Abstract
A growing body of evidence suggests that moderate to vigorous activity levels can affect quality of life, cognition, and brain structure in patients diagnosed with schizophrenia. However, physical activity has not been systematically studied during the period immediately preceding the onset of psychosis. Given reports of exercise-based neurogenesis in schizophrenia, understanding naturalistic physical activity levels in the prodrome may provide valuable information for early intervention efforts. The present study examined 29 ultra high-risk (UHR) and 27 matched controls to determine relationships between physical activity level, brain structure (hippocampus and parahippocampal gyrus), and symptoms. Participants were assessed with actigraphy for a 5-day period, magnetic resonance imaging (MRI), and structured clinical interviews. UHR participants showed a greater percentage of time in sedentary behavior while healthy controls spent more time engaged in light to vigorous activity. There was a strong trend to suggest the UHR group showed less total physical activity. The UHR group exhibited smaller medial temporal volumes when compared to healthy controls. Total level of physical activity in the UHR group was moderately correlated with smaller parahippocampal gyri bilaterally (right: r=.44, left: r=.55) and with occupational functioning (r=−.36; of negative symptom domain), but not positive symptomatology. Results suggest that inactivity is associated with medial temporal lobe health. Future studies are needed to determine if symptoms are driving inactivity, which in turn may be affecting the health of the parahippocampal structure and progression of illness. Although causality cannot be determined from the present design, these findings hold important implications for etiological conceptions and suggest promise for an experimental trial.
Keywords: Activity, Exercise, Hippocampus, Prodrome, Ultra High-risk, Psychosis, Schizophrenia
Accumulating evidence from animal models (Wolf, Melnik, & Kempermann, 2011), healthy populations (Erickson et al., 2011), and schizophrenia studies (Berle, Hauge, Oedegaard, Holsten, & Fasmer, 2010; Farrow, Hunter, Wilkinson, Green, & Spence, 2005; Pajonk et al., 2010) suggests that regular exercise positively affects integral functions such as hippocampal neurogenesis and memory. Likewise, moderate to vigorous activity has been associated with improved quality of life and lower symptom levels in patients with schizophrenia (Gorczynski & Faulkner, 2010). In a landmark study, Pajonk and colleagues (2010) found that a brief period (i.e., 30 minutes per each session, three times a week for 12 weeks) of cardiovascular activity significantly increased hippocampal volume in patients diagnosed with schizophrenia when compared to patient controls who did not exercise. Taken together, these data suggest that increased physical activity has several positive effects spanning neural, cognitive, and psychosocial domains. However, to date there have been no studies of activity in ultra-high risk youth (UHR), a group at imminent risk for a formal psychotic disorder that is characterized by recently emergent or escalating positive attenuated symptoms (e.g., suspiciousness, seeing shadows out of the corner of one's eye, hearing sounds when nobody is home) and a decline in socio-occupational functioning (Miller et al., 1999). Understanding activity profiles and the structural correlates of inactivity in the period immediately prior to the onset of schizophrenia is integral, as the prodrome is a viable period of intervention in which considerable brain development is still occurring (Dahl, 2004; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Spear, 2004). If empirical research can quantify activity/inactivity and determine how it may be associated with the structural abnormalities and symptomatology in UHR youth (Fusar-Poli et al., 2011; Fusar-Poli et al., 2007; Mittal, Walker, et al., 2010; Seidman et al., 2010), this information may potentially inform etiological models and serve as the groundwork for future intervention trials in this important group.
There are several promising avenues of research that suggest activity levels may be tied to neural-maintenance and cognition in healthy individuals. Investigators have observed that various types of learning and training improve neuroplasticity (Draganski et al., 2004). In mice, exercise has been found to result in increased blood volume and neurogenesis in the medial temporal lobe (Cotman & Berchtold, 2002; Wolf et al., 2011). Research also shows that exercise and fitness level can significantly alter blood oxygen level dependent activation in the parahippocampal gyrus (Holzschneider, Wolbers, Roder, & Hotting, 2012; Janse Van Rensburg, Taylor, Hodgson, & Benattayallah, 2009), a grey matter cortical region that encapsulates the hippocampus and plays an integral role in memory coding and retrieval. Because voluntary exercise has been found to stimulate adult medial temporal neurogenesis (Parker et al., 2011), and the hippocampal and parahippocampal gyrus abnormalities have been widely observed in structural, functional and neurocognitive studies of schizophrenia (Allen et al., 2011; Walker, Mittal, & Tessner, 2008; Wible et al., 1995), physical activity may be in an important factor in schizophrenia. Further, symptoms such as avolition affect motivation and may make individuals with schizophrenia increasingly prone to the deleterious effects of inactivity. Researchers have observed that exercise can improve symptoms of depression, low self-esteem, and social anhedonia in patients with schizophrenia, which reflects a potential bi-directional relationship between exercise and symptoms (Gorczynski & Faulkner, 2010). This may speak to the role of hippocampal abnormalities in contributing to symptoms (Anderson et al., 2002) and/or changes in inflammatory markers (Kop, Weinstein, Deuster, Whittaker, & Tracy, 2008).
