SUMMARY
Bone mineral density (BMD) testing is used to diagnose osteoporosis, assess fracture risk and monitor changes in BMD over time. A variety of devices and technologies are used to measure BMD or other surrogate markers of bone strength. Measurements obtained with these devices are often reported according to different proprietary standards, and the comparability of values obtained with different instruments is often poor. In addition, there is a high degree of variability in the skills of the technologists performing the tests and the clinicians interpreting the results. Heterogeneity in the guidelines for using BMD measurements together with poor-quality BMD testing and reporting can result in inappropriate clinical decisions, causing unnecessary worry and expense for the patient and possible harm due to unnecessary treatment or treatment being withheld. This Review describes and discusses the mistakes commonly made in BMD testing, and emphasizes the importance of maintaining high-quality standards in order to optimize patient management.
Keywords: BMD, diagnosis, DXA, osteoporosis, pitfalls
INTRODUCTION
Osteoporosis is a common disease that is associated with increases in fracture risk, morbidity and mortality rates, and healthcare expenses.1 Bone mineral density (BMD) testing is a key component in the management of patients with osteoporosis. A number of devices are currently available for the measurement of BMD and other surrogates of bone strength and fracture risk. The technologies used in bone densitometry devices include dual-energy X-ray absorptiometry (DXA), quantitative ultrasound (QUS), and quantitative CT (QCT). However, the differences in the devices used to measure BMD and report the results are so great that even when the underlying technology is the same, it is impossible to compare the findings of the different devices without cross-calibration.2 Cross-calibration is a mathematical method of correlating BMD measurements after repeatedly scanning either a phantom or multiple patients on both systems. A phantom is an object that is used to test a device’s mechanical operations or calibration, or any shift or drift in measured BMD values.
In order to compare serial BMD studies on the same device, precision assessment conducted according to well-recognized standards3 is necessary to calculate the precision error and least significant change (LSC). Precision error is inherent in the BMD measurement itself and is largely dependent on the skill of the technologist in placing the patient in the same position for different scans. Precision represents the reproducibility of the BMD measurement and is typically calculated by measuring BMD in 15 patients three times or 30 patients twice on the same day, repositioning the patient after each scan. The LSC, a value that is derived from the precision calculation, is the smallest BMD change that is statistically significant with a 95% level of confidence. Unfortunately, many DXA facilities have not done precision assessment, and quantitative comparison of BMD measurements cannot, therefore, be performed. Furthermore, there is often a lack of adherence to manufacturers’ recommendations for device maintenance and quality control, and the education and training of bone densitometry technologists and interpreters varies widely. For all these reasons, mistakes in BMD testing are commonly seen (Table 1), sometimes with adverse effects on patient care.
Table 1.
Common mistakes in BMD testing.
| Category | Mistake | Examples/comments |
|---|---|---|
| Indication | Not doing bone density test in a high-risk patient Doing bone density test when it is unlikely to change clinical management |
Healthy 67-year-old woman not tested Healthy 35-year-old woman is tested |
| Quality control | Failure to follow manufacturers’ recommendations for system maintenance and phantom measurement Failure to identify and correct significant change in calibration Failure to do precision assessment and calculate LSC |
Phantom scanning never done Results of phantom scanning not reviewed or instrument servicing not requested when calibration has changed It is not possible to quantitatively compare BMD tests if LSC is not known |
| Acquisition | Improper patient positioning Wrong scan mode Invalid skeletal site Artifacts not removed from scanned area Incorrect demographic information |
Spine not parallel to edges of DXA table or hip not sufficiently internally rotated Scan mode may alter BMD and is manually or automatically selected, depending on the instrument used BMD measured at hip with total hip replacement Spine scanned when patient is wearing underwired bra or has belly button ring in place Man entered as woman, or incorrect age used |
| Analysis | Failure to review and correct improper default identification of bone edges and regions of interest Incorrect labeling of vertebral bodies |
Computer includes large osteophyte in area of measured spine Helpful markers are the iliac crest, usually at the L4–L5 interspace, and lowest set of ribs, usually at T12 |
| Interpretation | Incorrect application of WHO diagnostic T-score criteria and ISCD Official Positions Invalid BMD comparison Stating that bone has been lost when there is only one BMD test Fracture risk incorrectly represented |
Reporting T-scores in a healthy premenopausal woman and applying the WHO diagnostic criteria may result in faulty assessment of fracture risk LSC not known, different instruments used, different bone area scanned, different labeling of vertebral bodies, left hip compared with right hip, comparing T-scores instead of BMD, different scan modes Bone loss can only be identified when serial BMD tests have been done and the LSC is known Expressing fracture risk as relative risk will overestimate fracture probability if the comparator population is at low fracture risk |
This is not a complete list but is representative of typical mistakes made in clinical settings. Abbreviations: BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; ISCD, International Society for Clinical Densitometry; LSC, least significant change.
