologic impairment. B16 and AGN2a tumors were monitored by caliper measurements and mice were considered moribund and euthanized with tumor masses >250 mm2. A body score was used to determine when mice bearing EL4, A20, or C1498 were moribund.
Antibodies and flow cytometry
Fluorochrome-labeled monoclonal antibodies to the following cell surface or intracellular proteins were obtained from eBioscience (San Diego, CA): CD11c (N418), CD19 (1D3), CD3 (145-2C11), CD4 (GK1.5), CD62L (MEL-14), CD8 (53-6.7), FoxP3 (FJK-16s), H-2Kb (AF6-88.5.5.3), I-Ab (AF6-120.2), NK1.1 (PK136), PD-1 (J43), and PD-L1 (MIH5). Isotype control antibodies included Armenian hamster IgG and rat IgG2a kappa. Anti-rat IgG2b (G15-337) and antibodies specific to CD44 (IM7) and CD45.1 (A20) were obtained from BD Biosciences (Franklin Lakes, NJ). For cell surface and viability staining, cells were resuspended in PBS + 0.5% BSA (PBS/BSA) and stained with antibody and 7-aminoactinomycin D (7AAD) (Calbiochem, San Diego, CA) for 10 minutes on ice. Unbound antibody was washed off by centrifugation, and cells were resuspended in PBS/BSA. Intracellular staining for FoxP3 was performed using eBioscience's FoxP3/Transcription Factor Staining Buffer Set according to the included instructions. For apoptosis analysis, cells were resuspended in Annexin V binding buffer (eBioscience) and stained with a combination of Annexin V (eBioscience) and propidium iodide (BD Biosciences) or 7AAD to discriminate viable from non-viable cells. After staining, cells were either analyzed within 4 hours or resuspended and fixed in PBS/BSA + 1% paraformaldehyde for later analysis. Fluorescence-minus-one (FMO) and/or isotype controls were used as negative controls for flow cytometric analysis. Flow cytometry was performed on an LSRII flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software version 6.4.7 (TreeStar Inc., Ashland, OR).
In vivo lymphocyte depletion
Purified anti-PD-L1 (10F.9G2), control rat IgG2b (LTF2), anti-CD4 (GK1.5), anti-CD8 (2.43), and anti-NK1.1 (PK136) antibodies were obtained from Bio X Cell (West Lebanon, NH). Mice were treated with 200 μg of anti-PD-L1 or control IgG, and/or 250 μg of anti-CD4, anti-CD8 or anti-NK1.1, in 200 μl PBS intraperitoneally (i.p.) at the indicated time points.
Hematopoietic stem cells and T cell enrichment
Femurs and tibias from either KaLwRij or (KaLwRij × C57BL/6.SJL) F1 mice were harvested, and bone marrow cells were processed into single cell suspensions. T cells for adoptive cell transfer (ACT) were enriched from splenocytes of either myeloma-bearing (eight days post-inoculation with 2×106 tumor cells i.v.) or naïve mice. Spleens were processed into single cell suspensions by pressing through wire mesh screens. Red blood cells were lysed by brief exposure to a hypoosmotic solution. Total T cells were negatively enriched using an autoMACs separator (Miltenyi Biotec, Bergisch Gladbach, Germany) and the murine Pan T Cell Isolation Kit II (Miltenyi Biotec) according to the manufacturer's directions. T cell purity was typically >85% based on flow cytometric analysis (data not shown).
Irradiation, HSC transplantation, and adoptive T cell transfer
In general, myeloma-bearing recipient mice were given total body irradiation as a single sublethal (500 cGy) or lethal (1100 cGy) dose. Radiation was administered by a Shepherd Mark I Cesium Irradiator in accordance with established guidelines. Twenty-four hours after total body irradiation, some mice received HSC transplantation and ACT as a single i.v. injection of 2.5-10×106 bone marrow cells plus/minus 5-6×106 enriched T cells.
IFN-γ ELISPOT
To assess for the presence of tumor-reactive IFN-γ secreting CD8 T cells, T cells harvested from spleens and bone marrow were purified by immunomagnetic sorting as described above. ELISPOT assays were done using the mouse IFN-γ ELISPOT Kit (BD Biosciences) as described previously (26).
Statistics
Survival curves were compared using the log rank (Mantel Cox) test. Other experiments were compared using Student's T-test or another test as noted in the figure legends. P-values of <0.05 were considered significant. Statistical analysis was done using Prism version 5.0a software (GraphPad Software, La Jolla, CA).
RESULTS
PD-1 expression is increased on tumor specific T cells
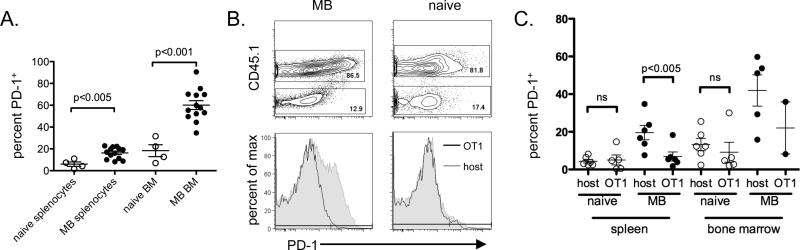
Our previous work demonstrated that the PD-1/PD-L1 pathway is important in suppressing immune responses to 5T33 MM and that PD-1 expression on splenic T cells is related to 5T33 burden (4). However, we did not know if PD-1 were upregulated globally on T cells or only on myeloma-specific cells. To examine this, we compared expression of PD-1 on T cells from separate locations in moribund myeloma-bearing mice. As expected, T cells from myeloma-bearing mice had increased PD-1 expression in both the spleen and bone marrow as compared to naïve mice (Fig. 1A). Since PD-1 expression on T cells is known to be related to cell activation, we hypothesized that PD-1+ T cells in the bone marrow and spleen of myeloma-bearing mice represent tumor-specific T cells as has been shown with PD-1+ tumor-infiltrating lymphocytes (TILs) from melanoma patients (27). 5T33 tumor antigens have not been identified, preventing us from directly addressing PD-1 expression levels on tumor antigen-specific T cells. Instead, we used an indirect approach where CD8 T cells from OT-1 mice served as a source of tumor non-specific T cells. OT-1 T cells express a transgenic TCR specific for the epitope SIINFEKL which is not found in 5T33 cells. We hypothesized that if PD-1 expression is increased on all T cells in the tumor microenvironment, then transferred OT-1 T cells will upregulate PD-1 equivalently to host T cells. CD45.1− OT-1 T cells were adoptively transferred into sublethally irradiated host F1 (CD45.1+) mice, and the mice were challenged with 5T33 MM one week later. T cells were analyzed when the mice became moribund, approximately 4-5 weeks after tumor challenge. In myeloma-bearing (MB) mice, while host CD8 T cells upregulated PD-1 expression in both the spleen and bone marrow, OT-1 CD8 T cells did not (Fig. 1B, C). In naïve mice, no differences were seen in the expression of PD-1 by either host or donor T cells. These results suggest that 5T33 myeloma contributes to the upregulation of PD-1 on tumor-specific T cells but not on tumor non-specific T cells.
