Abstract
Although formaldehyde (FA) has been classified as a human leukemogen, the mechanisms of leukemogenesis remain elusive. Previously, using colony-forming assays in semi-solid media, we showed that FA exposure in vivo and in vitro was toxic to human hematopoietic stem/progenitor cells. In the present study, we have applied new liquid in vitro erythroid expansion systems to further investigate the toxic effects of FA (0–150 µM) on cultured mouse and human hematopoietic stem/progenitor cells. We determined micronucleus (MN) levels in polychromatic erythrocytes (PCEs) differentiated from mouse bone marrow. We measured cell growth, cell cycle distribution, and chromosomal instability, in erythroid progenitor cells (EPCs) expanded from human peripheral blood mononuclear cells. FA significantly induced MN in mouse PCEs and suppressed human EPC expansion in a dose-dependent manner, compared with untreated controls. In the expanded human EPCs, FA slightly increased the proportion of cells in G2/M at 100 µM and aneuploidy frequency in chromosomes 7 and 8 at 50 µM. Our findings provide further evidence of the toxicity of FA to hematopoietic stem/progenitor cells and support the biological plausibility of FA-induced leukemogenesis.
Keywords: formaldehyde, erythroid progenitor, micronuclei, aneuploidy
1. Introduction
Formaldehyde (FA), the simplest aldehyde, is a high production volume industrial chemical and a ubiquitous environmental pollutant (NTP, 2010a). FA is highly reactive and causes multiple toxic effects in humans. The International Agency for Research on Cancer (IARC) classified FA as a human carcinogen that causes nasopharyngeal cancer in 2006 (IARC, 2006) and human leukemia, particularly myeloid leukemia, in 2009 (Baan et al., 2009). Recently, the U.S. National Toxicology Program also classified FA as human leukemogen (NTP, 2011).
Though the mechanisms underlying FA-induced leukemogenesis remain elusive, FA exposure has been associated with adverse effects on hematopoiesis. Our review of the Chinese literature reported that decreased white blood cell counts were observed in most studies of FA-exposed workers; in the largest study, exposed workers had higher percentages of blood abnormalities (decreased white blood cell and platelet counts and abnormal hemoglobin levels) (Tang et al., 2009). We also reported that Chinese factory workers exposed to high levels of FA had significantly lower counts of granulocytes, platelets, red blood cells, and lymphocytes, compared with unexposed controls (Zhang et al., 2010). The suppression of multiple lineages indicates a toxic effect of FA exposure on hematopoietic stem / progenitor cells (HSC/HPC), the target cells of leukemogenesis.
Colony-forming assays in semi-solid substrate enable the direct examination of the adverse effects of FA on HSC/HPC circulating in peripheral blood. Using these assays, we previously reported that FA exposure in vivo was associated with decreased formation of colonies from colony forming units – granulocyte and monocyte (CFU-GM) cells and the induction of leukemia-related aneuploidies monosomy 7 and trisomy 8 in CFU-GM in a subset of the subjects (Zhang et al., 2010). Further, we showed that FA exposure at toxicologically relevant concentrations in vitro decreased formation of CFU-GM, burst forming units – erythrocyte (BFU-E) and the more primitive colony forming units – granulocyte, erythrocyte, monocyte, and megakaryocyte (CFU-GEMM) colonies, the latter in a linearly dose-dependent manner (Zhang et al., 2010). These data supported the inhibitory effect of FA on myeloid progenitor cells indicated by the blood count data. However, limited mechanistic studies could be conducted as the colonies were formed in semi-solid medium.
Recently, in vitro methodologies were developed that utilize cytokines to drive differentiation or expansion and yield large numbers of mouse and human erythroid progenitor cells, facilitating the analysis of multiple endpoints. An in vitro liquid culture method that recapitulates erythropoietic differentiation from mouse bone marrow progenitors, producing polychromatic erythrocytes (PCEs) after 2–3 days in culture, was established in 2007 (Shuga et al., 2007). This method forms the basis of an in vitro micronucleus (MN) genotoxicity assay that was found to generate similar results as the widely used in vivo MN genotoxicity assay, thus generating physiologically relevant data (Shuga et al., 2007). Recently, we validated this assay in a study in which we found increased MN frequency in PCEs cultured from mouse bone marrow exposed to 2,5- dimethylfuran (Fromowitz et al., 2012).
A liquid culture approach to expand human erythroid progenitor cells (EPCs) from unfractionated peripheral blood was recently described (Filippone et al., 2010). The authors confirmed the functional competence of the expanded EPCs by showing their permissivity to B19 parvovirus infection. We recognized in this model a unique opportunity to test human stem/progenitor cell toxicity of known and suspected leukemogens. To our knowledge, we are the first researchers to use this new erythroid expansion model for this purpose.
In the present study, we employed both of these in vitro liquid culture systems to test the effects of FA on mouse PCEs and human EPCs. We measured MN frequency in FA-treated and untreated mouse PCEs and the expansion of FA-treated and untreated human EPCs. We also examined the effects of FA on cell proliferation and chromosomal instability in the expanded human EPCs.
2. Methods
2.1. Mouse erythropoietic culture
The experimental procedures in mice were approved by the Committee on Animal Research at the University of California, Berkeley. The mouse erythropoietic culture method was detailed previously (Fromowitz et al., 2012; Shuga et al., 2007). In brief, bone marrow (BM) cells were isolated from the hind legs of C57BL/6J mice and were labeled with biotin-conjugated α-Lin Abs, consisting of α-CD3e, α-CD11b, α- CD45R/B220, α-Ly6G/Ly6C, and α-TER-119 Abs (2 µl of each Ab/106 cells; BD Pharmingen, San Diego, CA). Lineage-marker-negative (Lin−) cells were purified through a 0.3-in StemSep negative selection column as per the manufacturer’s instructions (StemCell Technologies, Vancouver, BC, Canada). Purified cells were immediately seeded in fibronectin-coated (2 µg/cm2) tissue culture treated 24-well polystyrene plates (BD Falcon, BD Biosciences San Jose, CA) at a cell density of 105 cells/ml in modified IMDM with L-glutamine (500 µL per culture well) containing basal supplements consisting of: 15% FBS, 1% detoxified BSA, 200 µg/ml holotransferrin, 10 µg/ml recombinant human insulin (Sigma, St Louis, MO), 100 µM β-mercaptoethanol, 50 units/ml penicillin G, and 50 µg/ml streptomycin (Invitrogen, Carlsbad, CA); as well as soluble erythropoietic factors including erythropoietin (EPO, Amgen, Thousand Oaks, CA) at 7.5 units/ml and stem cell factor (SCF, R&D Systems, Minneapolis, MN) at 10 ng/ml. After 24 h of culture, the media was replaced with erythroid-differentiation medium (EDM) (IMDM, with 20% FBS, and 100 µM β-mercaptoethanol).