A recent survey examined perceptions of the quality of DXA reports among almost 6,000 members of the International Society for Clinical Densitometry (ISCD), a non-profit professional society devoted to the advancement of excellence in the assessment of skeletal health.4 Responses were received from 21% of clinicians (743 of 3,488) and 32% of technologists (754 of 2,362) to whom surveys were sent. Most clinicians (71%) and a large number of technologists (45%) reported viewing an incorrect DXA interpretation at least once a month. Almost all the clinicians (98%) felt that poor-quality DXA reports were harmful to patient care. Furthermore, technologists commonly found that demographic information (e.g. age or sex) had been entered incorrectly before the DXA scan, potentially resulting in a misleading or incorrect report.
Since it is likely that incorrect measurements will result in poor clinical decisions, all stakeholders in healthcare systems have a vested interest in assuring the validity of results. This Review identifies the common pitfalls encountered in BMD testing and outlines ways to avoid them; representative patient case scenarios are also included. In addition, current bone densitometry standards to assure high-quality testing and optimize clinical outcomes are presented.
SELECTING THE RIGHT TOOL FOR THE JOB
A 72-year-old woman has an ultrasound of the heel at a community hospital as part of a health awareness campaign. She is congratulated on having a ‘normal’ T-score of −1.0 and told that she is in good health. One year later she presents with an acute T12 vertebral fracture caused by bending over to pick up her granddaughter.
BMD devices differ in their clinical utility, cost, portability and use of ionizing radiation (Table 2). DXA is widely considered to be the ‘gold standard’ technology for measurements of BMD5 for a number of reasons. Biomechanical studies have shown a strong correlation between bone mechanical strength and BMD measured by DXA,6 and epidemiological studies have revealed a robust relationship between fracture risk and DXA-measured BMD.7 In addition, the WHO classification of BMD for the diagnosis of osteoporosis is based primarily on reference data obtained by DXA (Table 3 and Box 1).8 Most randomized clinical trials showing a reduction in fracture risk with drug therapy have selected participants based on BMD measured by DXA.9 Some studies have demonstrated a relationship between reduced fracture risk with drug therapy and increased DXA-measured BMD.10 Furthermore, the accuracy and precision of DXA is excellent, and radiation exposure is very low.11,12 For these reasons, and the widespread use of this technique in clinical practice, DXA is the principal technology discussed in this Review.
Table 2.
Bone mineral density testing technologies.
| DXA | pDXA | QUS | QCT | pQCT | |
|---|---|---|---|---|---|
| Diagnosis (WHO classification) | Yes | Limiteda | No | No | No |
| Prediction of fracture risk | Yes | Yes | Yes | Yes | Yes |
| Monitoring changes over time | Yes | No | No | Yes | No |
| Ionizing radiation | ++ | + | 0 | +++ | ++ |
| Cost | ++ | + | + | +++ | ++ |
Clinical applications of different technologies are listed with approximate comparison of associated radiation and cost, where 0 = none, + = low, ++ = moderate, +++ = highest. apDXA of the distal one-third radius (33% radius) may be used with the WHO classification. Abbreviations: DXA, dual-energy X-ray absorptiometry; pDXA, peripheral dual-energy X-ray absorptiometry; pQCT, peripheral quantitative CT; QCT, quantitative CT; QUS, quantitative ultrasound.
Table 3.