Figure 1. Increased PD-1 expression on CD8 T cells in the spleens and bone marrow of myeloma-bearing mice occurs preferentially on T cells with the potential for tumor antigen reactivity.
A. KaLwRij mice were inoculated with 2×106 5T33 cells i.v. Myeloma-bearing (MB) mice became moribund and were euthanized between days 28-40 after inoculation. Splenocytes and femoral bone marrows were harvested and the CD8 T cells analyzed by flow cytometry for PD-1 expression. At the same time points, naïve (non-myeloma-bearing) mice were analyzed as controls. CD8 T cells were gated as CD3+CD8+7AAD−. PD-1 percentage was based on isotype controls. Data are combined from 4 independent experiments; n=13 (MB) and 4 (naive) mice. B. and C. (KaLwRij × B6.SJL) F1 mice were sublethally irradiated (500 cGy) and given 1.5×106 purified OT-1 CD8 splenocytes i.v. Some mice were inoculated 1-2 weeks later with 2×106 5T33 cells i.v. Myeloma-bearing (MB) mice were euthanized when they became moribund, between days 28-35 after inoculation; naive (non-myeloma-bearing) control mice were euthanized at the same time points. Splenocytes and femoral bone marrow cells were analyzed by flow cytometry to determine PD-1 expression levels on host (CD45.1+) versus OT-1 (CD45.1−) CD8 T cells. CD8 T cells were gated as CD3+CD8+7AAD−. B. Representative flow cytometry dot/contour plots and histograms depicting PD-1 expression on host and transferred OT-1 CD8 T cells from MB and naïve mice. C. Percentages of PD-1+ host and OT-1 CD8 T cells in individual mice are shown. Each group contains of 2-6 mice from 2 independent experiments, and the mean +/− SEM for each group is shown. OT-1 cells could only be found in the bone marrow of 2/5 MB mice and 5/6 naive mice.
Anti-PD-L1 mAb treatment synergizes with lymphodepletive irradiation to facilitate a T cell-mediated anti-myeloma response
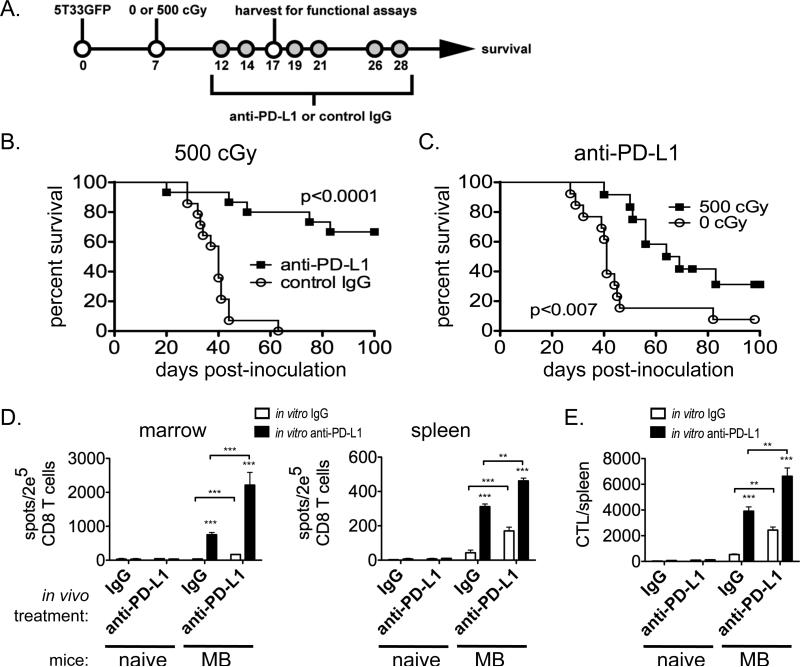
Based on the results from Figure 1, we hypothesized that PD-1+ T cells in myeloma-bearing mice represent functionally exhausted tumor-specific T cells capable of mediating anti-tumor immune responses upon blockade of the PD-1/PD-L1 pathway. Since PD-L1/PD-1 blockade alone is not able to facilitate the elimination of established myeloma (4), we tested our hypothesis by using a combined approach of non-myeloablative total body irradiation and anti-PD-L1 antibody treatment. Mice with established myeloma were treated with 500 cGy total body irradiation one week after tumor cell inoculation, followed by a series of six anti-PD-L1 or control IgG injections as illustrated in Figure 2A. Remarkably, sublethal total body irradiation and anti-PD-L1 treatment induced the rejection of myeloma in approximately two-thirds of mice (Fig. 2B). Mice treated with control IgG all died from myeloma progression. Subsequent experiments confirmed that irradiation was necessary for the anti-myeloma response, as mice given anti-PD-L1 treatment alone nearly all succumbed to myeloma progression (Fig. 2C). These results demonstrated a potent synergism between non-myeloablative total body irradiation and PD-L1 blockade.
Figure 2. Sublethal-irradiation and anti-PD-L1 administration facilitate the rejection of myeloma.