UHR patients and corroborating parents often report growing concern with an increasing lack of motivation and activity (Lencz, Smith, Auther, Correll, & Cornblatt, 2004; Pelletier & Mittal, 2013). However, beyond clinician ratings there have been no naturalistic investigations of how this may impact UHR samples. Further, a number of studies have indicated that volumetric decreases and signs of hippocampal and parahippocampal gyrus dysfunction throughout the prodromal period precede transition to schizophrenia (Dickey, McCarley, & Shenton, 2002; Dickey et al., 1999; Mittal & Walker, 2011; Pantelis et al., 2003; Pantelis et al., 2007). In addition, because etiological models implicate the hippocampus as a critical feature in the development of psychosis (Corcoran et al., 2003; Walker et al., 2008), intervention may prove vital. As there have been challenges raised to many of the available psychotherapy and pharmacological interventions (Corcoran, First, & Cornblatt, 2010), and an increasing level of promise found in neuroplasticity-based interventions in this group (Fisher, Holland, Merzenich, & Vinogradov, 2009), understanding respective brain-behavior relationships in a UHR sample holds promise.
Investigating the activity level of UHR youth and the relationships between activity, medial temporal volumes, and symptomatology in the high-risk period is important for advancing our understanding of psychosis as this is an integral step in highlighting any deficits and activity-related risk behaviors, and pinpointing potential etiological mechanisms and treatment targets for future study. In the present study, 29 UHR and 27 matched healthy controls wore an actigraph wristwatch for a five-day period and the percentages of time spent in a range of activities levels were attained (ranging from sedentary to vigorous activity). Participants were scanned utilizing magnetic resonance imaging (MRI) and hippocampi and parahippocampal gyri volumes were computed. Consistent with the available literature (Dickey, McCarley, & Shenton, 2002; Dickey et al., 1999; Mittal & Walker, 2011; Pantelis et al., 2003; Pantelis et al., 2007), we predicted that UHR adolescents and young adults would show smaller hippocampal and parahippocampal gyral volumes. In addition, based on the supporting literature suggesting UHR youth show deficits in motivation and activity (Lencz, Smith, Auther, Correll, & Cornblatt, 2004; Pelletier & Mittal, 2013), we predicted that the UHR patients would show a higher percentage of sedentary behavior, and a lower level of light-to-vigorous activity when compared to controls. Finally, based on evidence from an experimental design study demonstrating that regular prescribed activity is associated with increased medial temporal lobe volumes in patients with schizophrenia (Pajonk et al., 2010), we predicted that lower levels of total physical activity would associated with smaller medial temporal lobe volumes in the UHR participants (this was also examined in an exploratory analysis in the controls). Because there is not yet a strong guiding literature to inform predictions, the relationships between activity and domains of positive and negative symptomatology (as well as negative subdomains that are related to movement including avolition and occupational functioning) are examined in exploratory analyses.
Methods
Participants
Participants were recruited at the University of Colorado Boulder's Adolescent Development and Preventive Treatment (ADAPT) research program (see Table 1 for demographic characteristics of this sample). Adolescent and young adult control and UHR participants (mean age = 18.09) were recruited by Craigslist, email postings, newspaper ads, and community professional referrals. Exclusion criteria included history of head injury, the presence of a neurological disorder, lifetime substance dependence (including illicit substances but not nicotine), and the presence of any contraindication to the magnetic resonance imaging environment (e.g. current pregnancy or metal in the body). The presence of an Axis I psychotic disorder (e.g., schizophrenia, schizoaffective disorder, schizophreniform) was an exclusion criterion for UHR participants. To be included in the study, UHR individuals needed to meet criteria for a prodromal syndrome. To meet this criteria, participants showed 1) recent onset or escalation of moderate levels of attenuated positive symptoms (a score of 3–5) and/or 2) a decline in global functioning over the last 12 months accompanying the presence of schizotypal personality disorder (SPD), and/or 3) a decline in global functioning over the last 12 months accompanying the presence of a first-degree relative with a psychotic disorder such as schizophrenia (Miller et al., 1999). Based on the noted inclusion and exclusion criteria a total of 48 individuals were screened to yield the final UHR sample. The presence of a psychotic disorder in a first-degree relative or meeting for an Axis I disorder were exclusionary criteria for controls. Healthy control participants were recruited through flyers and newspaper announcements (advertised as a study of neuroimaging and healthy development for volunteers with no psychiatric symptoms and no family history of psychosis). The protocol and informed consent procedures were approved by the University Institutional Review Board.