WHO classification of bone mineral density.9
| Classification | T-score |
|---|---|
| Normal | −1.0 or greater |
| Low bone mass (osteopenia) | Between −1.0 and −2.5 |
| Osteoporosis | −2.5 or less |
| Severe osteoporosis | −2.5 or less with a fragility fracture |
Box 1. Definitions of T-score and Z-score.
The T-score compares the patient’s BMD to that of a young-adult sex-matched reference population, and is used to classify BMD in postmenopausal women and men ≥50 years old. It is calculated with the following equation:
A Z-score, which is not used with the WHO classification, compares the patient’s BMD to an age-, ethnicity- and sex-matched reference population. Z-scores, rather than T-scores, are preferred for reporting the results for premenopausal women, men under the age of 50 years and children, using the following equation:
Abbreviation: BMD, bone mineral density.
QUS is used on the peripheral skeleton, most often the calcaneus (other sites include phalanges, radius and tibia). Its advantages over DXA include lower cost, portability and lack of ionizing radiation. The technology is used to measure two parameters that are associated with bone strength—the speed of sound and broadband ultrasound attenuation—and studies with validated QUS devices have shown a good correlation between these parameters and fracture risk (i.e. as these values decrease, fracture risk increases).7,13 However, QUS cannot be used for diagnostic classification and it is not clinically useful for monitoring the effects of therapy.14 Common mistakes made with QUS include inappropriate application of the WHO diagnostic criteria to T-score values derived from speed of sound and broadband ultrasound attenuation measurements, and misdirected attempts to monitor the effects of therapy. QUS T-score values at the calcaneus are not equivalent to those measured by DXA at the hip or spine because of differences in technology, skeletal site, reference databases and other device-specific factors.14,15 A calcaneus QUS T-score is commonly higher than a central DXA T-score16 and could give the patient or uninformed clinician the false impression that bone strength is better than it really is.
In the case described above, QUS was inappropriately used for diagnostic classification, leading to a false sense of security concerning skeletal health. Had it been recognized that a ‘normal’ QUS T-score of −1.0 can be associated with an elevated fracture risk, further investigation might have resulted in treatment and a reduced risk of fracture.
BMD of the distal one-third radius can be measured either by a dedicated peripheral DXA device or a central DXA with the appropriate software. The measurement can be used both for diagnostic classification when using the WHO criteria and to assess fracture risk; however, it is generally not clinically useful in monitoring the effects of treatment.17
QCT and peripheral QCT (pQCT) can measure trabecular and cortical volumetric BMD at the axial skeleton and peripheral skeletal sites, respectively. QCT technology has proven to be a valuable research tool to enhance our understanding of the pathophysiology of osteoporosis and the mechanisms of action of the pharmacological agents used to treat osteoporosis. Furthermore, QCT has been shown to predict fracture risk, although the correlation varies according to the skeletal site and bone compartment measured, the type of fracture predicted and the population assessed.18 For example, the ISCD Official Positions state that “spinal trabecular BMD as measured by QCT has at least the same ability to predict vertebral fractures as AP [anteroposterior] spinal BMD measured by central DXA in postmenopausal women”.18 However, there is a “lack of sufficient evidence to support this position in men”. In addition, “pQCT of the forearm at the ultra-distal radius predicts hip, but not spine, fragility fractures in postmenopausal women”, but again with a “lack of sufficient evidence to support this position in men”.18 QCT is more expensive than both DXA and QUS; it uses higher levels of ionizing radiation and cannot be used for diagnostic classification, since the WHO diagnostic criteria were established using DXA. Furthermore, QCT T-scores are likely to be lower than those reported with DXA,16 and therefore the prevalence of osteoporosis will be overestimated if these scores are incorrectly used in association with the WHO criteria.
WHEN IS IT APPROPRIATE TO MEASURE BONE MINERAL DENSITY?
A 62-year-old woman with rheumatoid arthritis has been taking prednisone 10 mg/day for the past 3 years. She also takes a multivitamin daily but no other nutritional supplement. She trips on a garden hose, falls and fractures her right wrist.
Many societies have released guidelines on the indications for BMD testing, usually with the intention of selecting groups or individuals at high risk (e.g. postmenopausal women ≥65 years old or patients starting high-dose long-term glucocorticoids). The ISCD Official Positions include what is perhaps the most comprehensive list of indications (Box 2).19 Failure to order a BMD test when it is indicated represents a missed opportunity to identify and treat a patient who may be at high risk of fracture. Conversely, carrying out a BMD test in a patient for whom it is not indicated and not likely to influence therapy represents misuse of limited healthcare resources.