A. Experimental design: Myeloma-bearing KaLwRij (B) or (KaLwRij × B6.SJL) F1 (C) mice received either 500 cGy or no irradiation (0 cGy) seven days after tumor cell inoculation. Treatment with anti-PD-L1 or control IgG (200 μg i.p.) was initiated 5 days later, and specifically given 12, 14, 19, 21, 26 and 28 days after tumor inoculation. Some mice were euthanized at day 17 for use in IFN-γ ELISPOT assays (D & E). B & C. Survival curves from showing the combined data from 3 (B) or 4 (C) independent experiments; n=12-15 mice per experimental group. D & E. CD8 T cells were isolated from spleens and BM 17 days after tumor inoculation or 10 days after irradiation in naïve mice treated with anti-PD-L1 or control IgG. The CD8 T cells were assayed in IFN-γ ELISPOT assays with tumor cell stimulators to determine tumor-reactive IFN-γ secreting cell frequencies. Anti-PD-L1 or control IgG (10 μg/mL) was added to the assays in vitro. The graphs are representative of 2 independent experiments in which the CD8 T cells for each group were pooled from 5 individual mice. ***p<0.001; **p<0.01.
Some of the 500 cGy treated mice were euthanized at day 17 post-tumor cell inoculation (or at the equivalent time post-irradiation for naïve mice), CD8 T cells isolated from spleen and bone marrow, and IFN-γ ELISPOT assays performed to determine frequencies of 5T33-specific T cells in these tissues. Myeloma-bearing mice treated with anti-PD-L1 had significantly greater frequencies of tumor-reactive CD8 T cells in both the spleen and bone marrow than IgG-treated control mice (Fig. 2D). This effect was augmented when anti-PD-L1 was added in vitro to the assay indicating that PD-L1/PD-1 blockade affects tumor cell/CTL interactions directly. The increase in 5T33-reactive CD8 T cells was tumor-specific and not due to a generalized increase of T cell reactivity after anti-PD-L1 therapy as few 5T33-reactive T cells were found in 5T33-naïve mice treated with PD-L1.
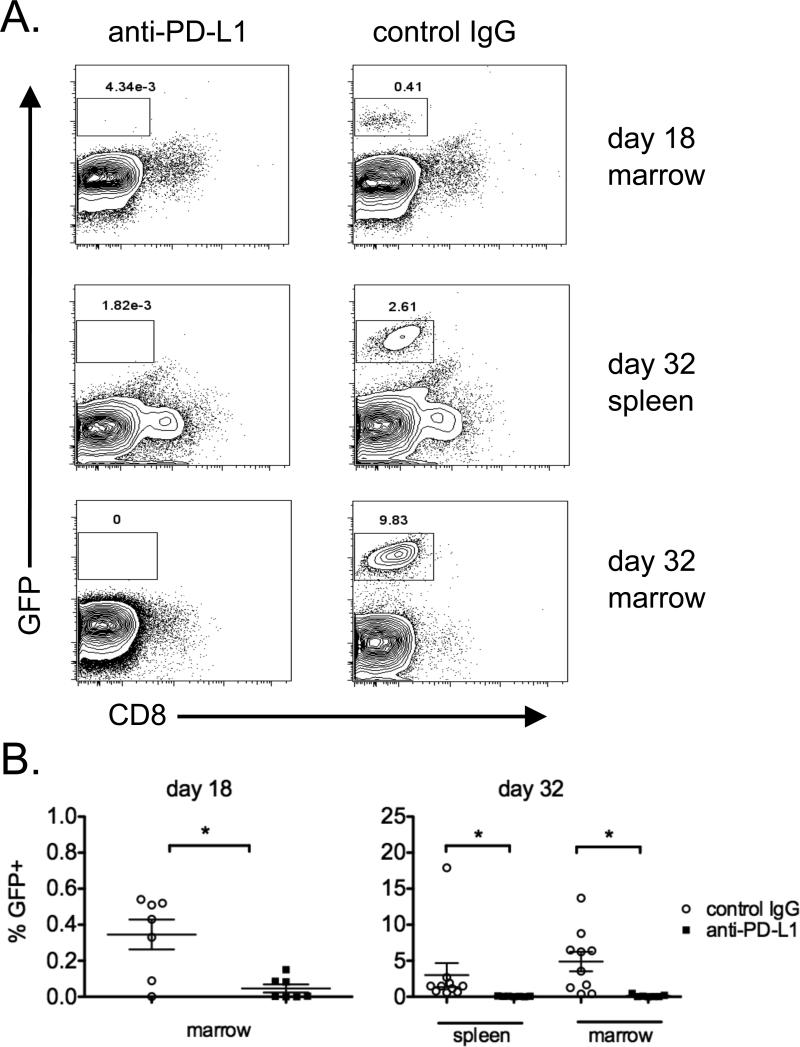
Experiments were performed to assess the timing of the anti-myeloma response after total body irradiation and anti-PD-L1 treatment. For these experiments, mice were irradiated and treated as illustrated in Figure 2A, and cohorts were euthanized at days 18 or 32 after 5T33GFP inoculation to assess tumor burden. Myeloma cells could not be distinguished from background until day 18 in the bone marrow, as shown in representative flow cytometric histograms (Fig. 3A; control IgG). By day 32 after tumor inoculation, tumor cells could be detected in both bone marrow and spleen. Unexpectedly, total body irradiation and anti-PD-L1 treatment resulted in the elimination of tumor cells in the bone marrow by day 18, which was only 5 days after the first anti-PD-L1 treatment (Fig. 3B).
Figure 3. The anti-myeloma effect induced by sublethal irradiation and anti-PD-L1 occurs relatively quickly.
Myeloma-bearing mice were treated with 500 cGy irradiation on day 8 after 5T33GFP inoculation, and anti-PD-L1 or control IgG was administered on days 13, 15, 20, 22, 27 and 29. Mice were euthanized on days 18 and 32 and spleens and BM were harvested. The tissues were analyzed by flow cytometry to detect the presence of 5T33GPF. A. Representative flow cytometry dot/contour plots depicting GFP expression (tumor cells) on the y-axis and CD8 on the x-axis. B. The percentages of GFP+ 5T33 myeloma cells in the tissues of individual experimental mice are shown. Spleens were not analyzed on day 18 due to the inability to detect myeloma at this time point. The data is representative of 2 separate experiments; n=7-10 mice per group at each time point. Groups were compared using the Mann Whitney test.
