Abstract
The strong association of HLA-DR2b (DRB1*15:01) with multiple sclerosis (MS) suggests this molecule as prime target for specific immunotherapy. Inhibition of HLA-DR2b-restricted myelin-specific T cells has the potential to selectively prevent CNS pathology mediated by these MHC molecules without undesired global immunosuppression.
Here we report development of a highly selective small molecule inhibitor of peptide binding and presentation by HLA-DR2b. PV-267, the candidate molecule used in these studies, inhibited cytokine production and proliferation of myelin-specific HLA-DR2b-restricted T cells. PV-267 had no significant effect on T cell responses mediated by other MHC class II molecules including HLA-DR1, -DR4, or -DR9. Importantly, PV-267 did not induce nonspecific immune activation of human PBMC. Lastly, PV-267 showed treatment efficacy both in preventing experimental autoimmune encephalomyelitis (EAE) and in treating established disease.
The results suggest that blocking the MS-associated HLA-DR2b allele with small molecule inhibitors may be a promising therapeutic strategy for the treatment of MS.
Introduction
A widely accepted concept of autoimmune disease is that self-derived cellular proteins trigger activation of pathogenic T cells via the presentation of autoantigens by MHC class II molecules. The resulting recognition of MHC:antigen complexes triggers proliferation and cytokine production of autoreactive T cells, resulting in inflammatory processes and subsequent destruction of normal tissues such as the CNS in multiple sclerosis (MS). In the case of MS, peptides derived from myelin antigens such as myelin basic protein (MBP) or myelin oligodendrocyte glycoprotein (MOG) bind to the strongly disease-associated MHC class II allele HLA-DR2b (DRB1*15:01) (1-3), resulting in an immune response and attack on the myelin nerve sheath, leading to disease symptoms and eventual disability. Despite the understanding of this fundamental mechanism and its inherent potential, no drugs based on the direct inhibition of antigen binding have been successfully developed to date.
First-generation MS therapeutics (e.g., beta-interferons and glatiramer acetate) are widely used (4-6), but do not act on the critical mechanism of antigen presentation, but rather modify downstream inflammatory responses. Beta-interferons have multiple targets, including modifying multiple inflammatory cytokines, while glatiramer acetate shifts the population of T cells from pro-inflammatory Th1 types to regulatory Th2 types and may also mimic myelin. However, these compounds have only modest effects on the disease in some patients, but on the positive side, are relatively safe.
Over the past several decades, much attention has been directed toward the concept of specific immunotherapy, i.e., the removal or silencing of specific disease-inducing pathogenic T cell clones in a manner that would not compromise the ability of the rest of the immune system to respond to foreign pathogens. Among the strategies that have been previously pursued are induction of oral tolerance via ingestion of myelin proteins, altered peptide ligands, T cell vaccination, and recombinant T cell receptor ligands (7-14). Clinical trials of these approaches in MS have met with limited success, due in part to unexpected immune reactions to the agents used (15).
In contrast to these approaches at specific immunotherapy, development of disease-modifying drugs that affect T-cell migration or agents that deplete immune cells in general or that alter cytokine profiles have been more efficacious and have led to approval of several second-generation therapeutics for MS, although posing significant safety risks. Natalizumab (a VLA-4 inhibitor) blocks T cell migration and is effective in MS, but the drug has led to serious safety concerns over progressive multifocal leukoencephalopathy (PML), an infectious brain disorder due to activation of JC virus in patients treated with the drug (16). The first oral MS drug, fingolimod, acts on S1P receptors and prevents certain activated T cells from leaving lymph nodes, thus suppressing their entry into the brain (17,18). While initial results from clinical trials support therapeutic efficacy, fingolimod has concerns with cardiac side effects, increased incidence of certain malignancies, and a somewhat increased risk of infection. Teriflunomide and alemtumzumab have migrated to MS from cancer chemotherapy and suppress MS but their overall utility is also compromised by safety concerns (19-21). A number of other drugs are in development or recently approved, including dimethyl fumarate (Tecfidera, BG-00012), an oral agent originally used in the treatment of psoriasis (22).
An attractive alternative strategy that focuses on the key step of antigen binding would be to block the activation of pathogenic T cells at the critical step of antigen binding to the disease-associated MHC class II molecule HLA-DR2b (DRB1*15:01). In those MS patients who express the DR2b gene (ca. 60% of all MS patients, (23)) selectively blocking this target is expected to disrupt the autoimmune process but not generally compromise the immune system. Early attempts at developing peptide inhibitors of MHC binding for HLA-DR1 and DR4 had been made but failed for several reasons. First, their natural peptide nature left them susceptible to rapid degradation by proteolytic enzymes in plasma and particularly toward cathepsins in the loading compartment of antigen presenting cells (APCs). Initially, it was hypothesized that a large molecular size (similar in peptide length to the antigens) would be required in order to satisfy all the molecular recognition sites in the binding groove of the MHC (24,25). However, such long peptides turned out to be unstable as noted, but in addition were also potentially immunogenic. Finally, these investigations and compounds also did not achieve sufficiently high binding affinity to compete efficiently with antigens in cellular assays (26). Here, we describe an approach to design MHC inhibitors guided by structure-based methods to generate compounds with high affinity and specificity for DRB1*15:01. The compounds are designed as small molecular structures and are stabilized against proteolytic cleavage. We describe the design, binding studies, structure-activity relationships, activity in animal models, immunological evaluations, and efficacy in human cells that have led to the selection of a development candidate, PV-267.
Materials and Methods
Human PBMCs and cell line
Characterized, HLA-typed cryopreserved human PBMCs were purchased from CTL (Cellular Technology Limited) and Astarte Biologics. Donor #4 PBMCs (DRB1*0101/DRB1*15:01) and donor #9 PBMCs (DRB1*0401/DRB1*0901) were from CTL. Donor #003 (DRB1*15:01/DRB1*14) PBMCs were from Astarte Biologics. A cryopreserved myelin basic protein-specific T cell line (BC3) was purchased from Astarte Biologics (originally derived from donor #003 PBMCs). All cryo-preserved human cells were thawed using CTL-Thaw Solution and were resuspended in CTL-Test Medium before being used in experiments.
PV-267 and HLA-DR restricted peptides
Compound PV-267 and all modified low-affinity compounds were designed and synthesized by Provid Pharmaceuticals Inc. HLA-DR2b (DRB1*15:01)-restricted MBP peptide 87-99 (VHFFKNIVTPRTP) for recall of BC3 T cells was purchased from United Biochemical Research. HLA-DR1 (DRB1*0101) restricted Influenza A Matrix Protein peptide 17-31 (SGPLKAEIAQRLEDV), HLA-DR9 (DRB1*0901) restricted Tetanus Toxin peptide 830-843 (QYIKANSKFIGITE), and HLA-DR4 (DRB1*0401) restricted Influenza Hemagglutinin peptide 307-319 (PKYVKQNTLKLAT) were purchased from GenScript. HLA-DR2b restricted Tetanus Toxin peptide 830-844 (QYIKANSKFIGITEL) was purchased from AnaSpec.
Cathepsin stability assay
Test compounds (1 mg) were dissolved in 20 μl of DMSO and diluted to 1 ml with acetate (pH 5.5) or citrate (pH 3.5) buffer. Cathepsin enzymes (B, Sigma; L, S, CalBio) were diluted in water to generate desired optimal working concentrations. Enzyme was added to the compound solution and incubated as indicated in Table 2 and aliquots were quenched with 50% TFA and frozen at −20°C until HPLC analysis.
TABLE 2.
Cathepsin Stability of selected p1 substitutions compared to the reference peptide AcVRFFKNI.
| Compound PV- | Sequence | Cathepsin/Cathepsin conc. (U/μmol compd) | pH | T1/2 (min) [Estimate] | Cleavage site (s) | |
|---|---|---|---|---|---|---|
| 004 | Ac-VRFFKNI-NH2 | B | 0.30 | 5.5 | 4.6 | Ac-VRF-//-FKNI-NH2 |
| 004 | Ac-VRFFKNI-NH2 | L | 0.15 | 5.5 | 4.4 | Ac-VRFF-//-KNI-NH2 |
| 004 | Ac-VRFFKNI-NH2 | S | 0.30 | 5.5 | 21 | Ac-VR-//-FF-//-K-//-NI-NH2 |
| 267 | Ac-V-Chg-R-Tic-F-NH2 | B | 0.30 | 5.5 | > 4 h [7 h] | Ac-V-Chg-R-Tic-F-//-NH2 |
| 267 | Ac-V-Chg-R-Tic-F-NH2 | S | 0.30 | 5.5 | > 4 h [7h] | Ac-V-Chg-R-Tic-F-//-NH2 |
| 267 | Ac-V-Chg-R-Tic-F-NH2 | S | 0.15 | 5.5 | 20 h | ND |
| 267 | Ac-V-Chg-R-Tic-F-NH2 | L | 0.01 | 5.5 | 24 h | Ac-V-Chg//-R-Tic-F-NH2 |
Test compound and cathepsin solutions were prepared as described in Materials and Methods. Cathepsin enzymes at indicated concentrations were added to the compound solution and incubated for 4, 8, 16, 32, 64, 128, 256 min and 24 hr for analysis. Chg, cyclohexylglycine, Tic, tetrahydroisoquinolin-3-carboxylic acid, ND, no cleavage detected, all are tested as C-terminal amides.