2.2. Human erythropoietic culture
Approval for human subject protocols and blood sample collection was obtained from the Institutional Review Board at UC Berkeley. The number of blood donors for each experimental endpoint ranged from 3–5 and the number of independent experiments ranged from 3–7, as described in the results section and figure legends. The method of EPC expansion from human peripheral blood was detailed previously (Filippone et al., 2010). In brief, peripheral venous blood was collected from healthy donors and peripheral blood mononuclear cells (PBMC) were isolated by a density gradient centrifugation using Ficoll-Paque. PBMC (5×105) were cultured in MEM (HyClone, Logan, UT, USA) supplemented with the serum substitute BIT 9500 (StemCell Technology, Vancouver, BC, Canada), diluted 1:5 for a final concentration of 10 mg/ml bovine serum albumin, 10 mg/ml rhu insulin, and 200 mg/ml iron-saturated human transferrin, enriched with 900 ng/ml ferrous sulfate (Sigma, St. Louis, MO, USA), 90 ng/ml ferric nitrate (Sigma), 1 mM hydrocortisone (Sigma), 3 IU/ml rhu erythropoietin (StemCell Technology), 5 ng/ml rhu IL-3 (R&D Systems, Minneapolis, MN, USA), and 100 ng/ml rhu stem cell factor (R&D Systems). The cells were maintained at 37°C in a 5% CO2 moist atmosphere and observed daily with an inverted microscope for phenotypic changes. Upon observation of small cell clusters on day 5±1, the cultures were split (1:5) with fresh media.
2.3. FA treatment of mouse and human erythroid cultures
2.3.1. Vehicle controls
FA was diluted from a 37% solution (Sigma, stabilized with 10–15% methanol) immediately before treatment. Phosphate-buffered saline (PBS) was used as the vehicle control in both mouse and human cultures but 0.1% methanol was added into the PBS only in human cells, resulting in a final methanol concentration of 0.001% in all cultures.
2.3.2. FA concentrations
For mouse erythroid cultures, FA was added to final concentrations of 0, 25, 50, 75 and 100 µM, 23 h after seeding in the first medium. Cultures were incubated for 1 h, after which the media was replaced with EDM according to the protocol described in section 2.1. The cells were harvested after 24 h of culture in EDM.
For human erythroid cultures, FA was added to final concentrations of 0, 25, 50, 100, and 150 µM, immediately after seeding the PBMC into the EPC expansion culture media and the media was changed on ~day 5, as described in section 2.2. As FA is reactive and interacts with cellular components rapidly (within ~1 hour), we did not change the media after FA treatment. This concentration range was chosen as it spans the dose of ~80 µM reported in human blood (Heck et al., 1985) and it has been used in many in vitro studies, including in cultured human blood cells (Neuss and Speit, 2008; Schmid and Speit, 2007). All endpoints were analyzed after 10 days of culture.
2.4. MN assay in mouse PCEs
Harvested cells were centrifuged onto slides (Statspin Cytofuge 2; Iris Sample Processing Westwood, MA), air-dried, and fixed with 25°C methanol for 10 min. The slides were then stained in acridine orange (Sigma, St Louis, USA) at a concentration of 20 µg/ml in staining buffer (19 mM NaH2PO4 and 81 mM Na2HPO4) for 10 min at 4°C. Slides were scored for the presence of MN using an Axioplan 2 microscope (Carl Zeiss MicroImaging GmbH, Germany). Scorers were blinded to the treatment status of the cells on the slides. Three independent experiments were conducted and more than 2000 PCEs were scored for each dose in each experiment.
2.5. Analyses of human EPCs
2.5.1. Cell enumeration
Cells were enumerated using a hemocytometer and cell viability was determined by the trypan blue exclusion assay.
2.5.2. Erythroid marker expression
The expression levels of three erythroid surface markers, CD235a, CD36, and CD71, were analyzed in PBMC before culture and in the expanded cells after 10 days of culture. The cells were washed three times with 1× PBS containing 2% FCS and stained with FITC-labeled monoclonal antibody for CD235a, phycoerythrin (PE)-labelled monoclonal antibody for CD36, and APC-labelled monoclonal antibody for CD71 (BD Biosciences, San Jose, CA, USA) at 4°C for 30 min. Anti-isotype antibodies (BD Biosciences) were used in parallel. After staining, cells were washed three times with in 1× PBS and analyzed by flow cytometry (Beckman-Coulter FC-500, Becton Dickinson, Franklin Lakes, NJ, USA).
2.5.3. Cell cycle analysis
Cell cycle was analyzed by propidium iodide (PI) staining. Briefly, 1×106 cells were washed with cold 1× PBS and fixed in 70% cold ethanol at −20°C for 2 hours. After a gentle wash with cold 1× PBS, cells were resuspended in 1 ml PI staining solution (10 µg/ml) and 50 µl RNase A (10 mg/ml) was added. After incubation at 4°C in the dark for 3 h, cells were analyzed by flow cytometry at 488 nm. Cell cycle analysis was performed using FlowJo software (Tree Star, San Carlos, CA, USA). The proliferation index was calculated as (S+G2/M)/(G0/1+S+G2/M).
2.5.4. Aneuploidy of chromosomes 7 and 8
Aneuploidy of chromosomes 7 and 8 was examined by dual-color fluorescence in situ hybridization (FISH) in metaphases of the expanded erythroid progenitor cells after 10 days of culture. Briefly, Colcemid was added to the culture at a final concentration of 0.1 µg/ml, 2 hours before harvesting the cells. After hypotonic treatment (0.075 M KCl) for 30 min at 37°C, the cells were fixed three times with freshly prepared Carnoy’s fixative (methanol:glacial acetic acid = 3:1). The fixed cells were dropped onto glass slides, allowed to air dry and stored at −20°C prior to the FISH assay. Metaphase spreads on each slide were scanned and localized automatically using Metafer software (MetaSystems, Altlussheim, Germany) before hybridization. Whole-chromosome painting probes for chromosome 7 (directly labeled with SpectrumOrange, Vysis Inc., Downers Grove, IL) and for chromosome 8 (directly labeled with SpectrumGreen, Vysis Inc.) were used. The FISH procedure and scoring criteria were performed as previously detailed (Smith et al., 1998; Zhang et al., 1998). The stained slides were randomized and coded and scored in a blinded manner by one researcher.