Box 2. ISCD indications for BMD testing.19,a.
Women aged ≥65 years
Postmenopausal women <65 years of age with risk factors for fracture
Women during the menopausal transition with clinical risk factors for fracture, such as low body weight, prior fracture or high-risk medication use
Men aged ≥70 years
Men <70 years old with clinical risk factors for fracture
Adults with a fragility fracture
Adults with a disease or condition associated with low bone mass or bone loss
Adults taking medications associated with low bone mass or bone loss
Anyone being considered for pharmacologic therapy
Anyone being treated, to monitor treatment effect
Anyone not receiving therapy in whom evidence of bone loss would lead to treatment
aWomen discontinuing estrogen should be considered for BMD testing according to the indications listed above. Abbreviation: BMD, bone mineral density.
DXA is contraindicated in patients for whom it is unlikely to alter clinical decisions, as well as in women who are or might be pregnant. If the patient has received recent radio-opaque contrast material or radioactive compounds, DXA should be postponed until such material no longer represents a potential confounding factor. Calcium supplements should not be taken on the same day before the DXA procedure, as an unabsorbed calcium tablet located in a scanned area might affect the BMD measurement. A patient whose weight exceeds the limit for the DXA table (typically about 130 kg for older instruments; 180–200 kg for others) should not be put on the table in case of damage to the table frame or injury to the patient.
The patient described above was at high risk for fracture due to the presence of rheumatoid arthritis and long-term glucocorticoid use. BMD testing should have been done when prednisone was started, and pharmacological therapy to prevent bone loss and reduce fracture risk should have been considered at that time.20 Although it can be argued that treatment in a high-risk patient could be initiated without a baseline BMD test, this would preclude the use of BMD to monitor the effects of therapy. In addition, not carrying out a BMD test might be a missed opportunity to consider non-BMD-related causes of skeletal fragility, as might be suggested in a patient having fragility fractures and higher than expected BMD.
CARE AND MAINTENANCE OF DXA DEVICE
A 65-year-old woman has a baseline DXA that shows osteoporosis; she is treated with an oral bisphosphonate. A 1-year follow-up study on the same instrument shows BMD loss that is greater than the LSC for that facility. A routine service visit then reveals that the phantom has not been measured in the past 6 months. Subsequent measurement of a phantom shows that BMD measurement is outside the tolerable range of variation. Consequently, the device is indicating an acceptable response to therapy. The same medication was continued.
Failure to comply with manufacturers’ recommendations for routine device maintenance and quality control might result in unreliable BMD measurements. Device calibration may change suddenly (shift) or gradually (drift) in ways that cause BMD values to be higher or lower than those previously measured.4,21 Calibration shifts can occur after moving a DXA system or following reassembly or breakage of its components; calibration drift can occur with deterioration of device components over time. Regular measurement of a phantom will detect these changes before they become clinically significant, and the device can be recalibrated.