Human cytokine ELISPOT assays
Primary anti-human IFN-γ mAb (Thermo Scientific; 2G1) was diluted in PBS and coated on ELISPOT plate (Millipore) at 4 °C, overnight. After washing and blocking, autologous APCs (irradiated PBMCs) pretreated with different concentrations of PV-267 for 45 min were added on the plate (0.3 – 0.5 × 106 cells per well), followed by the addition of Ag or positive control using PHA (Sigma-Aldrich) or anti-human CD3 mAb (Bio X Cell; OKT3) along with BC3 T cells or enriched PBMC T cells (4,000 – 20,000 cells per well). Plates were incubated at 37 °C for 24 h, then washed and biotinylated anti-human IFN-γ mAb (Thermo Scientific; B133.5) was added at 4 °C, overnight. After that, plates were incubated with Streptavidin AP (Invitrogen) and developed with BCIP/NBT Phosphatase substrate (KPL) after washing. After developing of the plates, image analysis of ELISPOT assays was performed on a Series 2 ImmunoSpot analyzer and software (CTL) as described previously (27).
Generation of HLA-DR restricted Ag specific T cell lines from human PBMCs
Thawed human PBMCs were cultured in fresh CTL Test Medium at 3 × 106 cells per ml and stimulated with peptide Ag at 2 μg/ml for 4 days. Viable cells were counted and plated at 3 × 106 cells per ml in CTL Test Medium with recombinant human IL-2 at 2 units/ml. After 5 days, cells were again counted and plated at 3 × 106 cells per ml with fresh IL-2 at 2 units/ml, or alternatively, used for the experiments as detailed.
Flow cytometry and mAbs
Thawed or cultured human PBMCs were washed in cold Flow Buffer containing 2% FCS (Atlanta Biologicals) and PBS (Beckman Coulter), followed by blocking with human FcR binding inhibitor (eBioscience) for 20 min and staining in 100 μl volume with 0.3 – 1 × 106 cells per sample at 4°C for 45 min in dark. The cells were then washed twice and fixed before examination by flow cytometry (BD FACS Aria II). Most of the mAbs for staining were obtained from eBioscience: APC anti-human CD40 (5C3), PE anti-human CD69 (FN50), APC anti-human/mouse CD44 (IM7), PerCP-Cy5.5 anti-human CD28 (CD28.2), PE anti-human CD25 (BC96), PerCP-Cy5.5 anti-human CD45 (2D1), PerCP-eFluor710 anti-human CD86 (IT2.2), APC anti-human HLA-DR (LN3), APC anti-human CD4 (OKT4). Anti-human CD3 mAb (Bio X Cell; OKT3) was conjugated with either Alexa Fluor 488 or Alexa Fluor 594 dye using a mAb labeling kit according to the manufacturer’s instructions (Invitrogen/Life Technologies).
Cytokine multiplex assay (Bio-Plex)
Supernatants from human PBMC or cultured BC3 T cells were collected and analyzed for human cytokine levels by cytokine multiplex assay following the Bio-Plex Pro Human Cytokine Standard Group I 27-Plex manufacturer’s protocol (Bio-Rad).
Cell proliferation assay by CFSE
BC3 T cells (Astarte Biologics) were resuspended in pre-warmed 0.1% BSA/PBS (Sigma-Aldrich) at 1 × 106 cells per ml and incubated with CFSE (CellTrace CFSE Cell Proliferation kit, Invitrogen) solution for a final concentration of 10 μM at 37°C for 10 min. Stock CFSE was prepared fresh in DMSO following the manufacturer’s instructions. The staining was quenched by adding 5 × volumes of ice-cold culture media to the cells followed by incubation on ice for 5 min. CFSE labeled BC3 T cells were centrifuged and washed two times with culture media, then cultured with autologous APCs (irradiated human PBMCs, Astarte Biologics), 10 μg/ml of MBP87-99 peptide or mitogens such as PHA, anti-human CD3 mAb (OKT3) for 5 days at 37°C. Cells were collected and stained with APC anti-human CD4 mAb (OKT4) followed by flow cytometry analysis.
Mice
HLA-DR2b (DRB1*15:01) transgenic mice (27) backcrossed to MHC class II deficient mice (28) were maintained under specific pathogen-free conditions and all animal procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at San Antonio.
EAE induction and treatment with PV-267
Active EAE was induced in HLA-DR2b transgenic mice by subcutaneous (s.c.) injection of 200 μg MOG35-55 peptide (United Biochemical Research) in 50 μl of complete Freund’s adjuvant (CFA). Mice also received intraperitoneal (i.p.) injections of 200 ng Pertussis toxin (PTX) on d 0 and d 2. 30 mg/kg of PV-267 or vehicle (30% Captisol or Tween-80) were injected i.p. into mice once daily starting either one day before disease induction for 20 d or with 50 mg/Kg in PBS once daily upon disease onset for 10 d. Adoptive transfer EAE model was induced in HLA-DR2b transgenic mice by injecting recipient mice i.p. with 20 × 106 encephalitogenic donor cells. Briefly, HLA-DR2b donor mice were immunized s.c. with 50 μg of MOG35-55 in CFA. Splenocytes and draining lymph node (DLN) cells were harvested from donors on d 10 and cultured for 3 d in complete DMEM medium (10% fetal bovine serum, L-glutamine) in the presence of MOG35-55 peptide (20 μg/ml), recombinant mouse IL-12 (30 ng/ml; R&D system), and anti-mouse-IFN-γ mAb (10 μg/ml; R4-6A2; Bio X Cell). After the culture, donor cells were harvested and resuspended in warm serum-free DMEM medium and injected i.p. into HLA-DR2b recipients. 50 mg/kg of PV-267 or control (0.02% PBS-Tween-80) were injected i.p. into the mice daily starting two days before disease induction for 15 d.
EAE evaluation
Mice were monitored and graded daily for clinical signs of EAE using the following scoring system (27,29): 0, no abnormality; 1, limp tail; 2, moderate and hind limb weakness; 3, complete hind limb paralysis; 4, quadriplegia or premoribund state; 5, death.
Mouse cytokine ELISPOT
ELISPOT plates (Millipore) were coated with functional-grade purified anti-mouse IFN-γ mAb (eBioscience; AN-18) and anti-mouse IL-17 mAb (Bio X Cell; 17F3) at 4°C overnight. Mouse splenocytes (1×106 cells per well) and brain infiltrating mononuclear cells (isolated by Lympholyte-M media, Cedarlane; 2-5 ×104 cells per well) were collected and restimulated with MOG35-55 peptide or PV-267 as control on ELISPOT plates in HL-1 medium (Lonza BioWhittaker, Fisher Scientific) at 37°C for 24 h. Biotinylated anti-mouse IFN-γ mAb (eBioscience; R4-6A2) and anti-mouse IL-17 mAb (BioLegend; TC11-8H4) were then added to the plate overnight at 4°C followed by incubation with streptavidin alkaline phosphatase (Invitrogen) for 2 h at RT and developing with BCIP/NBT Phosphatase substrate (KPL). After developing of the plates, image analysis of ELISPOT assays was performed on a Series 2 ImmunoSpot analyzer and software (CTL) as described previously (27).
Serum Ag-specific IgG ELISA
ELISA plates (Corning Costar 9018) were coated with MOG35-55 peptide or PV-267 compound at 20 μg/ml and incubated at 4°C overnight. After blocking with 1%BSA in PBS/Tween-20 buffer, serum titrations were added to the plates and incubated at 4°C overnight. The plates were then washed with PBS/Tween-20 and incubated with detection antibody (HRP conjugated goat anti-mouse IgG; Thermo Scientific) for 1 h RT. Plates were then washed and incubated with 1×TMB substrate solution (eBioscience) for 15 min and stopped by 2 N H2SO4. Plates were read at OD 450 nm for absorbance on a Synergy HT microplate reader (BioTek).
Statistical analysis
Sigma Stat (Systat Software) was used to perform all tests of significance. t-test was used for comparison between groups. A value of p < 0.05 was considered statistically significant. Since clinical scores in the EAE model are non-parametric the data were also treated statistically using the median scores and with a Mann-Whitney test. In the EAE assays, the difference in the median values between the two treatment and control groups was significant (prevention mode, P <0.006; treatment mode, P<0.001) (30). The cumulative EAE score was calculated by adding up daily EAE scores from the onset of disease until the end of study or before recovery for individual mice and dividing by the number of mice within each group (31). Statistical significance was calculated by t-test.