2.6. Statistical analysis
Negative binomial regression was used to test for differences in MN formation between FA doses and controls and for the dose-response trend, since this outcome is a count variable. Multiple-way ANOVA (MANOVA) was used to test for differences in human EPC expansion and cell cycle distribution between FA doses and controls; blood donors and experiment dates were included in the model. Linear regression was used to test for the dose-response trends. Since aneuploidy in chromosomes 7 and 8 is a count outcome and a rare event, the data of four experiments were pooled and a chi-squared test or Fisher's exact test was used to test for the differences in aneuploidy between FA doses and control. Differences were considered significant at P < 0.05.
3. Results
3.1. FA-induced MN frequency in mouse PCEs
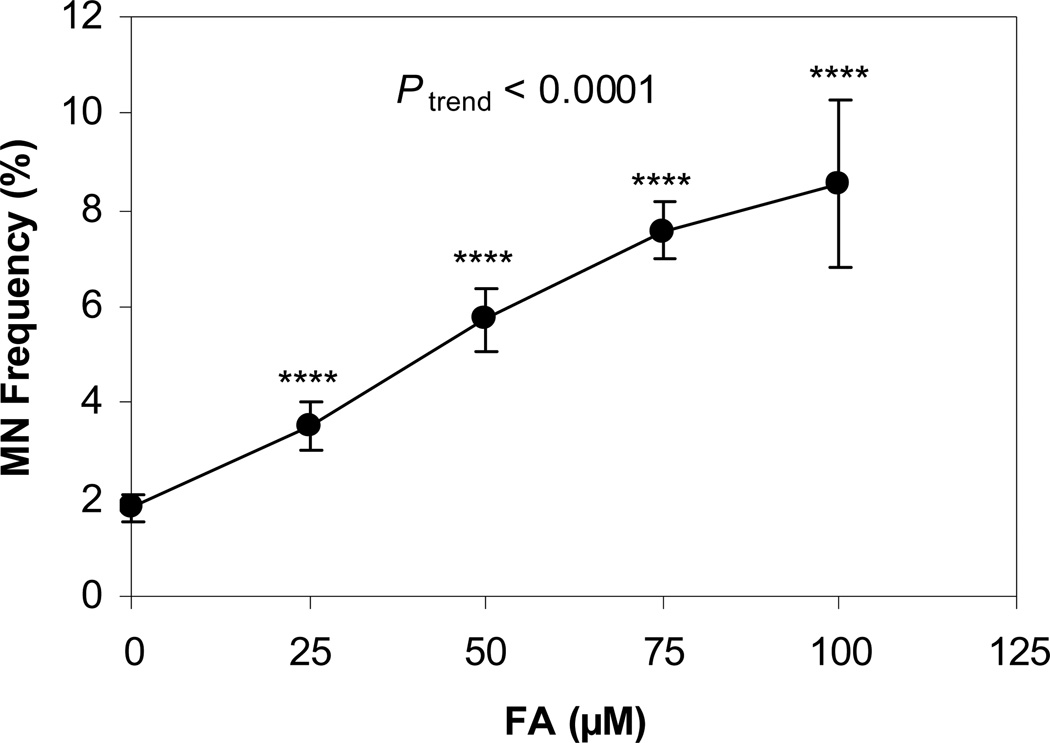
MN frequency was measured in PCEs generated by the induction of erythroid-differentiation in FA-treated and untreated (vehicle control) mouse BM, in a liquid culture system. Three independent experiments were conducted. As shown in Figure 1, 24 h after FA treatment, the MN frequencies in the mouse PCEs generated from bone marrow stem/progenitor cells treated with 25, 50, 75, and 100 µM FA were 3.52%, 5.74%, 7.56%, and 8.54%, respectively. The frequency observed at each FA dose was significantly higher than the level in the control cells 1.83% (P < 0.0001) and the increased frequencies occurred in a dose-dependent manner (Ptrend < 0.0001).
Figure 1. MN frequency in mouse erythropoietic cells after FA treatment.
Three independent experiments were conducted. Data is presented as mean ± standard error (SE). **** represents P < 0.0001, compared to the untreated control and Ptrend represents the p-value of the dose-response trend test. MN frequencies were significantly increased at each dose of FA and across all doses in a dose-dependent manner.
3.2. Effects of FA on human EPC expansion
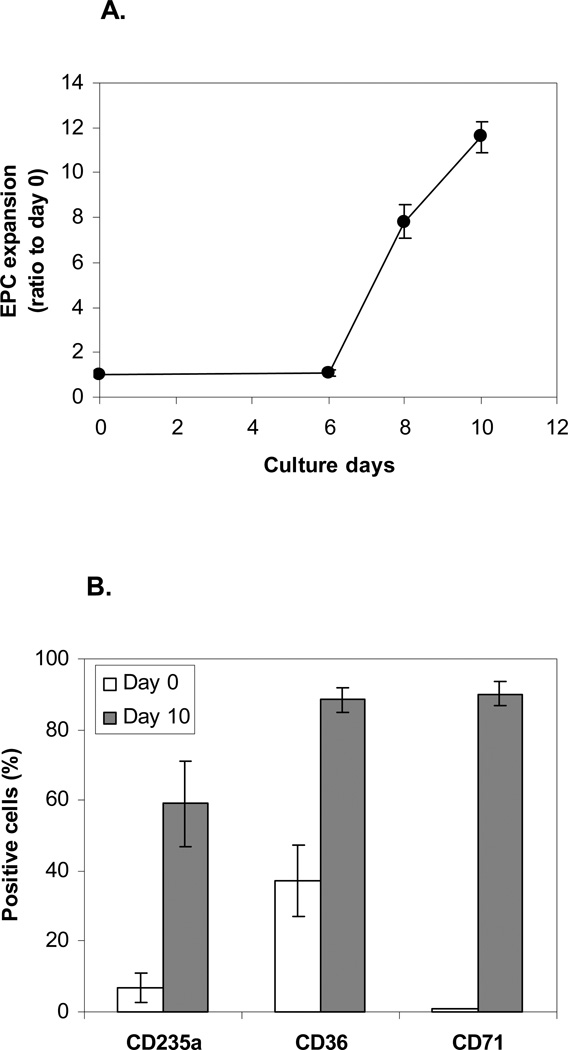
We expanded human EPCs from PBMCs isolated from three donors, in three separate experiments. As shown in Figure 2A, the cell population started to expand at day 6 and was increased 7.8 fold and 11.6 fold by days 8 and 10, respectively, relative to day 0. In the initial PBMC population, the proportions of cells positive for three erythroid markers, CD235a, CD36, and CD71, were 6.9%, 37.3%, and 0.9%, respectively, while in the expanded population after 10 days of culture, the proportions were 59.0%, 88.3%, and 89.9%, respectively (Figure 2B), indicating that the majority of the expanded cells were of the erythroid lineage.