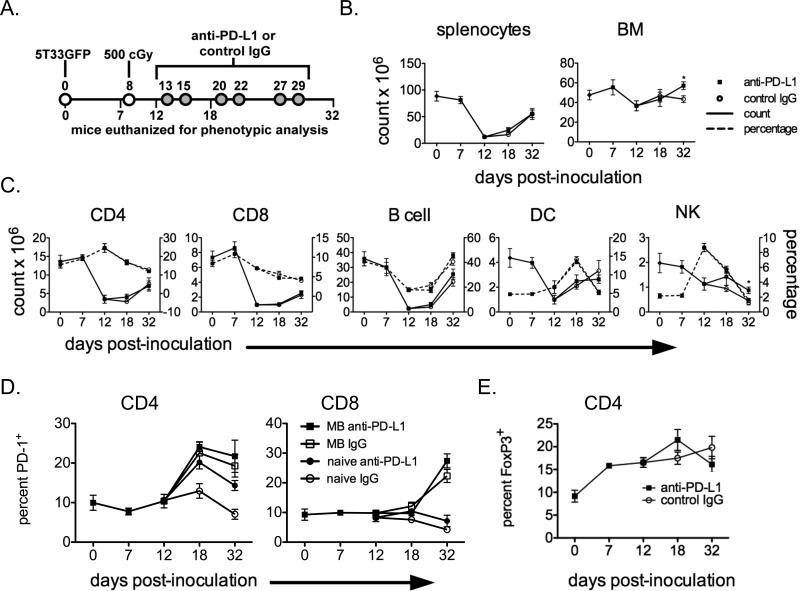
To determine the extent of lymphodepletion achieved by 500 cGy total body irradiation, groups of mice were treated as shown in Figure 4A. At days 0, 7, 12, 18, and 32 after myeloma inoculation, mice from each group were euthanized, spleen and bone marrow cellularity assessed, and the splenocytes and marrow cells were phenotyped by flow cytometry. After irradiation, absolute cell counts in the spleen dropped dramatically and then largely recovered by day 32 (Fig. 4B). Absolute cell counts in the bone marrow were less affected by irradiation. Little difference was seen between anti-PD-L1 and control IgG treated mice, except for an increased cellularity at day 32 in the bone marrow of anti-PD-L1 mice. This difference is likely due to different tumor burdens present in the two groups of mice since MM infiltration in bone marrow displaces normal cells and decreases cellularity. Absolute numbers (Fig. 4C; solid lines) and percentages (Fig. 4C; dashed lines) of immune cell populations in the spleen (CD4 and CD8 T cells, B cells, NK cells, and DC) generally followed the same pattern of decreased counts immediately post-irradiation followed by gradual reconstitution. Although the different immune cell types had different sensitivities to irradiation, anti-PD-L1 treatment had no impact on cell reconstitution, with the exception of NK cells. No treatment-related differences were seen in the percentages of cells undergoing apoptosis or in naïve/effector memory/central memory T cell frequencies (based on CD62L and CD44 immunophenotyping) (data not shown).
Figure 4. The time course of irradiation-induced lymphodepletion and the effect of irradiation and anti-PD-L1 treatment on PD-1 and FoxP3 expression.
A. Experimental design: Myeloma-bearing (KaLwRij × B6.SJL) F1 mice were euthanized on the indicated days during treatment with irradiation and anti-PD-L1 or control IgG. Splenocytes and bone marrow cells were harvested. B. Absolute numbers of splenocytes and bone marrow (femurs and tibias) are shown. C. Absolute numbers (solid lines) and percentages (dotted lines) of the indicated splenocyte immune cell populations. T cells were gated as 7AAD−CD3+, B cells were gated as 7AAD−CD3−CD19+, DC were gated as 7AAD−CD3−CD11c+, and −NK cells were gated as 7AAD−CD3−NK1.1+. The effect of treatment at specific time points was compared using the Student's T test (*p<0.05). D. percentages of splenic PD-1+ CD4 and CD8 T cells. E. Percentages of splenic Foxp3+ CD4 T cells. The data are the combined results of 1-3 separate experiments; n=4-14 mice per group at each time point.
We also examined the spleens of both myeloma-bearing and 5T33-naïve mice treated with sublethal total body irradiation and anti-PD-1 for possible effects on the percentages of PD-1+ T cells and Foxp3+ regulatory T cells over time in the spleen. The percentages of T cells expressing PD-1 were significantly increased over time in myeloma-bearing mice, but not in 5T33-naïve mice. In myeloma-bearing mice, anti-PD-L1 treatment did not result in increased PD-1 expression on T cells whereas in 5T33-naïve mice, the percentage of CD4 T cells expressing PD-1 was increased after anti-PD-L1 treatment (Fig. 4D). The PD-1/PD-L1 pathway has been shown to be important in the generation of regulatory T cells (28). Splenic CD4+Foxp3+ T cell percentages increased after tumor inoculation but were minimally affected by anti-PD-L1 treatment (Fig. 4E).
CD4 and CD8 T cells are required for effective anti-myeloma immunity after irradiation and anti-PD-L1 treatment
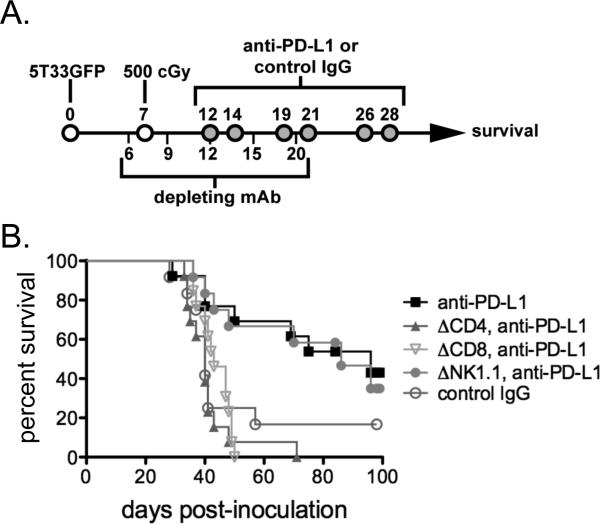
While both activated T and NK cells are known to upregulate PD-1, we postulated that re-activation of ‘exhausted’ T cells was responsible for the anti-myeloma effect induced by sublethal total body irradiation and anti-PD-L1. To determine which lymphocytes are involved in the anti-myeloma response, in vivo-depleting antibodies targeting CD4 T cells, CD8 T cells, or NK cells were administered to myeloma-bearing mice according to the schedule shown in Figure 5A. By day 22, mice were depleted of >95% of the corresponding cell type (data not shown). Surprisingly, depletion of either CD4 or CD8 T cells completely abrogated the therapeutic efficacy of irradiation plus anti-PD-L1 (Fig. 5B); depletion of NK cells did not significantly affect therapeutic efficacy. Since the 5T33 myeloma only expresses MHC class I proteins, these results suggest that CD8 effector T cells require the ongoing presence of CD4 T cell ‘help’ in order to mount an effective immune response.