Results
Generation of small molecule inhibitors for HLA-DR2 molecules
The interactions between peptides and MHC molecules have been previously well characterized in studies of high-resolution crystal structures (25,32,33). The Wiley group’s determination of the HLA-DR2 (DRA*0101/DRB1*15:01) structure with the MBP85-99 peptide bound provided a basis for the design of small molecule inhibitors used herein. The 15 amino acid MBP peptide lies in the binding groove with parts extending beyond the binding pocket (4-5 residues at each terminus) (25). Key interactions are with the hydrophobic pockets of DRB1*15:01 designated as P1 and P4. Since much of the interaction energy of a DR2 inhibitor was expected to be associated with hydrophobic interactions (dominant in P1 to P4), we explored truncation of the sequences, optimizing interactions by filling of the P1 and P4 pockets, and constraining the P3 group into a cyclic structure (for potency, reducing its exposure on the surface of the complex, and for cathepsin stabilization) through a series of 185 analogs. In analyzing the DR2 binding pocket, we noted that the region extending from the P5 pocket to the peptide antigen C-terminus was primarily polar. We reasoned that since those regions also mainly were interacting via a network of hydrogen bonds to the backbone of the MBP peptide, it might be possible to truncate the peptide ligand further from P4 to the C-terminus, essentially permitting solvent water to replace this region of the peptide. The N-terminus also seemed to extend out of the binding pocket and was associated with T cell activation, so was also a candidate for truncation. As shown in Table 1, removal of 4 residues from each of the N- and C-termini in the context of the MBP85-99 peptide (5 nM binding affinity) gave a minimally active sequence of only 7 residues, with a binding affinity of 1213 nM (compound 1). The 7-mer formed the basis for further modifications. Replacing the His at P2 by Arg (compound 2) enhanced binding (from 1213 nM to 707 nM) as anticipated from previous studies on DR4 ligands (32). We then examined the P1 pocket, which in MBP is valine (Val). Since the isopropyl side chain of Val does not fully occupy the P1 hydrophobic pocket, introducing cyclopentylglycine (Cpg, compound 3) or cyclohexylglycine (Chg, compound 4) in this 7-mer context to fill this space increased affinity to 16 and 4 nM respectively (Table 1). Constraining the backbone and P3 side chain by replacing the Phe of compound 2 by Tic (a constrained tetrahydroisoquinoline amino acid ) increased binding affinity for compound 5 to 17 nM compared to the original reference sequence (707 nM, compound 2). Extending the chain cap at the N-terminus to include Val at P(−1) enabled further truncation at the C-terminus (compound 7). Combining all of the features (P(−1) Val, P1 Chg, and P3 Tic) in a 5 residue sequence, we obtained PV-267 with an affinity of 2 nM vs. DRB1*15:01 and with high selectivity for DRB1*15:01 vs. DRB1*0101 and other alleles tested (data not shown). These modifications (particularly the Tic group that introduces a cyclic amino acid) impart high resistance to cathepsins as outlined in Table 2. Based on the potency of PV-267, this compound was selected as the lead for further studies.
TABLE 1.
Effect of truncation and individual amino acid substitutions on binding of selected inliibitors to DRBl *1501 (SAR evolution).
| Binding-Assay IC50 (nM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | P-2 | P-1 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | (DRB1*1501) | (DRB1*0101) |
| MBP | ENP | V | V | H | F | F | K | N | IVTPR | 5 | |
| 1 | Ac | V | H | F | F | K | N | I | 1213 | ||
| 2 | Ac | V | R | F | F | K | N | I | 707 | ||
| 3 | Ac | Cpg | R | F | F | K | N | I | 16 | ||
| 4 | Ac | Chg | R | F | F | K | N | I | 4 | ||
| 5 | Ac | V | R | Tic | F | K | N | I | 17 | ||
| 6 | Ac | V | R | Tiq | F | K | N | I | 18 | ||
| 7 | Ac | V | V | H | F | K | N | I | 52 | ||
| 8 | Ac | V | Chg | R | F | F | K | 3 | |||
| PV-267 | Ac | V | Chg | R | Tic | F | 2 | 862 | |||
The reference peptide (15 residues) was converted to a minimally active 7-mer and further optimized by modification of the P1 anchor (from Val to Cha), adding conformational constraints at the P3 group (Phe->Tic), His->Arg substitution at P2, and adding the N-terminal cap (Ac-Val) resulted in PV-267, a 2 nM binding affinity compound with only 5 residues. Cpg, cyclopentylglycine, Chg, cyclohexylglycine, Tic, tetrahydroisoquinolin-3-carboxylic acid, Tiq, tetrahydroisoquinoline-1-carboxylic acid, all are tested as C-terminal amides.
Inhibition of HLA-DR2b-restricted T cell responses by PV-267
PV-267 was chosen as a candidate MHC II inhibitor due to its high in vitro binding affinity for HLA-DR2 (2 nM) and selectivity against other alleles (>400-fold) combined with stability to degradation by cathepsins (Table 1 & 2) and with good half-life and pharmacokinetics following IP or SC administration (data not shown). To test the inhibitory effect of PV-267 on HLA-DR2b-restricted T cell responses, we activated a human MBP83-99-specific T cell line (BC3) derived from an HLA-DR2b+ MS patient with its cognate MBP peptide in the presence or absence of the inhibitor and determined T cell responses by cytokine ELISPOT and CFSE-based proliferation assays.
A shown in Fig. 1A, BC3 T cells cultured with the minimal-epitope MBP87-99 peptide in the presence of autologous APCs showed strong production of IFN-γ as measured by cytokine ELISPOT assay. Importantly, incubation of BC3 T cells with MBP87-99 and autologous APC pretreated with increasing concentrations of PV-267 (0 – 200 μg/ml) significantly inhibited cytokine production (Fig. 1A, 1B). Optimal inhibition was achieved at 50 μg/ml of PV-267 (Fig. 1A, 1B). Importantly, no significant inhibitory effect by PV-267 was observed when BC3 T cells were stimulated with anti-CD3 mAb, indicating that the compound did not affect viability at the concentrations used and that inhibition was specific for MHC:peptide mediated signaling (Fig. 1C). Additionally PV-267 did not affect the viability of resting and Ag activated BC3 T cells or autologous PBMCs at concentrations of up to 400 μg/ml for 24 h (data not shown).
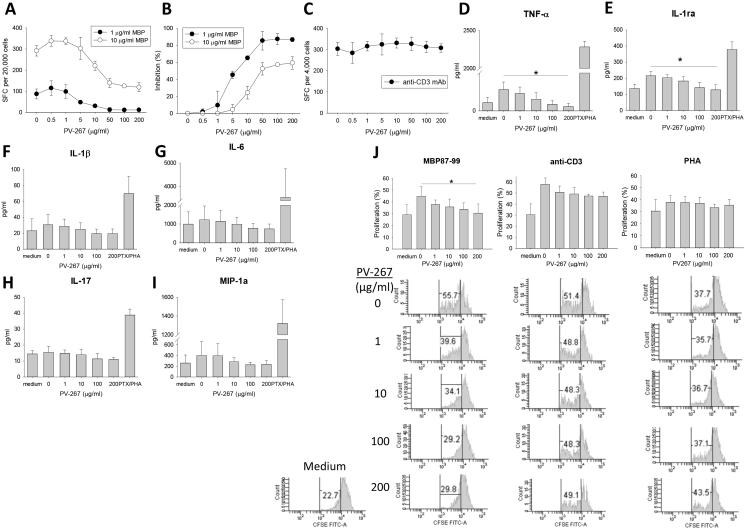
Figure 1.
PV-267 inhibits MBP Ag specific pro-inflammatory cytokine production and proliferation of BC3 T cells. MBP specific BC3 T cells were co-cultured with PV-267 pre-treated autologous APCs and MBP87-99 peptide for human IFN-γ ELISPOT assay as described in Materials and Methods. Shown are representative results of (A) quantified spots at each PV-267 titration, (B) percentage of inhibited response from at least 3 independent experiments, and (C) effect of PV-267 on BC3 T cells in response to anti-CD3 mAb stimulation as control (MEAN±SD). After MBP specific BC3 T cells were co-cultured with PV-267 pretreated autologous APC and medium, Ag or with positive control (PTX/PHA) for 24 h; supernatants were collected and analyzed using Bio-Plex assay for human cytokines. Shown are pooled results of proinflammatory cytokine production of (D) TNF-α, (E) IL-1ra, (F) IL-1β, (G) IL-6, (H) IL-17 and (I) MIP-1a from 3 independent experiments (mean ± SD). (J) MBP specific BC3 T cells were labeled with CFSE then co-cultured with PV-267 pretreated autologous APC and MBP87-99 or mitogen for 5 days followed by flow cytometry analysis as described in Materials and Methods. Shown are pooled results (top) of percentage of proliferated BC3 T cells in response to MBP87-99, anti-CD3 mAb and PHA (MEAN±SD), as well as representative flow cytometry histograms (bottom) from 3 - 4 independent experiments. * indicates significant difference between 0 μg/ml PV-267 and 200 μg/ml PV267 treated groups (p < 0.05, t-test).