Figure 2. Human EPC expansion from peripheral blood.
Three experiments on three blood donors were conducted. A. Ratio of EPCs relative to day 0 during the course of the culture. Expansion began at day 6 and increased by ~12-fold by day 10. B. Expression levels (%) of erythroid markers after expansion (day 10). EPCs expanded ~100-fold by day 10. Data is presented as mean ± standard error (SE).
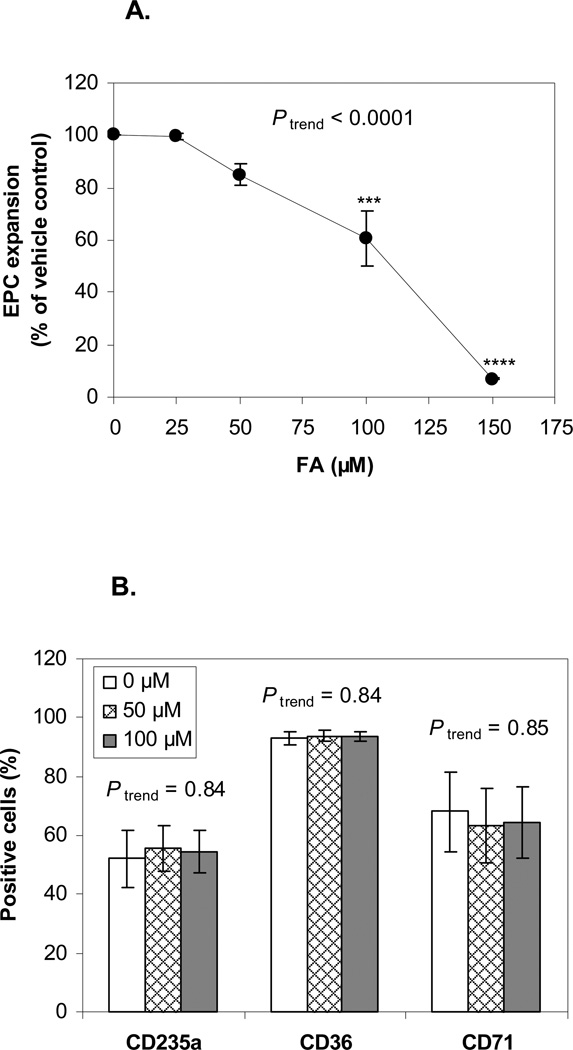
We tested the effects of FA on EPC expansion in six experiments from five blood donors. As shown in Figure 3A, after 10 days of culture, the numbers of EPCs generated from cells that had been treated with 100 µM and 150 µM FA were significantly lower than the numbers of EPCs generated from untreated control cells (P < 0.001, and P < 0.0001, respectively). FA at 150 µM potently decreased the expansion to 7.1% of the untreated control. The suppression occurred in a dose-dependent manner (Ptrend < 0.0001). Exposure to FA at 50 or 100 µM did not alter the expression of CD235a, CD36, and CD71, after 10 days in culture (Figure 3B). The limited number of cells available after expansion precluded testing of higher doses.
Figure 3. Human EPC expansion after FA treatment.
Six experiments on five blood donors were conducted. A. Ratio of EPCs relative to vehicle control on day 10. *** and **** represent P < 0.001 and P < 0.0001, respectively, compared to the untreated control and Ptrend represents the p-value of the dose-response trend test. The number of EPCs generated as a percentage of untreated control were significantly reduced by 100 and 150 µM FA and across the dose-range in a dose-dependent manner. B. Expression levels (%) of erythroid markers after expansion (day 10). Levels were unchanged by FA. Data is presented as mean ± SE.
3.3. Cell cycle distribution
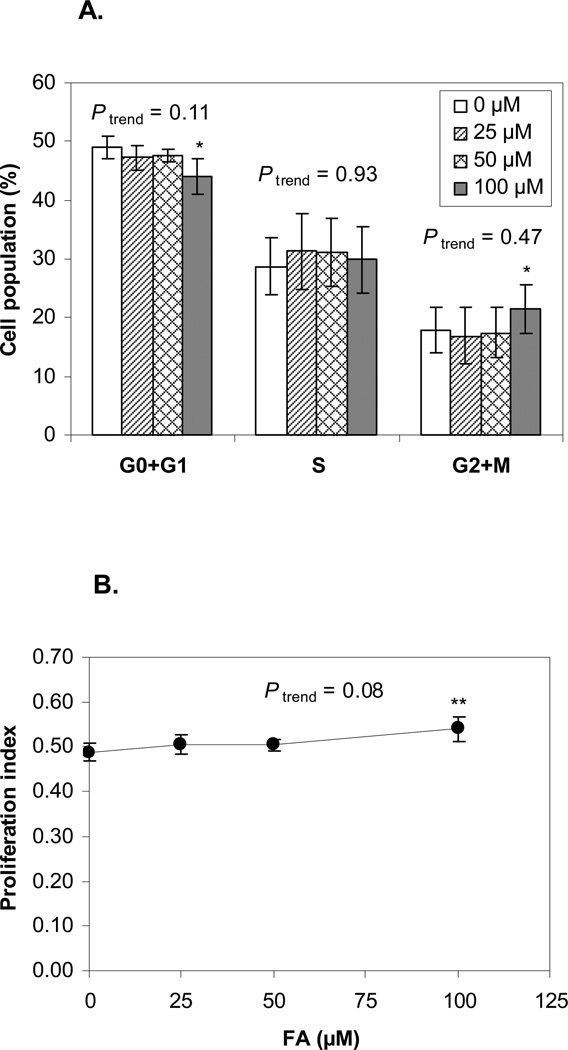
We examined the cell cycle distribution of the expanded EPCs at day 10 (Figure 4). As shown in Figure 4A, the percentage of cells in G0 and G1 phase from cultures treated with 100 µM FA (44.1%) was significantly decreased (P < 0.05) compared to that of the untreated control (48.9%). Meanwhile, the percentage of cells in G2 and M phase from cultures treated with 100 µM FA (21.5%) was significantly increased (P < 0.05) compared to that of the untreated control (17.9%; P < 0.05). The proportion of cells in S phase was not significantly changed by FA treatment. As shown in Figure 4B, we found that the proliferation index was significantly increased (P < 0.05) at 100 µM (0.54) compared to the untreated control (0.49). However, the effects of FA on cell cycle distribution or proliferation index did not occur at the lower levels of FA treatment or in a significantly dose-dependent manner.