Although DXA software updates do not usually affect BMD measurements, T-score and Z-score values (Box 1) can change due to differences in the mean BMD and/or standard deviation of reference databases; such differences may not be readily apparent.22 This scenario might explain an observed change in T-score or Z-score that does not correspond to BMD variations. Therefore, bone density comparisons should be made using differences in absolute BMD (g/cm2) rather than percentage differences in BMD, T-score or Z-score.23
ACQUIRING DXA DATA
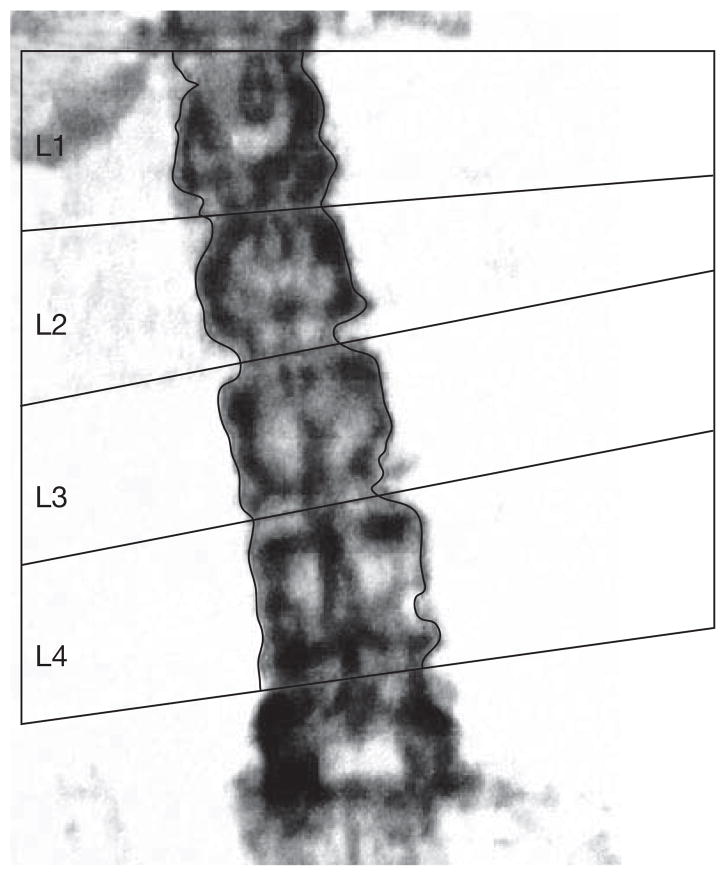
A 74-year-old woman has a follow-up DXA study 2 years after starting therapy. Comparison of BMD values at L1–L4 shows an unexpected loss of BMD. A review of the DXA images shows good positioning of the lumbar spine in the first DXA scan but tilting of the spine in the follow-up scan (Figure 1). With correct positioning, a repeat study shows that BMD is stable, suggesting an acceptable response to therapy.
Figure 1.
DXA of the lumbar spine with poor positioning. This spine is tilted off-center. In order to obtain a valid bone mineral density measurement the lumbar spine should be perpendicular to the long edges of the DXA table. This patient should be repositioned and rescanned. Abbreviation: DXA, dual-energy X-ray absorptiometry.
Correct positioning, which primarily depends on the skill of the technologist, is critically important in obtaining a valid BMD study. The spine must be positioned in the middle of the table, parallel to the long edges of the table, with visible benchmarks to help with labeling vertebral bodies. The L4–L5 interspace is usually at approximately the level of the pelvic brim, and L1 is typically, but not always, the vertebral body below the lowest ribs associated with T12. The bone images can be used to check that the patient is correctly positioned. These images should be reviewed before the patient leaves in order to allow for a repeat scan with correct positioning, if necessary. In the patient described above, positioning of the spine was not correct, resulting in an apparent BMD that was in fact an artifact of positioning.
The hip should be internally rotated approximately 15 °, as suggested by a small or absent protuberance of the lesser trochanter, and the shaft of the femur should be parallel to the long edge of the table unless otherwise recommended by the manufacturer. For serial BMD tests, it is critical that patient positioning is as similar as possible. The ‘scan mode’ should be correct for the size of the patient and BMD; a larger patient with denser bones may require a scan mode with a longer scan time than a smaller patient with less dense bones.
ANALYZING THE DATA
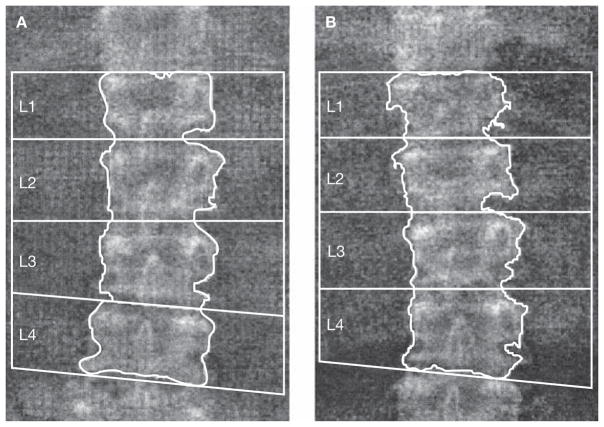
A 68-year-old man is referred for evaluation of bone loss at the lumbar spine while being treated for osteoporosis with an oral bisphosphonate. A review of the spine images shows differences in the labeling of the vertebral bodies in the two studies (Figure 2). When the second scan is labeled in the same way as the first, a re-analysis of the images shows that BMD is stable, representing a good response to therapy. The patient is advised to continue therapy.