To test whether PV-267 inhibited production of other proinflammatory cytokines by BC3 T cells supernatants were collected after stimulation of the cells with MBP87-99 peptide in the presence of autologous APCs and incubation with PV-267 at increasing concentrations. Supernatants were assayed by cytokine multiplex assay (human 27-multiplex, Fig. 1D-I and Table 3). The results show that PV-267 also inhibited the production of TNF-α and IL-1ra (Fig. 1D, 1E). Production of IL-6, IL-1β, MIP-1α, and IL-17 showed a trend to be decreased as PV-267 concentration increased (Fig. 1F-I), but the results did not reach statistical significance. Similarly, several other cytokines and chemokines including IFN-γ showed a trend to be decreased by PV-267 but the results did not achieve statistical significance (Table 3). Of note, BC3 T cells produced very low levels of IL-17, which we confirmed by cytokine ELISPOT assay (data not shown). No significant inhibition was observed for other cytokines tested (data not shown).
TABLE 3.
Summary of cytokine production of BC3 T cells cultured with Ag and PV-267.
| Ag stimulated cytokine production (pg/ml) |
0 μg/ml PV-267 |
200 μg/ml PV-267 |
Statistical difference *(p value) or NS |
|---|---|---|---|
| IL-8 | 17427.5 ± 10983.3 | 14791.0 ± 7191.8 | NS |
| MIP-1b | 3137.5 ± 2642.7 | 2409.0 ± 652.9 | NS |
| MCP-l(MCAF) | 1701.7 ± 1272.3 | 2001.4 ± 1079.3 | NS |
| IL-6 | 1228.6 ± 729.9 | 738.8 ± 261.0 | NS |
| RANTES | 905.5 ± 350.3 | 1124.9 ± 268.4 | NS |
| IP-10 | 703.8 ± 734.8 | 283.3 ± 302.2 | NS |
| MIP-1a | 399.5 ± 258.8 | 233.2 ± 69.2 | NS |
| PDGF-bb | 312.8 ± 50.5 | 415.9 ± 291.7 | NS |
| TNF-a | 225.8 ± 79.3 | 45.7 ± 34.9 * | p = 0.023 |
| IL-1ra | 215.6 ±27.2 | 128.2 ± 31.5 * | p = 0.022 |
| IL-13 | 126.7 ± 55.5 | 60.1 ± 78.5 | NS |
| VEGF | 96.2 ± 20.4 | 90.7 ± 33.9 | NS |
| IFN-g | 77.5 ± 22.9 | 46.2 ± 17.8 | NS |
| IL-9 | 35.3 ± 9.9 | 27.3 ± 9.1 | NS |
| IL-1b | 30.6 ± 13.2 | 19.7 ± 5.7 | NS |
| IL-10 | 17.9 ± 3.1 | 6.5 ± 2.4 | NS |
| G-CSF | 16.5 ± 9.5 | 11.8 ± 4.3 | NS |
| IL-17 | 15.5 ± 3.6 | 11.0 ± 1.2 | NS |
| Eotaxin | < 16.4 | < 23.5 | NS |
| IL-12(p70) | 9.7 ± 5.4 | 7.2 ± 5.2 | NS |
| FGF | 6.0 ± 1.1 | 2.1 ± 0.6* | p = 0.005 |
| IL-2 | 4.4 ± 0.4 | < 2.7* | p = 0.018 |
| IL-4 | 3.7 ± 0.5 | 2.5 ± 0.9 | NS |
| IL-7 | 2.0 ± 0.4 | < 3.7 | NS |
| IL-5 | < 3.3 | < 0.3 | NS |
| GM-CSF | < 0.5 | < 2.8 | NS |
| IL-15 | < 0.3 | < 0.2 | NS |
MBP specific BC3 T cells were cultured with Ag MBP87-99 or PHA as positive control and autologous APC pretreated with PV-267 at different concentrations for 24 h . Supernatant were collected and analyzed using Bio-Plex assay for human cytokines. Shown are the pooled result of the production of 27 human cytokines when BC3 T cells were cultured with Ag and PV-267 (0 – 200 μg/ml) from at least 3 independent experiments (MEAN±SD). NS, not significant.
Next, we tested whether PV-267 inhibited antigen-specific proliferation of BC3 T cells. As shown in Fig. 1J, PV-267 inhibited MBP87-99-induced proliferation of BC3 T cells in a dose-dependent manner as measured by CFSE-based proliferation assay. In contrast, proliferation induced by anti-CD3 mAb or PHA was not inhibited by PV-267. Taken together, the data showed that PV-267 inhibited antigen-induced cytokine production and proliferation of human HLA-DR2-restricted T cells.
Inhibition by PV-267 is specific for HLA-DR2b molecules
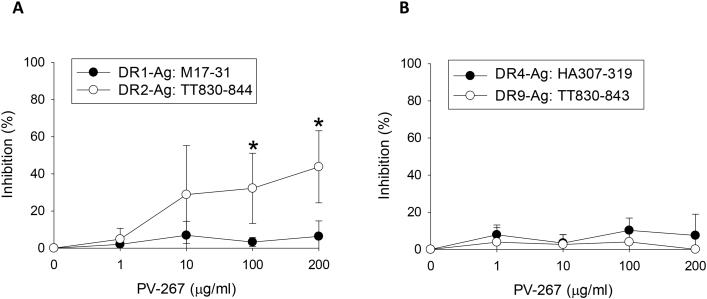
PV-267 inhibited human T cell responses to myelin peptides presented by HLA-DR2b molecules. To determine the specificity of the inhibition for HLA-DR2b as compared with other human MHC class II alleles, T cells were enriched from PBMC of healthy donors that expressed other MHC molecules including HLA-DR1 (DRB1*0101), HLA-DR4 (DRB1*0401), HLA-DR9 (DRB1*0901), with or without HLA-DR2b (DRB1*15:01). The PBMCs were activated with influenza or tetanus toxin peptides restricted by HLA-DR1, -DR4, -DR9, or -DR2b, respectively and tested by IFN-γ ELISPOT assay in the presence of increasing concentrations of PV-267.
As shown in Fig. 2A, PV-267 did not inhibit M17-31 peptide-induced IFN-γ cytokine production by HLA-DR1-restricted T cells. Similarly, T cell responses to the HLA-DR4-restricted HA307-319 and the HLA-DR9-restricted TT830-843 peptide, respectively, were also not inhibited by PV-267 in PBMCs co-expressing these MHC molecules (Fig. 2B). In strong contrast, T cell responses to the HLA-DR2b-restricted TT830-844 peptide were significantly inhibited by PV-267 (p < 0.05), confirming the selectivity of the MHC-blocker for HLA-DR2b. In addition, in vivo studies using HLA-DR4 (DRB1*04:01) transgenic mice (34) showed that PV-267 treatment did not inhibit Ag-specific cytokine response restricted by non-DR2b HLA molecules, consistent with its HLA-DR2b-specific inhibitory effect (Supplementary Fig. 1).
Figure 2.
PV-267 specifically inhibits DR2- but not DR1-, DR4-, or DR9-mediated T cell responses. T cell enriched human PBMCs were co-cultured with PV-267 pre-treated autologous APCs and the indicated Ag and tested by human IFN-γ ELISPOT assay as described in Materials and Methods. Shown are representative results of the percentage of inhibited response of (A) CTL donor#4 PBMCs (DRB1*0101/DRB1*15:01) to DR1 restricted Ag Influenza A Matrix Protein (M17-31) or DR2 restricted Ag Tetanus Toxin (TT830-844), and (B) CTL donor#9 PBMCs (DRB1*0401/DRB1*0901) to DR4 restricted Ag Influenza A Hemagglutinin (HA307-319) or DR9 restricted Ag Tetanus Toxin (TT830-843) from at least 3 independent experiments (mean ± SD). * indicates significant difference between the inhibition of response to DR1 and DR2 restricted peptide from donor#4 PBMCs (p< 0.05, t-test).
Taken together, the results showed that PV-276 specifically inhibited T cell responses mediated by HLA-DR2b, but not by HLA-DR1, -DR4, or DR9 molecules. The data support the selectivity of the inhibitor for HLA-DR2b.
PV-267 does not induce non-specific immune activation of human PBMC
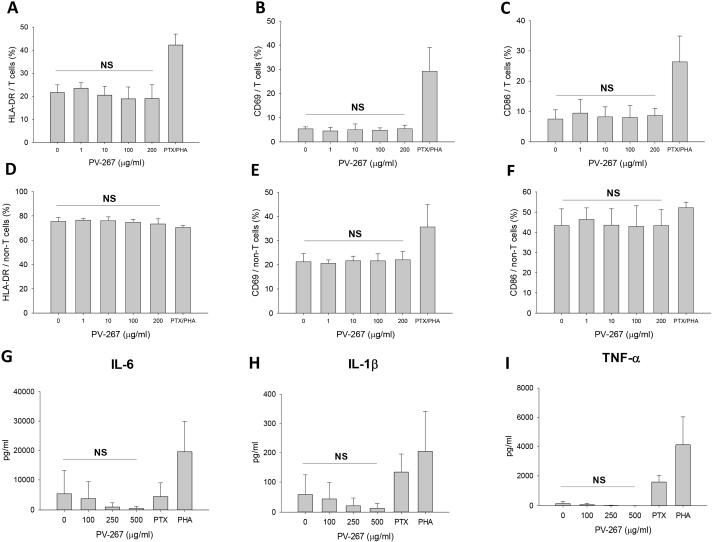
An important concern with immunomodulatory treatments is the induction of undesired detrimental side effects (16,35-37). Therefore we asked whether PV-267 had non-specific immune-activating effects on T cells or other PBMCs. To address this issue, HLA-DR2b-expressing PBMCs were incubated with increasing concentrations of PV-267 or with the mitogens PHA and PTX and expression of activation markers and surface molecules and production of cytokines by CD3+ T cells and non-T cells was determined by flow cytometry analysis and cytokine multiplex assay.