Figure 4. Cell cycle distribution of the expanded human EPCs after FA treatment.
Four experiments on three blood donors were conducted. Data is presented as mean ± SE. A. Cell cycle distribution. At 100 µM FA, the percentage of cells in G0 and G1 phase was significantly decreased (44.1%) compared to that of the untreated control (48.9%, P < 0.05) and the percentage of cells in G2 and M phase was significantly increased (21.5%) compared to that of the untreated control (17.9%, P < 0.05). The proportion of cells in S phase was not significantly changed by FA treatment. B. Proliferation index. The index was significantly increased at 100 µM FA (0.54) compared to the untreated control (0.49, P < 0.05). * represents P < 0.05, compared to the untreated control and Ptrend represents the p-value of the dose-response trend test.
3.4. Numerical alterations in chromosomes 7 and 8
We analyzed aneuploidy of chromosomes 7 and 8 in the expanded EPCs after FA treatment. As shown in Table 1, compared with the untreated control, the rate of monosomy 7 (one copy of chromosome 7) was not significantly changed by 50 and 100 µM FA. The rate of monosomy 8 was increased at 50 µM compared to untreated control and approached significance (P = 0.06). The rate of trisomy 7 (three copies of chromosome 7) at 50 µM FA was five times that of the untreated control, but the difference was not statistically significant, despite the large number of cells scored, due to the very low frequency of this aberration. Trisomy 8 was increased at 50 µM FA (P = 0.07), also at a very low frequency. Combined analysis of monosomies 7 and 8 and of trisomies 7 and 8 revealed significant increases at 50 µM FA (P < 0.05 for each). Monosomy and trisomy rates for chromosomes 7 and 8 were not significantly altered by 100 µM FA either in individual or combined analyses. The level of structural chromosome aberrations was not changed by treatment with 50 or 100 µM FA.
Table 1.
Aneuploidies of chromosomes 7 and 8 in the expanded EPCs after FA treatment a
| FA | Cell # | Monosomy |
Trisomy |
Monosomies | Trisomies | Structural | ||
|---|---|---|---|---|---|---|---|---|
| (µM) | Sorted | 7 | 8 | 7 | 8 | 7 & 8 | 7 & 8 | CAe |
| 0 | 7733 | 60 (0.78)b | 52 (0.67) | 1 (0.01) | 1 (0.01) | 112 (1.45) | 2 (0.03) | 3 (0.04) |
| 50 | 7494 | 74 (0.99) | 71 (0.95)c | 4 (0.05) | 6 (0.08)c | 145 (1.93)d | 10 (0.13)d | 4 (0.05) |
| 100 | 6871 | 46 (0.67) | 39 (0.57) | 1 (0.01) | 5 (0.07) | 85 (1.24) | 6 (0.09) | 4 (0.06) |
Four experiments on four blood donors were conducted.
Data are presented as n (%).
represent P < 0.1 and P < 0.05, respectively, compared to untreated controls.
CA: Chromosome aberrations
4. Discussion
The mechanisms underlying FA-induced leukemogenesis remain elusive but may involve toxicity to hematopoietic stem/progenitor cells (HSC/HPC). Previously, we found that FA was toxic to myeloid progenitor cells in vivo and in vitro using colony-forming assays (Zhang et al., 2010). In the present study, using relatively new in vitro liquid culture protocols that enable the analysis of multiple mechanistic endpoints in large numbers of erythroid progenitor cells, we have detected several toxic effects of FA in mouse PCEs and human EPCs. We found that FA induced MN in mouse PCEs in a dose-dependent manner. MN in mouse PCEs is a widely used biomarker for genotoxicity (Fromowitz et al., 2012; Hayashi et al., 1994) and MN frequency in human peripheral lymphocytes has been associated with cancer risk (Bonassi et al., 2011). Our finding shows that FA is genotoxic to mouse HSC/HPC in vitro, suggesting that chromosomal damage may be one potential mechanism underlying FA induced leukemogenesis.
From our human erythroid culture experiments, we reported that FA suppressed the expansion of EPCs from circulating stem/progenitor cells in the peripheral blood. This is consistent with our previous finding in FA-exposed workers and in peripheral blood treated with FA (100–200 µM) in vitro, using colony-forming assays in semi-solid substrate (Zhang et al., 2010), and further supports the adverse effects of FA on HSC/HPC. Kuehner et al. also found suppression of colony formation from peripheral blood treated with FA (10–100 µM) in vitro (Kuehner et al., 2012). It is possible that only a proportion of the stem/progenitor cells in peripheral blood survived FA treatment and was available for expansion. However, we did not examine apoptosis in stem/progenitor cells following FA treatment because these cells are rare among PBMC and require separation from other blood cell types for analysis. When we examined apoptosis in the expanded EPCs from FA-treated and untreated peripheral blood, we did not detect any differences (Supplemental Figure 1). Erythroid differentiation, determined by the proportion of cells with CD235a, CD36, and CD71 expression, was unchanged by FA exposure, suggesting that erythroid progenitor cells that survived FA treatment retained this capability.