Figure 5. The anti-myeloma effect of sublethal irradiation and anti-PDL1 is dependent upon both CD4 and CD8 T cells.
A. Experimental design: Myeloma-bearing KaLwRij mice received in vivo depleting antibodies (250 μg i.p. of anti-CD4, anti-CD8, or anti-NK1.1) on days 6, 9, 12, 15, and 20 after 5T33 inoculation. The mice were irradiated (500 cGy) and treated with anti-PDL1 blocking antibody as illustrated. Peripheral blood from some mice was obtained after administration of depleting antibodies to verify depletion. In each case, >95% of the targeted cell population was depleted (data not shown). B. The survival curves depict the combined results of 3 independent experiments; n=12-13 mice per experimental group.
Myeloma-bearing hosts contain tumor-reactive T cells in their lymphoid tissues as early as 8 days after tumor inoculation
Our laboratory previously showed that treatment of myeloma-bearing mice with a combination of lethal total body irradiation, HSC transplantation (bone marrow plus added splenocytes), and PD-L1 blockade completely eliminated myeloma in up to 40% of animals, but co-treatment with a tumor vaccine was required to observe this effect (4). In those experiments, naïve splenocytes were added to the bone marrow graft to provide a source of T cells, since murine bone marrow is relatively T cell-deficient. Unlike lethal total body irradiation, the sublethal irradiation used in earlier figures provides non-myeloablative lymphodepletion in which some endogenous lymphocytes survive irradiation and contribute to the reconstitution of the immune compartment. We speculated that some endogenous tumor antigen-experienced T cells survive the sublethal irradiation and are responsible for the anti-tumor response after anti-PD-L1 treatment, as suggested by the absence of tumor at day 18 in sublethal irradiation/anti-PD-L1-treated mice. As a way to begin addressing this possibility, we asked whether transfer of T cells from the spleens of myeloma-bearing mice (8 days after 5T33 inoculation) could contribute to the anti-myeloma response in lethally irradiated (1100 cGy) recipients.
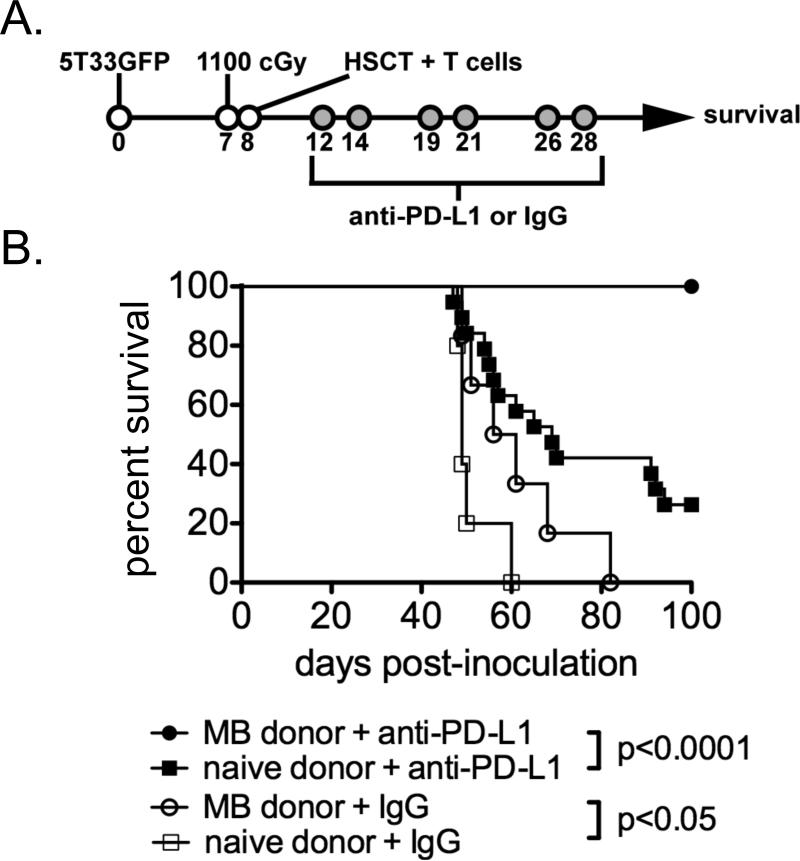
An experimental design similar to that previously used in our laboratory was employed. Briefly, mice with established myeloma were treated with lethal irradiation (1100 cGy) seven days after 5T33 MM inoculation. Mice were rescued one day later by transfer of syngeneic bone marrow supplemented with 5-6×106 T cells from either myeloma-bearing (8 days after 5T33 inoculation) or naïve syngeneic mice. Mice in each treatment group were then given a series of six anti-PD-L1 or control IgG treatments at the times indicated in Figure 6A. The effect of T cell transfer from myeloma-bearing donors was surprisingly impressive in anti-PD-L1-treated mice. Whereas less than 25% of anti-PD-L1-treated mice given naïve T cells survived long-term, 100% of anti-PD-L1-treated mice given T cells from myeloma-bearing mice survived to day 100 (Fig. 6B). In mice treated with control IgG, T cells from myeloma-bearing donors did statistically improve survival, but there were no long-term (100 day) survivors as seen in anti-PD-L1 treated mice. Together, the improved survival seen after transfer for tumor-experienced T cells indicates that tumor antigen-reactive T cells are present in the lymphoid tissues of myeloma-bearing mice within 8 days after tumor inoculation.
Figure 6. ACT with T cells from syngeneic myeloma-bearing donor mice provides superior anti-myeloma immunity in combination with anti-PD-L1 treatment.