Figure 2.
Differences in vertebral body labeling. Comparison of lumbar spine BMD with differences in vertebral body labeling is like comparing ‘apples with oranges’. Comparison of the L1–L4 BMD of these two studies showed an apparent loss of BMD at the time of the second DXA. When the vertebral bodies were labeled consistently, BMD was found to be stable. (A) The first DXA scan gives a BMD of 0.767 g/cm2. (B) The second scan gives a BMD of 0.730 g/cm2. Vertebral body labeling is different to that in the first DXA scan, leading to an invalid BMD comparison. Abbreviations: BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry.
The technologist must evaluate and, if necessary, correct the default computer-selected bone edges and markers of bone regions of interest. Vertebral body labeling, selection of bone edges and placement of the lines through the intervertebral spaces should be correct and consistent with serial BMD tests. Likewise, hip bone edges and regions of interest should be correct, with particular attention paid to the location of the femoral neck box (Figure 3). Other responsibilities of the DXA technologist include reviewing the patient’s skeletal health history, entering demographic data into the computer and performing and analyzing the scan. Historical information could be of importance in the selection of skeletal sites to be scanned; for example, if one hip has been surgically replaced, then the other should be scanned. Demographic information (e.g. age and sex) must be correctly entered into the computer. Since the computer selects a sex-matched reference database for calculation of the T-score, entry of the wrong sex will result in an incorrect T-score. For example, if a man is tested but entered into the computer as a woman, a female reference database will be selected resulting in a higher T-score than had a male reference database been used; this error could result in an inaccurate diagnostic classification and assessment of fracture risk.
Figure 3.
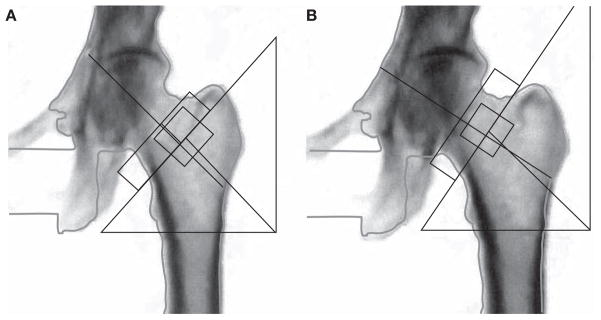
Femoral neck box placement. Each manufacturer has its own standards for correct femoral neck box placement. The dual-energy X-ray absorptiometry technologist must be familiar with the recommendations for the instrument that is used and place the neck box in the same position in serial studies. Here, both images are the same, with the femoral neck box correctly placed on the right. If these were different scans done to evaluate possible changes over time, any comparison would be invalid. (A) Incorrect analysis: femoral neck T-score = −3.2. (B) Correct analysis: femoral neck T-score = −3.0.
The ISCD currently recommends calculating T-scores using a uniform sex-matched (white) young-adult database for patients of all ethnicities in the USA, recognizing that other countries might use alternative databases according to local requirements.19 Regarding Z-scores, the ISCD recommends databases that are matched for sex, ethnicity and age. Although there is no established standard for using or not using weight adjustment for Z-scores, the evidence seems to favor not using weight adjustment.24
DATA INTERPRETATION AND REPORTING
A healthy 35-year-old woman undergoes DXA after a heel QUS at a health fair shows a low T-score. The DXA study reveals a femoral neck T-score of −2.5, leading to a diagnosis of osteoporosis. The patient is started on an oral bisphosphonate. Two years later, she is denied disability insurance cover because of the diagnosis of osteoporosis. Subsequently, a consultation with an osteoporosis specialist showed that the patient did not meet the criteria for a diagnosis of osteoporosis in the baseline DXA study. A thorough evaluation for factors contributing to low BMD is unrevealing, and the correct diagnosis is found to be “BMD below the expected range for age”.19 This condition is usually attributable to low peak bone mass25 and is primarily genetically determined. In these patients, BMD is usually stable and the fracture risk is low. The patient’s bisphosphonate therapy is discontinued and she is counseled on the importance of a healthy lifestyle and good nutrition. Re-application for disability insurance again results in denial.