Damage induced by FA may have led to cell-cycle arrest of the HSC/HPC. Oxidative stress is a potential mechanism of FA-induced toxicity and leukemogenesis. Bone marrow HSC, in which leukemia originates (Passegue et al., 2003), are sensitive to oxidative stress, exposure to which causes DNA damage, premature senescence, and loss of stem cell function (Yahata et al., 2011). FA was previously shown to induce oxidative stress in multiple tissues, including lymphocytes, in exposed rats and mice (Gulec et al., 2006; Lino-dos-Santos-Franco et al., 2011; Lino-Dos-Santos-Franco et al., 2010; Matsuoka et al., 2010; NTP, 2010b; Wang et al., 2012). In two recent new studies, we reported the significant dose-dependent induction of oxidative stress in multiple organs, including bone marrow, of mice exposed to FA (0.5–3.0 mg/m3) by nose-only inhalation (Ye et al., 2013; Zhang et al., 2013). Our current study in erythroid liquid cultures and our previous study in exposed workers have examined the effects of FA on PBMC-derived stem/progenitor cells in vitro and in vivo. Future studies should examine markers of oxidative stress and other potential mechanisms of toxicity in bone marrow-derived HSC of FA-exposed animals.
Leukemia is characterized by a cell differentiation block leading to accumulation of immature cells (Olsson et al., 1996) that is further driven by an imbalance between proliferation and death rates (Chiorazzi, 2007). In our study, EPCs expanded from FA-treated (100 µM) stem/progenitor cells appeared to proliferate faster than EPCs expanded from untreated cells, apparent from cell cycle distribution and proliferation index data. Thus, accelerated proliferation might contribute to FA induced leukemogenesis; this requires further confirmation, however, due to the lack of a dose-dependent response. In normal erythropoiesis, progenitors are critically dependent on growth factors for survival and proliferation (Koury and Bondurant, 1990) while growth factor independence is a hallmark of cancer cells (Hanahan and Weinberg, 2000; Howell et al., 1998a; Howell et al., 1998b; Sporn and Roberts, 1985; Sporn and Todaro, 1980; Ziober et al., 1993). While expanded EPCs from FA-treated cultures may have acquired a growth advantage, they did not exhibit more growth factor independence than untreated cells (Supplemental Figure 2). Future studies should examine the effects of a culture time longer than 24 hours in the absence of exogenous growth factors on FA-treated and untreated EPCs.
Aneuploidy, an abnormal number of chromosomes resulting from loss or gain, is a common characteristic of cancer cells that is thought to promote tumorigenesis (Ganmore et al., 2009; Weaver and Cleveland, 2009) and is a common phenomenon in therapy-related leukemia (Pedersen-Bjergaard et al., 2008; Qian et al., 2010) and leukemia related to exposure to benzene, an established human leukemogen (Zhang et al., 2002). Monosomy 7 and trisomy 8 are associated with myeloid leukemia (Johnson and Cotter, 1997; Paulsson and Johansson, 2007) and have been reported in workers exposed to benzene (Kim et al., 2004; Smith et al., 1998; Zhang et al., 2011; Zhang et al., 1998; Zhang et al., 2005). Previously, we reported the induction of monosomy 7 and trisomy 8 in the CFU-GM cultured from a small number of workers (n=10) exposed to FA in vivo, compared with unexposed control workers (n=12) (Zhang et al., 2010). In the present study, monosomy and trisomy of chromosomes 7 and 8 were not significantly increased in expanded EPCs at either 50 µM or 100 µM FA, when each endpoint was analyzed separately. Similar to our EPC findings, Kuehner et al. did not find increased aneuploidy of chromosomes 7 and 8 in myeloid colonies cultured from peripheral blood treated with 10–50 µM FA in vitro; they did not report data for aneuploidy at 100 µM although they did see suppression of colony formation at this dose (Kuehner et al., 2012). We scored around five times more cells per dose (~7500 cells) than did Kuehner (1500 cells). We found significant induction of monosomy and trisomy in the expanded EPCs at 50 µM but not 100 µM FA when both chromosomes were analyzed together. However, we could not compare these findings with those of Kuehner et al. as they did not analyze aneuploidy in chromosomes 7 and 8 together. It is unclear why effects were apparent at 50 µM but not 100 µM in our study; at both doses, cell viability was similar in FA-treated cultures and similar numbers of cells were scored (Table 1). Further studies are needed to validate an effect of FA on the rate of monosomy and trisomy of chromosomes 7 and 8 at different doses.
Based on our findings, we speculate that HSC/HPC are sensitive to FA treatment, leading to cell death in a proportion of these cells and consequent suppression of EPC expansion. Further, a proportion of FA-exposed hematopoietic stem and/or progenitor cells likely sustain non-lethal damage (e.g. MN, aneuploidy) promoting their survival and possibly conferring a growth advantage, which is apparent in their differentiated progeny. Acquisition of additional toxic insults may be required for the development of leukemic stem cells.
We selected a FA dose range that spans the reported physiological level in the blood of humans, monkeys and rats (66.6 to 100 µM, 2 to 3 µg/g) (Casanova et al., 1988; Heck et al., 1985). Increased MN frequency in mouse PCEs, suppressed human EPC expansion and increased proliferation of human EPCs occurred at exposure to 100 µM exogenous FA. The actual FA levels in the treated cells in the current study are equivalent to the both the exogenous and naturally occurring, endogenous levels. The endogenous levels in the target HSC and HPC in culture are unknown. Methods to estimate the endogenous levels of cultured cells should be incorporated into future studies, so that the absolute FA levels in untreated and treated cells can be estimated.
One potential weakness of our study is that the expanded EPC populations are likely heterogeneous. Although the proportions of the erythroid cell markers CD 235a, CD36 and CD71, are the same in both FA-treated and untreated EPC, indicating that the FA-treated and untreated populations are comparable, we performed our assays on unselected populations. In future studies, we plan to select erythroid progenitor subsets during the early stages of differentiation by gating on different levels and combinations of these three erythroid markers, as employed by others (Williams et al., 2013). This selection, together with measurement of more mechanistic endpoints such as DNA-protein crosslinks and oxidative stress that we recently reported in the bone marrow of mice inhaled by nose-only exposure to FA in vivo (Ye et al., 2013; Zhang et al., 2013), will provide further insight into FA-induced toxicity and leukemogenesis.
In conclusion, FA induces genotoxicity in mouse erythropoietic cells and suppresses human EPC expansion in vitro, supporting the adverse effects of FA on HSC/HPC and the biological plausibility of FA-induced leukemogenesis.
Supplementary Material
Highlights.