A. Experimental design: Myeloma-bearing (KaLwRij × B6.SJL) F1 mice were lethally-irradiated (1100 cGy) seven days after 5T33 inoculation (some mice were irradiated on day 8). One day following irradiation, the mice were intravenously injected with 5-10×106 bone marrow cells from naïve mice and 6×106 purified T cells from myeloma-bearing (MB) or naïve donors. Experimental groups were treated with control IgG or anti-PD-L1 blocking antibody as depicted. B. The survival curves compare experimental groups of mice given T cells from MB versus naïve donors. Data for control IgG mice are from 1 experiment; n=5-6. Data for anti-PD-L1 mice are combined from 4 independent experiments; n=19-20 mice per experimental group.
Sublethal irradiation and anti-PD-L1 mAb treatment is significantly improves the survival of mice bearing A20 B cell lymphoma or C1498 leukemia, but not mice bearing B16F10 melanoma, AGN2a neuroblastoma, or EL4 T cell lymphoma
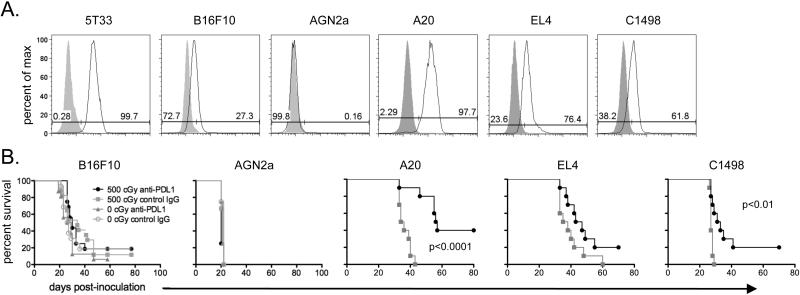
PD-L1 is commonly expressed by tumors other than MM. The murine tumor cell lines B16F10, A20, EL4, and C1498, but not AGN2a neuroblastoma, express PD-L1 although not as highly as 5T33 (Fig. 7A). PD-L1 expression was further increased after 48 hours of culture in 100 ng/ml IFN-γ (data not shown). We tested whether sublethal irradiation and anti-PD-L1 treatment is able to improve the survival of mice bearing these other tumors. When lethal doses of these tumor cells were tested in a similar experimental strategy to that used for 5T33 MM, sublethal irradiation and anti-PD-L1 treatment significantly improved survival in the A20 and C1498 models but was ineffective at eliminating tumors in the B16F10 and AGN2a models (Fig. 7b). Although EL4-bearing mice treated with irradiation and anti-PD-L1 survived longer, and tumor cells were eliminated in two mice, the improved survival was not statistically significant.
Figure 7. Sublethal irradiation and anti-PD-L1 is also effective in the treatment of other hematologic malignancies.
A. PD-L1 expression on cultured 5T33, B16F10, AGN2a, A20, EL4, and C1498 cells is depicted in flow cytometry histograms. Solid curves represent isotype controls. B. Survival curves are shown for mice inoculated with the indicated tumors. The experimental design followed that depicted in Figure 2A. Briefly, approximately one week after inoculation mice received 0 or 500 cGy irradiation and then were treated on six days with anti-PD-L1 or control IgG. Survival curves are from 1-3 independent experiments; n=8-20 mice per experimental group.
DISCUSSION
We report that irradiation-induced lymphopenia combined with PD-L1-specific antibody treatment results in elimination of established murine 5T33 MM. The anti-myeloma effect is T cell-dependent and requires both CD4 and CD8 T cell subsets. This combined therapy is potently synergistic as non-myeloablative irradiation or anti-PD-L1 alone has no effect on myeloma progression. We hypothesize that the lymphopenic environment allows for the functional recovery of ‘inactivated’ tumor-reactive T cells, while PD-L1 blockade prevents re-inactivation of the T cells via the PD-1/PD-L1 pathway. This is the first report to document the efficacy of this combined treatment for myeloma.
Anti-PD-L1 antibody therapy after irradiation-induced lymphopenia has been shown to improve anti-tumor responses in other studies, but efficacy was only seen in the presence of concurrent vaccine administration. Hallett et al. demonstrated improved survival in myeloma-bearing mice treated with tumor-based vaccine plus anti-PD-L1 after lethal total body irradiation and HSC transplantation, but they saw no response in the absence of vaccine administration (4). Although the current study used a similar experimental design, the transfer of bulk splenocytes in the previous study may have resulted in the transfer of regulatory cell populations that inhibited successful anti-tumor responses. To address this possibility, future experiments are planned to determine the cells responsible for anti-tumor responses in the different settings (sublethal versus lethal irradiation). Pilon-Thomas et al. investigated the addition of PD-L1 blocking antibody to a peptide-pulsed DC vaccine in the B16 and M05 murine melanoma models (29). Without total body irradiation, DC vaccination plus anti-PD-L1 had no impact on survival or tumor growth. However, when mice received 600 cGy total body irradiation, delayed tumor growth was seen with DC vaccination plus anti-PD-L1; delayed tumor growth was not observed when only total body irradiation and anti-PD-L1 were administered, similar to our results in the B16 model shown in Figure 7. Overall survival was significantly improved when tumor-specific or T cells from vaccinated donors were adoptively transferred into irradiated mice treated with the DC vaccine and anti-PD-Ll. B16 is known to be a poorly immunogenic tumor and it expresses lower levels of PD-L1 than 5T33. This may explain why anti-PD-L1 monotherapy fails and why vaccination and transfer of tumor-specific or tumor antigen-primed T cells are needed to achieve efficacious anti-tumor responses in the B16 model.
A lymphopenic environment can be caused by lethal (myeloablative) or sublethal (non-myeloablative) irradiation or by chemotherapy with drugs including cyclophosphamide, fludarabine, and melphalan (30-35). After lymphodepletion, lymphocytes undergo spontaneous, antigen-independent expansion called homeostatic proliferation that restores the pre-lymphodepletion lymphocyte compartment. In addition to endogenous lymphocytes, adoptively transferred T cells undergo homeostatic proliferation when placed in a lymphopenic environment (36). Although transferred HSCs can differentiate into T cells, thymopoiesis is inversely correlated with age and only low levels are expected to occur in most MM patients, especially in the two months following HSC transplantation; reconstitution of the lymphopenic compartment early after HSC transplantation is due to expansion of lymphopenia induction-resistant T cells and/or T cells present in the transplant inoculum (37-39). Adoptive cellular therapy after lymphodepleting conditioning is known to cause regression of established tumors in murine models (40-44). Although this effect has been noted for many years (45, 46), it was not until the past decade that the observation has been effectively translated to human trials. Early clinical studies using melanoma antigen (gp100)-specific autologous clonal CD8 T cells as ACT for the treatment of melanoma did not incorporate lymphodepletive conditioning and saw no complete responses (47). Subsequent phase I/II studies added chemotherapy-based non-myeloablative conditioning and demonstrated the importance of modulating the host environment through lymphodepletion. When rapidly-expanded TILs containing polyclonal CD4 and CD8 T cells were used for ACT in combination with non-myeloablative conditioning, objective responses reached 50 percent (31, 48). Lymphopenia contributes to effective ACT through several different mechanisms including an increased availability of immune stimulatory cytokines and the creation of an environment conducive to the disruption of T cell tolerance.