The responsibilities of the DXA interpreter, reporter or supervisor include regular and frequent collaboration with the technologist to establish standard operating procedures for the facility and resolve any issues that arise with procedures or DXA studies in individual patients. There must be a comprehensive understanding of current standards for BMD testing, interpretation and reporting as provided by the ISCD.19 In the case described above, an incorrect diagnosis of osteoporosis resulted in permanent economic harm (denial of disability insurance). In healthy premenopausal women it is recommended that Z-scores and not T-scores are used in the interpretation of the DXA; the WHO criteria for diagnostic classification using T-scores should not be used. The rationale for this recommendation is based on the likelihood that in healthy premenopausal women the relationship between BMD and fracture risk is not the same as it is in postmenopausal women. In addition, the WHO criteria are derived from fracture prevalence data in postmenopausal, not premenopausal women. Furthermore, the interpreter should take great care to ensure that BMD comparisons are valid and that a statement of bone loss or gain is based on knowledge of the LSC at their facility. Each report should include mention of the technical quality of the study, diagnostic classification and a statement on fracture risk.
When appropriate, fracture risk should be expressed as 10-year probability of fracture according to the WHO fracture risk algorithm (FRAX™).26 Fracture probability is more clinically useful than relative risk in expressing fracture risk. Although the fracture probability might be low, the relative risk of fracture can be high when the comparator population is at low risk of fracture. A common example encountered in clinical practice is that of an early postmenopausal woman with low BMD, such as a T-score of −2.0, and a relative risk of fracture that is four times that of an age-matched woman with a T-score of 0.0, but a 10-year fracture probability that is well below the threshold for pharmacological intervention. FRAX™, which uses femoral neck BMD and selected clinical risk factors for fracture, can be applied to untreated postmenopausal women and men aged 40–90. It has not been validated for clinical use in premenopausal women, children or patients who have been treated for osteoporosis. Fracture probability can be combined with country-specific economic assumptions to establish cost-effective intervention thresholds, such as that provided by the National Osteoporosis Foundation in the USA.27
CONCLUSION
Since healthcare providers depend on valid bone density reports to make patient care decisions, it is imperative that DXA facilities provide consistently high-quality data. Poor BMD reports may trigger clinical decisions that generate unnecessary medical expenses and result in therapeutic decisions that could be harmful to patients. Clinicians at DXA facilities must be educated and trained in bone densitometry and update their skills regularly. Demonstration of proficiency in bone densitometry by certification and/or accreditation gives healthcare providers and those who pay for these services some assurance that basic skills have been learned and can be applied.
REVIEW CRITERIA.
Published full-text, English-language articles were identified from a search of the PubMed database in June 2008 and by review of publications of relevant organizations with an interest in quality assessment of skeletal health. The terms included in the search were “bone mineral density testing”, “BMD testing”, “dual-energy X-ray absorptiometry”, “DXA”, “quantitative ultrasound”, “QUS”, “quantitative computed tomography”, and “QCT” with cross-referencing for “quality”, “pitfalls”, and “mistakes”. Emphasis was placed on results of randomized clinical trials and clinical practice guidelines from professional medical societies.
KEY POINTS.
Dual-energy X-ray absorptiometry (DXA) can be used to diagnose osteoporosis, assess fracture risk and monitor changes in bone mineral density (BMD) over time
Quality control, acquisition, analysis, interpretation and reporting of DXA studies require training and experience for the DXA technologist and interpreter
Quantitative comparison of BMD values on the same instrument cannot be made unless precision assessment has been done and the least significant change calculated
Quantitative comparison of BMD values obtained on different instruments cannot be made unless a cross-calibration study has been done
Poor-quality DXA reports may result in inappropriate patient care decisions that can be costly and sometimes harmful to patients
Footnotes
Competing interests
The authors declared no competing interests.