As expected, treatment of human PBMC with the mitogens PHA and PTX strongly upregulated the expression of the early activation marker CD69 on CD3+ T cells, as well as HLA-DR and CD86 (Fig. 3A-C). Furthermore, CD69 and CD86 molecules were upregulated by mitogen on non-T cells (Fig. 3E, 3F). In strong contrast, incubation of PBMC with PV-267 as high as 200 μg/ml did not result in the upregulation of these molecules (Fig. 3A-F). Furthermore, PV-267 also did not induce upregulation of CD25, CD28, and CD40 (data not shown).
Figure 3.
PV-267 does not non-specifically activate human PBMCs. Human PBMCs were cultured with PV-267 at different concentrations or with positive control (PTX+PHA). After 24 h, cells were collected and stained with fluorescent labeled mAb for HLA-DR, CD69 and CD86 cell surface markers followed by flow cytometry analysis. Shown are results for the expression of (A) HLA-DR, (B) CD69 and (C) CD86 on CD3+ T cells and (D) HLA-DR, (E) CD69 and (F) CD86 on non-T cells for at least 3 pooled independent experiments for each donor (mean ± SD). NS, no significant difference between 0 μg/ml PV-267 and 200 μg/ml PV-267 treated groups (t-test). Supernatants from the same cultures were collected and analyzed using cytokine multiplex assay (Bio-Plex). Shown are results of pro-inflammatory cytokine production of (G) IL-6, (H) IL-1β and (I) TNF-α from 3 pooled independent experiments (mean ± SD). NS, no significant difference between 0 μg/ml PV-267 and 500 μg/ml PV-267 treated groups (t-test).
To further establish whether or not PV-267 induced nonspecific immune activation of human PBMC, supernatants were collected from culture and analyzed by cytokine multiplex assay. The results show that incubation with PHA and PTX induced production of proinflammatory cytokines by human PBMC including IL-6, IL-1β, and TNF-α. In contrast, PV-267 did not induce significant production of cytokines at concentrations up to 500 μg/ml (Fig. 3G-I, Table 4). Of note, at very high concentrations of PV-267 (500 μg/ml) we observed induction of IP-10, RANTES, and PDGF-bb that was statistically not significant, but not of any other cytokine. Lastly, MIP-1β was significantly decreased by incubation with PV-267 (Table 4).
TABLE 4.
Summary of cytokine production of human PBMCs cultured with PV-267.
| Cytokine production (pg/ml) |
0 μg/ml PV-267 |
250 μg/ml PV-267 |
500 μg/ml PV-267 |
Statistical difference *(p value) or NS |
|---|---|---|---|---|
| IL-8 | 21481.3 ± 10022.3 | 15684.8 ± 14390.2 | 13472.2 ± 14245.7 | NS |
| IL-6 | 5303.1 ± 7899.7 | 964.0 ± 1373.6 | 488.7 ± 677.1 | NS |
| MCP-1(MCAF) | 4283.9 ± 3034.5 | 4848.5 ± 5346.3 | 4789.8 ± 4894.8 | NS |
| MIP-1b | 2064.8 ±283.6 | 917.5 ± 133.1 | 618.1 ± 168.3 | p < = 0.003 |
| MIP-1a | 568.4 ± 860.6 | 100.5 ± 129.7 | 46.4 ± 51.8 | NS |
| RANTES | 388.0 ± 328.9 | 549.3 ± 458.9 | 6991.3 ± 8147.6 | NS |
| IP-10 | 235.9 ± 166.4 | 1212.9 ± 961.2 | 8307.3 ± 4559.8 | NS |
| IL-1ra | 178.8 ± 90.4 | 201.1 ± 109.9 | 151.2 ± 69.4 | NS |
| PDGF-bb | 153.4 ± 114.4 | 250.0 ± 255.2 | 1251.4 ± 709.6 | NS |
| TNF-a | 133.2 ± 144.8 | 12.5 ± 15.3 | 7.0 ± 3.1 | NS |
| VEGF | 116.6 ± 38.1 | 111.3 ± 61.7 | 125.3 ± 58.3 | NS |
| G-CSF | 61.9 ± 86.6 | 9.6 ± 12.8 | 6.0 ± 7.6 | NS |
| IL-1b | 57.8 ± 66.7 | 20.5 ± 25.7 | 12.7 ± 15.9 | NS |
| IFN-g | 49.7 ± 42.9 | 28.0 ± 18.4 | 26.9 ± 6.2 | NS |
| Eotaxin | < 21.3 | < 15.7 | < 1.6 | NS |
| IL-10 | 19.7 ± 22.2 | 4.3 ± 2.9 | 2.0 ± 1.7 | NS |
| IL-9 | 16.7 ± 7.0 | 16.0 ± 8.1 | 12.3 ± 4.8 | NS |
| IL-17 | 11.5 ± 4.9 | 5.5 ± 4.7 | 4.3 ± 3.1 | NS |
| FGF | 9.9 ± 3.3 | < 4.5 * | 2.7 ± 1.2 | p < = 0.03 |
| GM-CSF | < 9.8 | < 6.9 | < 1.3 | NS |
| IL-12(p70) | 9.9 ± 0.9 | 4.5 ± 1.2* | 2.5 ± 2.0* | p < 0.004 |
| IL-2 | 3.0 ± 1.8 | 6.9 ± 3.3 | 6.4 ± 2.3 | NS |
| IL-7 | 2.6 ± 1.7 | 0.3 ±0.5 | 0.6 ± 0.5 | NS |
| IL-4 | 2.0 ± 1.3 | 1.2 ± 0.6 | 1.4 ±0.2 | NS |
| IL-13 | 1.3 ± 0.6 | < 0.3 * | < 0.3 * | p < = 0.043 |
| IL-15 | < 0.5 | < 0.5 | < 0.19 | NS |
| IL-5 | < 0.2 | < 0.2 | < 0.2 | NS |
Human PBMCs were cultured for 24 h with PV-267 at different concentrations, or with positive control (PTX or PHA). Supernatant were collected and analyzed using Bio-Plex assay for human cytokines. Shown are the summary of the level and profile of 27 human cytokines when PBMCs were cultured with PV-267 (0 – 250 μg/ml or maximum 500 μg/ml) from at least 3 independent experiments of 3 human PBMC donors. NS, not significant.
Collectively, the results show that PV-267 did not have undesired nonspecific effects on the activation of human T cells or other PBMCs (e.g., monocytes or NK cells) and the production of proinflammatory cytokines. In addition, mice treated daily with up to 400 mg/Kg of PV-267 for 2 weeks did not show a dose-limiting toxicity (data not shown) suggesting a promising general as well as immunological safety profile.
PV-267 ameliorates EAE in HLA-DR2b transgenic mice
Based on the property of PV-267 to inhibit proliferation and cytokine production by HLA-DR2b-restricted human myelin antigen-specific T cells in vitro we asked whether the compound showed efficacy in the prevention and/or treatment of EAE in HLA-DR2b transgenic mice as a preclinical model for human MS.
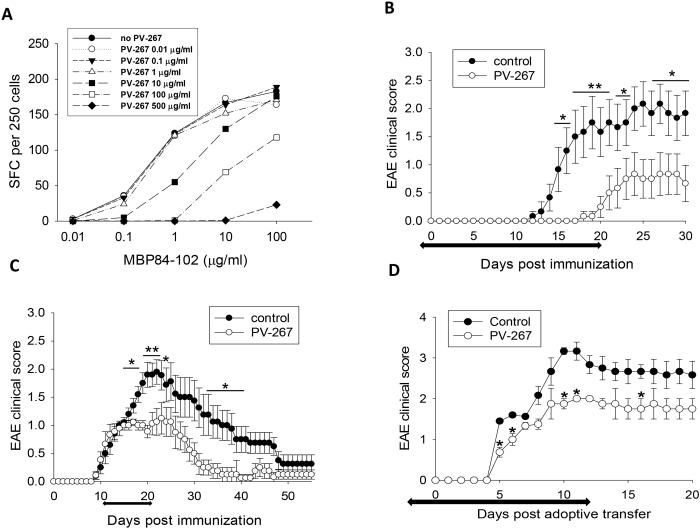
First, we tested whether PV-267 inhibited cytokine production by HLA-DR2b-restricted T cells generated in HLA-DR2b (DRB1*15:01) transgenic mice deficient for endogenous murine MHC class II molecules on the C57BL/6 background (27). To address this question, PV-267 was titrated against increasing concentrations of the cognate MBP peptide using a T cell clone specific for MBP84-102 generated from the HLA-DR2b transgenic mice. The results showed that PV-267 inhibited production of IFN-γ by MBP84-102 peptide-specific murine T cells in a dose-dependent fashion (Fig. 4A). In contrast, a non-DR2b-binding control compound (PV-015) did not inhibit T cell responses (data not shown).