We tested formaldehyde (FA) toxicity in liquid in vitro erythroid culture systems.
FA significantly induced micronuclei in cultured mouse polychromatic erythrocytes.
FA suppressed human erythroid progenitor cell (EPC) expansion.
FA increased proliferation in EPCs.
These data confirm that FA is toxic to hematopoietic stem and progenitor cells.
Acknowledgements
The authors are thankful to Prof. Christopher Vulpe for his guidance in the animal protocols in the animal facility at UC Berkeley, Dr. Joe Shuga for his assistance in the mouse PCE experiments and Dr. Cliona M. McHale for her critical editing of the manuscript. This research was supported in part by the NIEHS grant R01ES017452 (L. Zhang) and the Provincial Scholarship Fund of Guangxi Education Department, Guangxi, China (to X. Li).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim- Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part F: chemical agents and related occupations. Lancet Oncol. 2009;10:1143–1144. doi: 10.1016/s1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- Bonassi S, El-Zein R, Bolognesi C, Fenech M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: evidence from human studies. Mutagenesis. 2011;26:93–100. doi: 10.1093/mutage/geq075. [DOI] [PubMed] [Google Scholar]
- Casanova M, Heck HD, Everitt JI, Harrington WW, Jr, Popp JA. Formaldehyde concentrations in the blood of rhesus monkeys after inhalation exposure. Food Chem Toxicol. 1988;26:715–716. doi: 10.1016/0278-6915(88)90071-3. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N. Cell proliferation and death: forgotten features of chronic lymphocytic leukemia B cells. Best Pract Res Clin Haematol. 2007;20:399–413. doi: 10.1016/j.beha.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Filippone C, Franssila R, Kumar A, Saikko L, Kovanen PE, Soderlund-Venermo M, Hedman K. Erythroid progenitor cells expanded from peripheral blood without mobilization or preselection: molecular characteristics and functional competence. PLoS One. 2010;5:e9496. doi: 10.1371/journal.pone.0009496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromowitz M, Shuga J, Wlassowsky AY, Ji Z, North M, Vulpe CD, Smith MT, Zhang L. Bone marrow genotoxicity of 2,5-dimethylfuran, a green biofuel candidate. Environ Mol Mutagen. 2012;53:488–491. doi: 10.1002/em.21707. [DOI] [PubMed] [Google Scholar]
- Ganmore I, Smooha G, Izraeli S. Constitutional aneuploidy and cancer predisposition. Hum Mol Genet. 2009;18:R84–R93. doi: 10.1093/hmg/ddp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulec M, Songur A, Sahin S, Ozen OA, Sarsilmaz M, Akyol O. Antioxidant enzyme activities and lipid peroxidation products in heart tissue of subacute and subchronic formaldehyde-exposed rats: a preliminary study. Toxicol Ind Health. 2006;22:117–124. doi: 10.1191/0748233706th248oa. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Tice RR, MacGregor JT, Anderson D, Blakey DH, Kirsh-Volders M, Oleson FB, Jr, Pacchierotti F, Romagna F, Shimada H, et al. In vivo rodent erythrocyte micronucleus assay. Mutat Res. 1994;312:293–304. doi: 10.1016/0165-1161(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Heck HD, Casanova-Schmitz M, Dodd PB, Schachter EN, Witek TJ, Tosun T. Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am Ind Hyg Assoc J. 1985;46:1–3. doi: 10.1080/15298668591394275. [DOI] [PubMed] [Google Scholar]
- Howell GM, Humphrey LE, Awwad RA, Wang D, Koterba A, Periyasamy B, Yang J, Li W, Willson JK, Ziober BL, Coleman K, Carboni J, Lynch M, Brattain MG. Aberrant regulation of transforming growth factor-alpha during the establishment of growth arrest and quiescence of growth factor independent cells. J Biol Chem. 1998a;273:9214–9223. doi: 10.1074/jbc.273.15.9214. [DOI] [PubMed] [Google Scholar]
- Howell GM, Humphrey LE, Ziober BL, Awwad R, Periyasamy B, Koterba A, Li W, Willson JK, Coleman K, Carboni J, Lynch M, Brattain MG. Regulation of transforming growth factor alpha expression in a growth factor-independent cell line. Mol Cell Biol. 1998b;18:303–313. doi: 10.1128/mcb.18.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans. Formaldehyde; 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. International Agency for Research on Cancer. 2006;88:39–325. [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Cotter FE. Monosomy 7 and 7q--associated with myeloid malignancy. Blood Rev. 1997;11:46–55. doi: 10.1016/s0268-960x(97)90006-0. [DOI] [PubMed] [Google Scholar]
- Kim SY, Choi JK, Cho YH, Chung EJ, Paek D, Chung HW. Chromosomal aberrations in workers exposed to low levels of benzene: association with genetic polymorphisms. Pharmacogenetics. 2004;14:453–463. doi: 10.1097/01.fpc.0000114751.08559.7b. [DOI] [PubMed] [Google Scholar]
- Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Kuehner S, Schlaier M, Schwarz K, Speit G. Analysis of leukemia-specific aneuploidies in cultured myeloid progenitor cells in the absence and presence of formaldehyde exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2012;128:72–78. doi: 10.1093/toxsci/kfs126. [DOI] [PubMed] [Google Scholar]
- Lino-dos-Santos-Franco A, Correa-Costa M, Durao AC, de Oliveira AP, Breithaupt-Faloppa AC, Bertoni Jde A, Oliveira-Filho RM, Camara NO, Marcourakis T, Tavares-de-Lima W. Formaldehyde induces lung inflammation by an oxidant and antioxidant enzymes mediated mechanism in the lung tissue. Toxicol Lett. 2011;207:278–285. doi: 10.1016/j.toxlet.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Lino-Dos-Santos-Franco A, Domingos HV, Oliveira AP, Breithaupt-Faloppa AC, Peron JP, Bolonheis S, Muscara MN, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Differential effects of formaldehyde exposure on the cell influx and vascular permeability in a rat model of allergic lung inflammation. Toxicol Lett. 2010;197:211–218. doi: 10.1016/j.toxlet.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Takaki A, Ohtaki H, Shioda S. Early changes to oxidative stress levels following exposure to formaldehyde in ICR mice. J Toxicol Sci. 2010;35:721–730. doi: 10.2131/jts.35.721. [DOI] [PubMed] [Google Scholar]