Homeostatic proliferation of various lymphocyte subsets is driven and influenced by the common gamma chain cytokines IL-7 and IL-15 (36, 49, 50). Immune stimulatory cytokines are more available after lymphodepletion (30, 51), possibly due to the ablation of cell populations acting as cytokine sinks (ex. NK cells) or through upregulation of cytokine production by marrow and other cells in the periphery (44). In addition to affecting proliferation, IL-15 also enhances the anti-tumor effects of CD8 T cells used for ACT (52), and effective therapy by ACT positively corresponds with levels of common gamma chain cytokines in lymphodepleted hosts (43).
Recently, Schietinger et al. showed that tolerized CD8 transgenic T cells transferred into sublethally irradiated (500 cGy) hosts were reactivated during homeostatic proliferation and responded to antigenic-challenge (53). This response occurred whether or not the cognate antigen was present in the host. However, transferred T cells were re-tolerized after homeostatic proliferation ceased and the host's T cell compartment was reestablished. The authors attribute this finding to epigenetic changes in the tolerant T cells that are overcome during homeostatic proliferation, but re-assert themselves in the post-homeostatic proliferative environment, even in the absence of cognate antigen. We found in lethally irradiated mice that ACT with T cells from myeloma-bearing mice provided superior tumor rejection suggesting that they were able to break tolerance; however, PD-L1 blockade was required to ensure elimination of myeloma. Some surviving mice from our experiments were re-challenged with tumor 100 days after initial inoculation, and the animals uniformly rejected the secondary challenge without additional anti-PD-L1 treatment (data not shown). We hypothesize that tumor antigen-experienced T cells from the myeloma-bearing mice are able to break tolerance during homeostatic expansion and that re-tolerization is prevented by blockade of the PD-1/PD-L1 pathway, which could possibly alter pro-tolerization epigenetic changes. This hypothesis will be tested in future studies.
Our results demonstrate that the combination of lymphopenia induced by non-myeloablative irradiation and PD-L1/PD-1 blockade is sufficient for the generation of an effective anti-myeloma immune response. Since the intensive high-dose therapy used prior to autologous HSCT is associated with increased side effects and mortality, reduced-intensity conditioning is attractive, particularly since most myeloma patients are elderly and more susceptible to the side effects. A randomized trial of previously untreated multiple myeloma patients directly compared high-dose and reduced-intensity conditioning regimens (54). Reduced-intensity conditioning resulted in less mucositis, a smaller duration of neutropenia and thrombocytopenia, a shorter hospital stay, fewer red blood cell and platelet transfusions, and a decreased need for antibiotic administration. The possibility of fewer treatment-induced complications, however, must be weighed against data showing increased responses to ACT with higher intensity preparative regimens. For example, response rates to ACT with TILs for metastatic melanoma increased to 52 and 72 percent after addition of 200 and 1200 cGy total body irradiation, respectively, to the preparative regimen (51).
Bracci et al. showed that ex vivo depletion of CD4 T cells, but not CD8 T cells, NK cells, or macrophages from transferred splenocytes abrogated the enhanced anti-tumor response seen with lymphodepletion plus ACT in a murine melanoma model (44). Similarly, we found that in vivo depletion of CD4 cells eliminated the response to 5T33 MM that was induced by lymphodepletion and PD-L1 blockade, despite the fact that 5T33 cells only express MHC class I proteins (no class II). This finding was surprising since we hypothesize that the anti-myeloma effect is due to the re-activation of PD-1+ T cells, and not through the generation of new effectors. We therefore expected that depletion of only CD8 T cells would eliminate the anti-myeloma response. If our hypothesis is correct, the data suggests that CD4 T cells provide necessary ‘help’ to facilitate the re-activation of tolerized CD8 T cells. Just how this help is mediated is unclear. Much of the focus of early ACT clinical trials was on the transfer of autologous CD8 T cells only. The incomplete clinical efficacy of CD8 T cell-only ACT may reflect the need to transfer appropriate T helper cells (51, 55). Schmidmaier et al. showed that helper T cell numbers in autologous HSC transplants were positively correlated with event free survival in MM (56). Several studies have shown that CD4 T cells are required for the development of anti-tumor memory after ACT and HSC transplantation (26, 57, 58), further underscoring the need for CD4 T cells in ACT. Although high-intensity conditioning results in increased lymphodepletion, it is unlikely that irradiation or chemotherapy-based conditioning can completely remove immune regulatory elements. Therefore, other methods of overcoming regulatory elements, such as PD-1/PD-L blockade, could result in synergistic anti-tumor immunity, similar to the synergism observed here in our study.
In conclusion, our data show that lymphodepletion and PD-L1 blockade synergize to eradicate MM. While this therapy was highly effective at eliminating established myeloma, it did not eliminate disease in all of the animals. However, we believe that this combined immunotherapeutic approach could serve as an exciting clinical platform for other immunotherapies, including ACT, to achieve even better outcomes. The results we obtained in other tumor models (A20 and C1498) is encouraging as it demonstrates that the anti-tumor effect obtained with this combination therapy is not limited to murine myeloma and may have a role in the treatment of other hematologic malignancies. These observations provide support for the clinical testing of therapeutic strategies involving lymphodepletive-conditioning and PD-1/PD-L pathway blockade.
ACKNOWLEDGEMENTS
We thank the following for their assistance in providing cell lines for this study Drs. Samuel Hwang (MCW), William Murphy (UC Davis), and William Drobyski (MCW).