Figure 4.
PV-267 inhibits INF-γ production by HLA-DR2b-restricted, MBP 84-102-specific T cells and ameliorates EAE in HLA-DR2b mice. MBP84-102 specific T cell clone derived from HLA-DR2b transgenic mice was cultured with APCs, cognate MBP peptide at increasing concentrations and PV-267 was titrated as indicated and cytokine production was assessed by IFN-γ ELISPOT assay (A). HLA-DR2b transgenic mice were immunized with MOG35-55 peptide, then monitored and scored daily for clinical EAE as described in Materials and Methods. Mice were injected i.p. with (B) 30 mg/kg of PV-267 or control daily starting on d(-1) for 20 days, or (C) 50 mg/kg of PV-267 or control daily starting upon disease onset (d11) for 10 days (n = 10 -12 mice per group, mean ± SD) . D) HLA-DR2b transgenic recipient mice were adoptively transferred with encephalitogenic MOG35-55 peptide-specific donor cells as described in Materials and Methods. Recipients were also injected i.p. with 50 mg/kg of PV-267 or control daily starting on d(-2) for 15 days (n = 4 or 5, mean ± SEM). Shown are the representative results of 3 independent experiments. * indicates significant difference between PV-267 and vehicle treated groups, *, p < 0.05, **, p < 0.01, t-test.
Next, we investigated whether PV-267 was efficacious in treating EAE induced by active immunization with the MOG35-55 peptide in the HLA-DR2b transgenic mice. To test whether the compound was efficacious in a prevention mode, the HLA-DR2b transgenic mice were injected once daily with PV-267 (30 mg/kg) or vehicle control (30% Captisol) starting on d(−1) before induction of EAE and for 20 days after induction. Disease was induced with MOG35-55 peptide and the animals were observed for clinical signs of disease for 30 days as described (29). As shown in Fig. 4B and supplementary Table 1 (analysis of cumulative EAE scores) PV-267 efficiently prevented EAE and significantly delayed disease onset and lowered disease scores as compared to the control group. To test the efficacy of PV-267 for treatment of established EAE, disease was induced as outlined above and the mice randomized at the onset of EAE (d 11) into two groups. Subsequently, mice were injected once daily over 10 days with PV-267 (50 mg/kg) or carrier control and observed for clinical EAE. Importantly, treatment with PV-267 significantly ameliorated disease after onset of EAE and the animals recovered faster (Fig. 4C). Of note, the PV-267 treated mice did not show rebound disease after termination of the treatment (Fig. 4C). Analysis of cumulative EAE scores of PV-267 treated and control mice confirmed treatment efficacy of the treatment (supplementary Table 1). Lastly, PV-267 was efficacious when EAE was induced by adoptive transfer of MOG35-55-reactive T cells (Fig. 4D).
Lastly, we examined serum from the EAE mice for possible Ab production against PV-267 due to the injections. However, we did not detect substantial serum IgG production against PV-267 as compared with production of IgG against MOG35-55 in PV-267 treated DR2b transgenic mice (Supplementary Fig. 2).
Taken together, the results showed that PV-267 was safe and highly effective in preventing and treating EAE in HLA-DR2b transgenic mice.
Discussion
In this study we report development of a small molecule inhibitor of human HLA-DR2b molecules that can curtail myelin-reactive T cell responses. The lead compound used in these studies, PV-267, inhibited cytokine production and proliferation by MBP87-99 specific HLA-DR2b-restricted CD4+ T cells derived from an MS patient. Furthermore, PV-267 similarly inhibited murine MBP-reactive T cells in an HLA-DR2b transgenic mouse model and ameliorated EAE both in prevention and treatment mode. Importantly, PV-267 did not have non-specific immune-activating effects on human PBMCs in vitro and did not result in upregulation of activating/costimulatory receptors and production of cytokines.
The mode of action of PV-267 is proposed to be via specific binding of the molecule to the peptide groove of the HLA-DR2b molecule and thus blocking of antigen recognition by T cells. This interpretation is based on the observation that inhibition of T cell responses was only observed with HLA-DR2b-restricted T cell responses, but not with T cell responses mediated by other human MHC II molecules such as HLA-DR1, DR4, or DR9. In line with specific inhibition of HLA-DR2b:peptide presentation, PV-267 did not show an effect when T cells or PBMC were activated with anti-CD3 mAb, which circumvents peptide recognition by T cells. In addition, incubation of human PBMC or T cell lines with PV-267 did not result in increased T cell death, arguing against cytotoxic effects of the compound.
Silencing pathogenic T cells via inhibition of HLA-DR2b-mediated presentation of neuroantigens as shown here advances the concept of specific immunotherapy without invoking undesired immunomodulatory effects observed in clinical trials of oral tolerance, altered peptide ligands, T cell vaccination, recombinant T cell receptor peptide therapy etc. (8,12,13). Most of these approaches have shown activity in preclinical animal models but were of limited efficacy in clinical trials (8,15,38). Concerns were failure to show sufficient clinical efficacy as compared with placebo as well as development of serious side effects. Thus, most of these approaches have been abandoned and strategies have shifted to broad-based immunomodulatory therapies targeting widely expressed molecules, such as homing receptors and inflammatory mediators. However, targeting of molecules expressed by many cell types, such as VLA-4, brings with it the inherent risk of global immunosuppression, as well as the potential to have significant undesired immunomodulatory and/or immune activating effects. The potentially dire consequences were clearly evidenced by development of PML in MS patients treated with natalizumab due to reactivation of JC virus (16).
HLA-DR2b has repeatedly been confirmed as the most important gene complex lined to MS (39). While the exact reasons for this genetic linkage have not yet been elucidated, it is conceivable that the critical contribution of these molecules is via presentation of peptide antigens to myelin-reactive T cells resulting in their activation, proliferation, and production of pathogenic cytokines. Thus, inhibition of antigen presentation specifically by the MS-associated HLA-DR2b molecules is a conceptually attractive means to prevent the activation of pathogenic T cells in MS patients. This inhibition could take effect both in peripheral lymphatic tissues as well as directly in the CNS and would be independent of the nature of the autoantigenic peptides.
To block HLA-DR2b, several key factors needed to be addressed. First, an inhibitor would need to bind to HLA-DR2b with high affinity and selectivity to be able to compete with antigen binding in the endosomal loading compartment of APCs and in the presence of HLA-DM, a catalyst for peptide-MHC exchange (40,41). Second, since the endosomal compartment of the APCs is rich in cathepsin enzymes that process MHC molecules and degrade protein antigens into peptide fragments that bind the MHC, an inhibitor would need to be stabilized against cathepsins as well as show stability in plasma. Of note, once a peptide (antigen or inhibitor) is loaded, the MHC complex translocates to the cell surface where it has a very low off-rate and remains bound and is subject to a turnover rated ranging in days. Next, an effective inhibitor, to be differentiated from the other strategies described, needs to be highly selective in binding to the disease-associated DR2b allele (in MS) to avoid interfering with immune responses to pathogens via other MHC molecules. In effect, this would make this a personalized medicine approach targeted towards MS patients carrying the HLA-DR2b allele. Finally, the inhibitor needed to be immunologically inert as to not stimulate an immune response on its own.
In keeping with the above outlined key requirements, development of an HLA-DR2b-selective inhibitor appeared to be feasible based on previously established differences in key positions in the antigen binding pocket between different MHC class II molecules. The unique characteristics of the binding pocket of DRB1*15:01 may be attributed to the small Ala residue at position-71 (p71) of the MHC beta chain. This opens up the space for a larger P4 residue in the inhibitor sequence as a way of achieving selectivity vs. DRB1*0101 and DRB1*0401 (where p71 is positively charged (Arg or Lys) in these DR1/DR4 alleles, respectively, and the chain length blocks off space so that the Phe residue is a good fit to DR2b, but too large to bind DR1/DR4. Also, the hydrophobic nature of the P1 pocket is a characteristic of DR alleles, whereas this site is polar in MHC DQ alleles. Thus, a Chg group at P1 selects for DR vs DQ and a Phe at P4 selects for DR2b versus DR1/DR4. The small p71 Ala group is unique to DR2b so selectivity can be achieved with ligands that have larger P4 groups (e.g., Phe) that would not fit the corresponding pocket in DR1, where p71 is Arg, or DR4 where it is Lys.
An important consequence of selectively blocking HLA-DR2b in MS patients is that it should inhibit the autoimmune T cell responses associated with MS, but not affect T cell responses mediated via other MHC alleles. Thus, an HLA-DR2b inhibitor should not impair protective immunity towards microbial pathogens mediated by other MHC class II alleles. Our results support the feasibility of this presumption by showing that PV-267 did not impair T cell responses to peptides derived from microbial pathogens restricted by other MHC class II molecules such as HLA-DR1, -DR4, and DR9.
Another key conceptual benefit of inhibiting antigen presentation by the MS-associated DR2b allele is its utility in globally inhibiting autoreactive T cells irrespective of their specificity for particular autoantigenic peptides. In other words, by blocking HLA-DR2b itself it becomes irrelevant whether autoimmune T cells respond to MBP, MOG, or PLP peptides etc., as long as they are restricted by HLA-DR2b. Furthermore, this approach eliminates potential concerns over epitope spreading (42,43).