- Neuss S, Speit G. Further characterization of the genotoxicity of formaldehyde in vitro by the sister chromatid exchange test and co-cultivation experiments. Mutagenesis. 2008;23:355–357. doi: 10.1093/mutage/gen025. [DOI] [PubMed] [Google Scholar]
- NTP. Final Report on Carcinogens Background Document for Formaldehyde. Rep Carcinog Backgr Doc. 2010a:i-512. [PubMed] [Google Scholar]
- NTP. Report on Carcinogens Background Document for Formaldehyde. Research Triangle Park, NC: National Toxicology Program; 2010b. http://ntp.niehs.nih.gov/ntp/roc/twelfth/2009/November/Formaldehyde_BD_Final.pdf. [PubMed] [Google Scholar]
- NTP. Report on Carcinogens, Twelfth Edition. National Toxicology Program. 2011:195–205. [Google Scholar]
- Olsson I, Bergh G, Ehinger M, Gullberg U. Cell differentiation in acute myeloid leukemia. Eur J Haematol. 1996;57:1–16. doi: 10.1111/j.1600-0609.1996.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA 100 Suppl. 2003;1:11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson K, Johansson B. Trisomy 8 as the sole chromosomal aberration in acute myeloid leukemia and myelodysplastic syndromes. Pathol Biol (Paris) 2007;55:37–48. doi: 10.1016/j.patbio.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–248. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- Qian Z, Joslin JM, Tennant TR, Reshmi SC, Young DJ, Stoddart A, Larson RA, Le Beau MM. Cytogenetic and genetic pathways in therapy-related acute myeloid leukemia. Chem Biol Interact. 2010;184:50–57. doi: 10.1016/j.cbi.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid O, Speit G. Genotoxic effects induced by formaldehyde in human blood and implications for the interpretation of biomonitoring studies. Mutagenesis. 2007;22:69–74. doi: 10.1093/mutage/gel053. [DOI] [PubMed] [Google Scholar]
- Shuga J, Zhang J, Samson LD, Lodish HF, Griffith LG. In vitro erythropoiesis from bone marrow-derived progenitors provides a physiological assay for toxic and mutagenic compounds. Proc Natl Acad Sci U S A. 2007;104:8737–8742. doi: 10.1073/pnas.0701829104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Zhang L, Wang Y, Hayes RB, Li G, Wiemels J, Dosemeci M, Titenko-Holland N, Xi L, Kolachana P, Yin S, Rothman N. Increased translocations and aneusomy in chromosomes 8 and 21 among workers exposed to benzene. Cancer Res. 1998;58:2176–2181. [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. Autocrine growth factors and cancer. Nature. 1985;313:745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Tang X, Bai Y, Duong A, Smith MT, Li L, Zhang L. Formaldehyde in China: production, consumption, exposure levels, and health effects. Environ Int. 2009;35:1210–1224. doi: 10.1016/j.envint.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Wang HX, Wang XY, Zhou DX, Zheng LR, Zhang J, Huo YW, Tian H. Effects of low-dose, long-term formaldehyde exposure on the structure and functions of the ovary in rats. Toxicol Ind Health. 2012 doi: 10.1177/0748233711430983. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Cleveland DW. The role of aneuploidy in promoting and suppressing tumors. J Cell Biol. 2009;185:935–937. doi: 10.1083/jcb.200905098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KN, Szilagyi A, Conrad P, Halerz M, Kini AR, Li Y, Gamelli RL, Shankar R, Muthumalaiappan K. Peripheral blood mononuclear cell-derived erythroid progenitors and erythroblasts are decreased in burn patients. Journal of burn care & research : official publication of the American Burn Association. 2013;34:133–141. doi: 10.1097/BCR.0b013e3182642ccd. [DOI] [PubMed] [Google Scholar]
- Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, Ando K. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- Ye X, Ji Z, Wei C, McHale CM, Ding S, Thomas R, Yang X, Zhang L. Inhaled formaldehyde induces DNA-protein crosslinks and oxidative stress in bone marrow and other distant organs of exposed mice. Environmental and Molecular Mutagenesis. 2013 doi: 10.1002/em.21821. In Press. [DOI] [PubMed] [Google Scholar]
- Zhang L, Eastmond DA, Smith MT. The nature of chromosomal aberrations detected in humans exposed to benzene. Crit Rev Toxicol. 2002;32:1–42. doi: 10.1080/20024091064165. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lan Q, Guo W, Hubbard AE, Li G, Rappaport SM, McHale CM, Shen M, Ji Z, Vermeulen R, Yin S, Rothman N, Smith MT. Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis. 2011;32:605–612. doi: 10.1093/carcin/bgq286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rothman N, Wang Y, Hayes RB, Li G, Dosemeci M, Yin S, Kolachana P, Titenko-Holland N, Smith MT. Increased aneusomy and long arm deletion of chromosomes 5 and 7 in the lymphocytes of Chinese workers exposed to benzene. Carcinogenesis. 1998;19:1955–1961. doi: 10.1093/carcin/19.11.1955. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tang X, Rothman N, Vermeulen R, Ji Z, Shen M, Qiu C, Guo W, Liu S, Reiss B, Freeman LB, Ge Y, Hubbard AE, Hua M, Blair A, Galvan N, Ruan X, Alter BP, Xin KX, Li S, Moore LE, Kim S, Xie Y, Hayes RB, Azuma M, Hauptmann M, Xiong J, Stewart P, Li L, Rappaport SM, Huang H, Fraumeni JF, Jr, Smith MT, Lan Q. Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol Biomarkers Prev. 2010;19:80–88. doi: 10.1158/1055-9965.EPI-09-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang W, Hubbard AE, Smith MT. Nonrandom aneuploidy of chromosomes 1, 5, 6, 7, 8, 9, 11, 12, and 21 induced by the benzene metabolites hydroquinone and benzenetriol. Environ Mol Mutagen. 2005;45:388–396. doi: 10.1002/em.20103. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu X, McHale CM, Li R, Zhang L, Wu Y, Ye X, Yang X, Ding S. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One. 2013;8:e74974. doi: 10.1371/journal.pone.0074974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober BL, Willson JK, Hymphrey LE, Childress-Fields K, Brattain MG. Autocrine transforming growth factor-alpha is associated with progression of transformed properties in human colon cancer cells. J Biol Chem. 1993;268:691–698. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.