Contributor Information
E Michael Lewiecki, Clinical Assistant Professor of Medicine at the University of New Mexico School of Medicine and Osteoporosis Director of the New Mexico Clinical Research & Osteoporosis Center, Albuquerque, NM.
Nancy E Lane, Endowed Professor of Medicine and Rheumatology and Director of the Center for Aging at the University of California at Davis Medical School, Sacramento, CA, USA.
References
- 1.US Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 2.Shepherd JA, et al. Cross-calibration and minimum precision standards for dual-energy X-ray absorptiometry: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:31–36. doi: 10.1016/j.jocd.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Bonnick SL, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–110. doi: 10.1385/jcd:4:2:105. [DOI] [PubMed] [Google Scholar]
- 4.Lewiecki EM, et al. DXA quality matters. J Clin Densitom. 2006;9:388–392. doi: 10.1016/j.jocd.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Lewiecki EM. Update on bone density testing. Curr Osteoporos Rep. 2005;3:136–142. doi: 10.1007/s11914-996-0016-3. [DOI] [PubMed] [Google Scholar]
- 6.Lotz JC, et al. Fracture prediction for the proximal femur using finite element models: part I—linear analysis. J Biomechan Eng. 1991;113:353–360. doi: 10.1115/1.2895412. [DOI] [PubMed] [Google Scholar]
- 7.Marshall D, et al. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: WHO; 1994. [PubMed] [Google Scholar]
- 9.Cranney A, et al. Systematic reviews of randomized trials in osteoporosis: introduction and methodology. Endocr Rev. 2002;23:497–507. doi: 10.1210/er.2001-1002. [DOI] [PubMed] [Google Scholar]
- 10.Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85:231–236. doi: 10.1210/jcem.85.1.6267. [DOI] [PubMed] [Google Scholar]
- 11.Mazess R, et al. Enhanced precision with dual-energy X-ray absorptiometry. Calcif Tissue Int. 1992;51:14–17. doi: 10.1007/BF00296209. [DOI] [PubMed] [Google Scholar]
- 12.Njeh CF, et al. Radiation exposure in bone mineral density assessment. Appl Radiat Isot. 1999;50:215–236. doi: 10.1016/s0969-8043(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 13.Siris ES, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 14.Lewiecki EM, et al. Uses and misuses of quantitative ultrasonography in managing osteoporosis. Cleve Clin J Med. 2006;73:742–752. doi: 10.3949/ccjm.73.8.742. [DOI] [PubMed] [Google Scholar]
- 15.Krieg MA, et al. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:163–187. doi: 10.1016/j.jocd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Faulkner KG, et al. Discordance in patient classification using T-scores. J Clin Densitom. 1999;2:343–350. doi: 10.1385/jcd:2:3:343. [DOI] [PubMed] [Google Scholar]
- 17.Hans DB, et al. Peripheral dual-energy X-ray absorptiometry in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:188–206. doi: 10.1016/j.jocd.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Engelke K, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Baim S, et al. Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 Position Development Conference. J Clin Densitom. 2008;11:75–91. doi: 10.1016/j.jocd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum. 2001;44:1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Bonnick SL. Bone Densitometry in Clinical Practice—Application and Interpretation. 2. Totowa, NJ: Humana Press; 2004. [Google Scholar]
- 22.Binkley NC, et al. What are the criteria by which a densitometric diagnosis of osteoporosis can be made in males and non-Caucasians? J Clin Densitom. 2002;5 (Suppl):S19–S28. doi: 10.1385/jcd:5:3s:s19. [DOI] [PubMed] [Google Scholar]
- 23.Lenchik L, et al. What is the role of serial bone mineral density measurements in patient management? J Clin Densitom. 2002;5 (Suppl):S29–S38. doi: 10.1385/jcd:5:3s:s29. [DOI] [PubMed] [Google Scholar]
- 24.Leslie WD, et al. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:22–30. doi: 10.1016/j.jocd.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Lewiecki EM. Premenopausal bone health assessment. Curr Rheumatol Rep. 2005;7:46–52. doi: 10.1007/s11926-005-0008-9. [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA. Technical report. Sheffield: WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield; 2007. Assessment of osteoporosis at the primary health-care level. [Google Scholar]
- 27.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. [Google Scholar]