Since MS and other autoimmune diseases are chronic, life-long diseases they may require long-term treatment and thus accumulation of long-term side effects are a major concern. For example, treatments that deplete immune cells, even if specific for certain subsets of cells such as B cells, will incur the potential to cause significant harm if applied over a longer period of time. Often, it is difficult to predict serious side effects, as for example evidenced by development of lymphomas in rheumatoid arthritis or Crohn’s disease patients treated with TNF immunomodulators (44-46). Thus, treatments with as few side effects as possible are highly desirable, and, conceptually, MHC II inhibitors selective for one MHC class II allele could fulfill that tenet. First, as outlined earlier, protection from infectious microorganisms would still be mediated via the other MHC class II alleles expressed in an individual. Furthermore, since the only function of MHC blockers is to occupy the peptide binding groove of one specific MHC II allele without having any other effect on the immune system including being non-toxic, detrimental side effects are not expected even over a prolonged course of treatment. Our results showing a lack of effect of PV-267 on the activation or cytokine production by T cells or other PBMCs support this view and suggest that long-term treatment with this class of molecules may be feasible without resulting in detrimental side effects found with immunomodulatory drugs. Importantly, lack of side effects such as flu-like symptoms in patients treated with beta-interferons will facilitate treatment adherence and conceivably improve long-term outcomes. Furthermore, the lack of side effects may facilitate development of prevention strategies for people at risk but not yet affected by MS, such as monozygotic twins of MS patients who have a 25% risk to develop the disease (47,48) or in patients with diagnoses of clinically isolated syndrome (CIS). All in all, our results support the concept of MHC-specific immunotherapy based on blockade of autoantigen-presentation by autoimmune disease-associated MHC class II molecules such as HLA-DR2b.
Supplementary Material
Acknowledgments
We wish to acknowledge the following who have contributed to the efforts described in this work: (a) Peptide synthesis: Nallaganchu Rao, Hong Chen, Evita Sadimin, William May, Barbara Sluboski. (b) Biochemical assays: Bernard Maillere, Lora Hamuro (c) Modeling: Charles Cook. (d) DR2b transgenic mice: Lars Fugger. (e) EAE studies: Susan Cantin, Gabriela Garcia, and Belen Carillo-Rivas.
Footnotes
This work was supported by grants NS048731 (Provid) and G12MD007591 (T.G.F.) from the National Institute of Health, grant RG3701 from the National Multiple Sclerosis Society (T.G.F.), and by Fast Forward LLC. (Provid and T.G.F.).
Reference List
- 1.Krogsgaard M, Wucherpfennig KW, Canella B, Hansen BE, Svejgaard A, Pyrdol J, Ditzel H, Raine C, Engberg J, Fugger L. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85-99 complex. Journal of Experimental Medicine. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valli A, Sette A, Kappos L, Oseroff C, Sidney J, Miescher G, Hochberger M, Albert ED, Adorini L. Binding of Myelin Basic-Protein Peptides to Human Histocompatibility Leukocyte Antigen Class-Ii Molecules and Their Recognition by T-Cells from Multiple-Sclerosis Patients. Journal of Clinical Investigation. 1993;91:616–628. doi: 10.1172/JCI116242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergelli M, Kalbus M, Rojo SC, Hemmer B, Kalbacher H, Tranquill L, Beck H, McFarland HF, DeMars R, Long EO, Martin R. T cell response to myelin basic protein in the context of the multiple sclerosis-associated HLA-DR15 haplotype: Peptide binding, immunodominance and effector functions of T cells. Journal of Neuroimmunology. 1997;77:195–203. doi: 10.1016/s0165-5728(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, Myers LW, Panitch HS, Rose JW, Schiffer RB, Vollmer T, Weiner LP, Wolinsky JS, Bird SJ, Constantinescu C, Kolson DL, Gonzalezscarano F, Brennan D, Pfohl D, Mandler RN, Rosenberg GA, Jeffrey C, Barger GR, Gandhi B, Moore PM, Rogers LR, Lisak D, Smith L, Ellison GW, Baumhefner RW, Craig SL, Jalbut SS, Katz E, Conway KL, Burns JB, Shiba C, Giang DW, Petrie MD, Guarnaccia JB, Mckeon S. Anderson, A., Mccarthy M, Thomas AB, Vriesendorp FJ, Austin SG, Lindsey JW, Dimachkie M, Cerreta E, Kachuck N, Mccarthy KA, Fleming J, Parnell JH, Tamulevich J, Weasler C, Kadosh S, Halt H, Stark Y, Pinchasi I, Spiller N, Vandennoort S, Miller A, Mellits D, Hopkins J, Reingold S, Gomolin IH. Copolymer-1 Reduces Relapse Rate and Improves Disability in Relapsing-Remitting Multiple-Sclerosis - Results of A Phase-Iii Multicenter, Double-Blind, Placebo-Controlled Trial. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 5.Duquette P, Girard M, Despault L, Dubois R, Knobler RL, Lublin FD, Kelley L, Francis GS, Lapierre Y, Antel J, Freedman M, Hum S, Greenstein JI, Mishra B, Muldoon J, Whitaker JN, Evans BK, Layton B, Sibley WA, Laguna J, Krikawa J, Paty DW, Oger JJ, Kastrukoff LF, Moore GRW, Hashimoto SA, Morrison W, Nelson J, Goodin DS, Massa SM, Gutteridge E, Arnason BGW, Noronha A, Reder AT, Martia R, Ebers GC, Rice GPA, Lesaux J, Johnson KP, Panitch HS, Bever CT, Conway K, Wallenberg JC, Bedell L, Vandennoort S, Weinshenker B, Weiss W, Reingold S, Pachner A, Taylor W. Interferon Beta-1B Is Effective in Relapsing-Remitting Multiple-Sclerosis - Clinical-Results of A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 6.Ebers GC, Rice G, Lesaux J, Paty D, Oger J, Li DKB, Beall S, Devonshire V, Hashimoto S, Hooge J, Kastrukoff L, Krieger C, Mezei M, Seland P, Vorobeychi G, Morrison W, Nelson J, Freedman MS, Chrisie S, Nelson R, Rabinovitch H, Freedman C, Hartung HP, Rieckmann P, Archelos J, Jung S, Weilbach F, Flachenecke P, Sauer J, Hommes O, Jongen P, Brouwer S, McLeod J, Pollard J, Ng R, Sandberg-Wollheim M, Kallen K, Nilsson P, Ekberg R, Lundgren A, Jadback G, Wikstrom J, Multanen J, Valjakka M, Carton H, Lissoir F, Declerq I, Vieren M, Peeters E, Dubois B, Dekeersmaeker E, Van Herle A, Hughes RAC, Sharrack B, Soudain S, Panelius M, Eralinna J, Soilu-Hanninen M, Murto S, Medaer R, Broeckx J, Vanroose E, Bogaers A, Blumhardt LD, Edwards S, Liu C, Orpe V, Barnes D, Schwartz M, Stoy N, Harraghy C, Bertelsmann F, Uitdehaag B, Nasseri K, Chofflon M, Roth S, Kappos L, Huber S, Bellaiche Y, Senn C, King J, Jubert J, Whitten S, Newsom-Davis JM, Palace J, Lee M, Evangelou N, Pinto A, Cavey A, Sindic CJM, Monteyne P, Verougstraete D, Van Doorn PA, Moll W, Visser L, Willems M, Martina I, Buljevac D, Loman L, Bates D, Pandit D, Irving J, Rhodes B, Riddehough A, Zhao GJ, Wang X, Cheng Y, Ammoury N, Dupont F, Galazka A, Hyde R, Olson M, O. Pernin M, bdul-Ahad AK, Hommes O, Noseworthy J, Borden E, O'Brien P, Wolinsky J. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- 7.Whitacre CC, Gienapp IE, Meyer A, Cox KL, Javed N. Oral tolerance in experimental autoimmune encephalomyelitis. Oral Tolerance: Mechanisms and Applications. 1996;778:217–227. doi: 10.1111/j.1749-6632.1996.tb21130.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, Hafler DA. Double-Blind Pilot Trial of Oral Tolerization with Myelin Antigens in Multiple-Sclerosis. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 9.Brod SA, Alsabbagh A, Sobel RA, Hafler DA, Weiner HL. Suppression of Experimental Autoimmune Encephalomyelitis by Oral-Administration of Myelin Antigens .4. Suppression of Chronic Relapsing Disease in the Lewis Rat and Strain-13 Guinea-Pig. Annals of Neurology. 1991;29:615–622. doi: 10.1002/ana.410290608. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz PJ, Garren H, Hirschberg DL, Langer-Gould AM, Levite M, Karpuj MV, Southwood S, Sette A, Conlon P, Steinman L. Microbial epitopes act as altered peptide ligands to prevent experimental autoimmune encephalomyelitis. Journal of Experimental Medicine. 1999;189:1275–1283. doi: 10.1084/jem.189.8.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergelli M, Hemmer B, Utz U, Vogt A, Kalbus M, Tranquill L, Conlon P, Ling N, Steinman L, McFarland HF, Martin R. Differential activation of human autoreactive T cell clones by altered peptide ligands derived from myelin basic protein peptide (87-99) European Journal of Immunology. 1996;26:2624–2634. doi: 10.1002/eji.1830261113. [DOI] [PubMed] [Google Scholar]
- 12.Bourdette DN, Chou YK, Whitham RH, Buckner J, Kwon HJ, Nepom GT, Buenafe A, Cooper SA, Allegretta M, Hashim GA, Offner H, Vandenbark AA. Immunity to T cell receptor peptides in multiple sclerosis. III. Preferential immunogenicity of complementarity-determining region 2 peptides from disease-associated T cell receptor BV genes. Journal of Immunology. 1998;161:1034–1044. [PubMed] [Google Scholar]