This work was supported by the Midwest Athletes against Childhood Cancer Fund, by grant 8UL1TR000055 from the Clinical & Translational Science Award (CTSA) program of the National Center for Research Resources and the National Center for Advancing Translation Sciences (JAG), and by a Senior Research Grant from the Multiple Myeloma Research Foundation (BDJ).
Abbreviations used in this article
- PD-L1/2
programmed death ligand-1
- PD-1
programmed death-1
- MM
multiple myeloma
- MB
myeloma-bearing
- 7AAD
7-aminoactinomycin D
- ACT
adoptive cell transfer
- HSC
hematopoietic stem cell
- NK
natural killer cell
- DC
dendritic cell
- TIL
tumor infiltrating lymphocyte
Footnotes
DISCLOSURES
The authors have no financial conflict of interest.
REFERENCES
- 1.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 2.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, Svane IM. Increased Level of both CD4+FOXP3+ Regulatory T Cells and CD14+HLA-DR(−)/low Myeloid-Derived Suppressor Cells and Decreased Level of Dendritic Cells in Patients with Multiple Myeloma. Scand J Immunol. 2010;72:540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharabi A, Ghera NH. Breaking tolerance in a mouse model of multiple myeloma by chemoimmunotherapy. Adv Cancer Res. 2010;107:1–37. doi: 10.1016/S0065-230X(10)07001-6. [DOI] [PubMed] [Google Scholar]
- 4.Hallett WHD, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011;17:1133–1145. doi: 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 7.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clinical and Developmental Immunology. 2012;2012:656340. doi: 10.1155/2012/656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wüthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, Mayer L. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JA, Dorfman DM, Ma F-R, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 15.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 18.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 20.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radl J, Croese JW, Zurcher C, van den Enden-Vieveen MH, de Leeuw AM. Animal model of human disease. Multiple myeloma. Am J Pathol. 1988;132:593–597. [PMC free article] [PubMed] [Google Scholar]
- 24.Manning LS, Berger JD, O'Donoghue HL, Sheridan GN, Claringbold PG, Turner JH. A model of multiple myeloma: culture of 5T33 murine myeloma cells and evaluation of tumorigenicity in the C57BL/KaLwRij mouse. Br J Cancer. 1992;66:1088–1093. doi: 10.1038/bjc.1992.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson BD, Yan X, Schauer DW, Orentas RJ. Dual expression of CD80 and CD86 produces a tumor vaccine superior to single expression of either molecule. Cell Immunol. 2003;222:15–26. doi: 10.1016/s0008-8749(03)00079-0. [DOI] [PubMed] [Google Scholar]
- 26.Jing W, Gershan JA, Johnson BD. Depletion of CD4 T cells enhances immunotherapy for neuroblastoma after syngeneic HSCT but compromises development of antitumor immune memory. Blood. 2009;113:4449–4457. doi: 10.1182/blood-2008-11-190827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inozume T, Hanada K-I, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33:956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Y, Wu J, Bi L, Xiong S, Gao S, Yin L, Jiang L, Chen C, Yu K, Zhang S. Malignant B cells induce the conversion of CD4+CD25-T cells to regulatory T cells in B-cell non-Hodgkin lymphoma. PLoS ONE. 2011;6:e28649. doi: 10.1371/journal.pone.0028649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilon-Thomas S, Mackay A, Vohra N, Mulé JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrus MJ, Williams JF, Eckhaus MA, Gress RE, Fowler DH. An immunoablative regimen of fludarabine and cyclophosphamide prevents fully MHC-mismatched murine marrow graft rejection independent of GVHD. Biol Blood Marrow Transplant. 2000;6:182–189. doi: 10.1016/s1083-8791(00)70041-3. [DOI] [PubMed] [Google Scholar]
- 33.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels J-PH, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Condomines M, Veyrune J-L, Larroque M, Quittet P, Latry P, Lugagne C, Hertogh C, Kanouni T, Rossi J-F, Klein B. Increased plasma-immune cytokines throughout the high-dose melphalan-induced lymphodepletion in patients with multiple myeloma: a window for adoptive immunotherapy. J Immunol. 2010;184:1079–1084. doi: 10.4049/jimmunol.0804159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS ONE. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldrath AW. Cytokine Requirements for Acute and Basal Homeostatic Proliferation of Naive and Memory CD8+ T Cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 38.Bomberger C, Singh-Jairam M, Rodey G, Guerriero A, Yeager AM, Fleming WH, Holland HK, Waller EK. Lymphoid reconstitution after autologous PBSC transplantation with FACS-sorted CD34+ hematopoietic progenitors. Blood. 1998;91:2588–2600. [PubMed] [Google Scholar]
- 39.Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 40.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA, Restifo NP. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 45.Berenson JR, Einstein AB, Fefer A. Syngeneic adoptive immunotherapy and chemoimmunotherapy of a Friend leukemia: requirement for T cells. J Immunol. 1975;115:234–238. [PubMed] [Google Scholar]
- 46.Mulé JJ, Jones FR, Hellström I, Hellström KE. Selective localization of radiolabeled immune lymphocytes into syngeneic tumors. J Immunol. 1979;123:600–606. [PubMed] [Google Scholar]
- 47.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 50.Moniuszko M, Fry T, Tsai W-P, Morre M, Assouline B, Cortez P, Lewis MG, Cairns S, Mackall C, Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued Tolerant CD8 T Cells Are Preprogrammed to Reestablish the Tolerant State. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, Sotto J-J, Guilhot F, Marit G, Doyen C, Jaubert J, Fuzibet J-G, François S, Benboubker L, Monconduit M, Voillat L, Macro M, Berthou C, Dorvaux V, Pignon B, Rio B, Matthes T, Casassus P, Caillot D, Najman N, Grosbois B, Bataille R, Harousseau J-L, Intergroupe Francophone du Myélome Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 55.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidmaier R, Oversohl N, Schnabel B, Straka C, Emmerich B. Helper T cells (CD3+/CD4+) within the autologous peripheral blood stem cell graft positively correlate with event free survival of multiple myeloma patients. Exp Oncol. 2008;30:240–243. [PubMed] [Google Scholar]
- 57.Jing W, Yan X, Hallett WHD, Gershan JA, Johnson BD. Depletion of CD25+ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood. 2011;117:6952–6962. doi: 10.1182/blood-2010-12-326108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]