- 13.Bourdette DN, Edmonds E, Smith C, Bowen JD, Guttmann CRG, Nagy ZP, Simon J, Whitham R, Lovera J, Yadav V, Mass M, Spencer L, Culbertson N, Bartholomew RM, Theofan G, Milano J, Offner H, Vandenbark AA. A highly immunogenic trivalent T cell receptor peptide vaccine for multiple sclerosis. Multiple Sclerosis. 2005;11:552–561. doi: 10.1191/1352458505ms1225oa. [DOI] [PubMed] [Google Scholar]
- 14.Link JM, Rich CM, Korat M, Burrows GG, Offner H, Vandenbark AA. Monomeric DR2/MOG-35-55 recombinant TCR ligand treats relapses of experimental encephalomyelitis in DR2 transgenic mice. Clinical Immunology. 2007;123:95–104. doi: 10.1016/j.clim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nature Medicine. 2000;6:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 16.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of Natalizumab-Associated Progressive Multifocal Leukoencephalopathy. New England Journal of Medicine. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 17.Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. New England Journal of Medicine. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Oral fingolimod (FTY720) in multiple sclerosis Two-year results of a phase II extension study. Neurology. 2009;72:73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor PW, Li D, Freedman MS, Bar-Or A, Rice GPA, Paty DW, Stewart JA, Scheyer R. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66:894–900. doi: 10.1212/01.wnl.0000203121.04509.31. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS. Randomized Trial of Oral Teriflunomide for Relapsing Multiple Sclerosis. New England Journal of Medicine. 2011;365:1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 21.Coles AJ, Compston DAS, Selmaj KW, Lake SL, Moran S, Margolin DH, Norris K, Tandon PK. Alemtuzumab vs. Interferon beta-1a in early multiple sclerosis. New England Journal of Medicine. 2008;359:1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 22.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. New England Journal of Medicine. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt H, Williamson D, Ashley-Koch A. HLA-DR15 haplotype and multiple sclerosis: a HuGE review. Am. J. Epidemiol. 2007;165:1097–1109. doi: 10.1093/aje/kwk118. [DOI] [PubMed] [Google Scholar]
- 24.Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. Crystallographic analysis of endogenous peptides associated with HLA-DR1 suggests a common, polyproline II-like conformation for bound peptides. Proc. Natl. Acad. Sci. U. S. A. 1996;93:734–738. doi: 10.1073/pnas.93.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. Journal of Experimental Medicine. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishioka GY, Adorini L, Guery JC, Gaeta FC, LaFond R, Alexander J, Powell MF, Sette A, Grey HM. Failure to demonstrate long-lived MHC saturation both in vitro and in vivo. Implications for therapeutic potential of MHC-blocking peptides. J. Immunol. 1994;152:4310–4319. [PubMed] [Google Scholar]
- 27.Kawamura K, McLaughlin KA, Weissert R, Forsthuber TG. Myelin-reactive type B T cells and T cells specific for low-affinity MHC-binding myelin peptides escape tolerance in HLA-DR transgenic mice. Journal of Immunology. 2008;181:3202–3211. doi: 10.4049/jimmunol.181.5.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund's adjuvant: Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. Journal of Immunology. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Fleming KK, Bovaird JA, Mosier MC, Emerson MR, LeVine SM, Marquis JG. Statistical analysis of data from studies on experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;170:71–84. doi: 10.1016/j.jneuroim.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Graham GJ, Damodaran A, Hu T, Lira SA, Sasse M, Canasto-Chibuque C, Cook DN, Ransohoff RM. Cutting edge: the silent chemokine receptor D6 is required for generating T cell responses that mediate experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:17–21. doi: 10.4049/jimmunol.177.1.17. [DOI] [PubMed] [Google Scholar]
- 32.Bolin DR, Swain AL, Sarabu R, Berthel SJ, Gillespie P, Huby NJS, Makofske R, Orzechowski L, Perrotta A, Toth K, Cooper JP, Jiang N, Falcioni F, Campbell R, Cox D, Gaizband D, Belunis CJ, Vidovic D, Ito K, Crowther R, Kammlott U, Zhang XL, Palermo R, Weber D, Guenot J, Nagy Z, Olson GL. Peptide and peptide mimetic inhibitors of antigen presentation by HLA-DR class II MHC molecules. Design, structure-activity relationships, and X-ray crystal structures. Journal of Medicinal Chemistry. 2000;43:2135–2148. doi: 10.1021/jm000034h. [DOI] [PubMed] [Google Scholar]
- 33.Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC. Crystal-Structure of the Human Class-Ii Mhc Protein Hla-Dr1 Complexed with An Influenza-Virus Peptide. Nature. 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 34.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, Falcioni F, Vidovic D, Hammer J, Nagy ZA. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buttinelli C, Clemenzi A, Borriello G, Denaro F, Pozzilli C, Fieschi C. Mitoxantrone treatment in multiple sclerosis: a 5-year clinical and MRI follow-up. European Journal of Neurology. 2007;14:1281–1287. doi: 10.1111/j.1468-1331.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- 36.Charach G, Grosskopf I, Weintraub M. Development of Crohn's disease in a patient with multiple sclerosis treated with Copaxone. Digestion. 2008;77:198–200. doi: 10.1159/000143156. [DOI] [PubMed] [Google Scholar]
- 37.Turaka K, Bryan JS. Does fingolimod in multiple sclerosis patients cause macular edema? Journal of Neurology. 2012;259:386–388. doi: 10.1007/s00415-011-6367-4. [DOI] [PubMed] [Google Scholar]
- 38.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, Martin R. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nature Medicine. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 39.Link J, Kockum I, Lorentzen AR, Lie BA, Celius EG, Westerlind H, Schaffer M, Alfredsson L, Olsson T, Brynedal B, Harbo HF, Hillert J. Importance of Human Leukocyte Antigen (HLA) Class I and II Alleles on the Risk of Multiple Sclerosis. Plos One. 2012:7. doi: 10.1371/journal.pone.0036779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stratikos E, Wiley DC, Stern LJ. Enhanced catalytic action of HLA-DM on the exchange of peptides lacking backbone hydrogen bonds between their N-terminal region and the MHC class II alpha-chain. J. Immunol. 2004;172:1109–1117. doi: 10.4049/jimmunol.172.2.1109. [DOI] [PubMed] [Google Scholar]
- 41.Anders AK, Call MJ, Schulze MS, Fowler KD, Schubert DA, Seth NP, Sundberg EJ, Wucherpfennig KW. HLA-DM captures partially empty HLA-DR molecules for catalyzed removal of peptide. Nat. Immunol. 2011;12:54–61. doi: 10.1038/ni.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-Cell Autoimmunity to Cryptic Determinants of An Autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 43.Mcrae BL, Vanderlugt CL, Dalcanto MC, Miller SD. Functional Evidence for Epitope Spreading in the Relapsing Pathology of Experimental Autoimmune Encephalomyelitis. Journal of Experimental Medicine. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development - Twenty-six cases reported to the Food and Drug Administration. Arthritis and Rheumatism. 2002;46:3151–3158. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- 45.Geborek P, Bladstrom A, Turesson C, Gulfe A, Petersson IF, Saxne T, Olsson H, Jacobsson LTH. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Annals of the Rheumatic Diseases. 2005;64:699–703. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of Lymphoma Associated With Combination Anti-Tumor Necrosis Factor and Immunomodulator Therapy for the Treatment of Crohn's Disease: A Meta-Analysis. Clinical Gastroenterology and Hepatology. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen T, Skytthe A, Stenager E, Petersen HC, Bronnum-Hansen H, Kyvik KO. Concordance for multiple sclerosis in Danish twins: an update of a nationwide study. Multiple Sclerosis. 2005;11:504–510. doi: 10.1191/1352458505ms1220oa. [DOI] [PubMed] [Google Scholar]
- 48.Baranzini SE, Mudge J, van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, Zhang L, Farmer AD, Bell CJ, Kim RW, May GD, Woodward JE, Caillier SJ, McElroy JP, Gomez R, Pando MJ, Clendenen LE, Ganusova EE, Schilkey FD, Ramaraj T, Khan OA, Huntley JJ, Luo SJ, Kwok P, Wu TD, Schroth GP, Oksenberg JR, Hauser SL, Kingsmore SF. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010;464:1351–13U6. doi: 10.1038/nature08990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.