TABLE 1.
Participant Demographics, Symptoms, Role Functioning and Medial Temporal Lobe Volumes
| Healthy Control | Ultra High-Risk | Grand Total | Group Differences | |
|---|---|---|---|---|
| Gender | ||||
| Males | 12(44%) | 18(62%) | 30(54%) | N.S. |
| Females | 15(56%) | 11(38%) | 26(46%) | |
| Total | 27 | 29 | 56 | |
| Age | ||||
| Mean Years (SD) | 17.63(2.70) | 18.52(1.90) | 18.09(2.34) | N.S. |
| Parent Education | ||||
| Mean Years (SD) | 15.26(2.93) | 14.07(4.86) | 14.65(4.04) | N.S. |
| Symptoms | ||||
| Positive | ||||
| Mean (SD) | .93(1.54) | 11.72(4.86) | 6.52(6.54) | p ≤ 0.01 |
| Negative | ||||
| Mean (SD) | .81(1.39) | 11.82(7.49) | 6.52(7.77) | p ≤ 0.01 |
| Avolition | ||||
| Mean (SD) | .15(.60) | 2.41(1.59) | 1.32(1.66) | p ≤ 0.01 |
| Occupational Functioning | ||||
| Mean (SD) | .26(.59) | 2.38(1.81) | 1.36(1.73) | p ≤ 0.01 |
| Total Physical Activity | 458.17(222.76) | 364.30(177.31) | 409.10(203.51) | p = 0.06 |
| Hippocampus | ||||
| Right Mean % ICV (SD) | .28(.02) | .26(.02) | .27(.02) | p ≤ 0.01 |
| Left Mean % ICV (SD) | .28(.03) | .26(.03) | .27(.03) | p ≤ 0.01 |
| Parahippocampal Gyrus | ||||
| Right Mean % ICV (SD) | .14(.02) | .13(.01) | .13(.02) | p ≤ 0.01 |
| Left Mean % ICV (SD) | .15(.02) | .14(.02) | .14(.02) | p = 0.13 |
Note: not significant (N.S.); Positive and negative symptoms as well as Avolition and Occupational Functioning scales reflect total sums from domains from the Structured Interview for Prodromal Syndromes (SIPS); Global Role Functioning reflects scores on the Global Functioning Scale- Role (GFS-R); Brain volumes represent the respective structure divided by total intracranial volume. Brain volumes represent the respective structure divided by total intracranial volume.
A total of 29 UHR and 27 control adolescents and young adults participated in the study. Symptom data was available for all participants (see Table 1). Two participants elected not to participate in the imaging assessment (one UHR and one control participant). The total number of participants included in the group comparisons of imaging variables was 54 (UHR: mean age = 18.61, SD = 1.89, 64.3% male; Control: mean age = 17.60, SD = 2.74, 46.2% male). In addition, six UHR and six controls did not participate in the actigraph assessment portion of the investigation (three participants did not wear the watch long enough to provide valid data and nine participants did not elect to participate). The total number of participants included in group comparisons of activity variables was 44 (UHR: mean age = 18.61, SD = 1.95, 56.5% male; Control: mean age =17.43, SD = 2.90, 38.1% male). Correlational analyses comparing activity and brain volumes had a total of 42 participants or 22 in the UHR analyses (UHR: mean age = 18.73, SD = 1.91, 59.1% male) and 20 in the Control analysis (Control: mean age =17.35, SD = 2.96, 40. 0% male). There were no differences in symptoms, brain volumes, or activity levels between those with and without imaging or actigraph data.
Clinical Interviews
The Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 1999) was administered to diagnose a prodromal syndrome. As noted, UHR participants in the present study met criteria for a prodromal or high-risk syndrome. The SIPS gauges several distinct categories of prodromal symptom domains including positive and negative dimensions. A sum score for each category is used as an indicator of the respective dimensions of symptomatology. Among items comprising the negative symptom domain, the SIPS gauges Avolition (i.e., symptoms relating to impairment in the initiation, persistence, and control of goal-directed activities; low drive, energy, or productivity) and Occupational Functioning (i.e., symptoms relating to difficulty performing role functions that were previously performed without problems; having difficulty in productive, instrumental relationships with colleagues at work or school). As these items are of particular relevance to the present investigation of activity, they were subject to further analyses in addition to the broad negative symptom dimension. The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 1995) was also administered to rule out formal psychosis (a noted exclusionary criterion). This measure has been demonstrated to have excellent inter-rater reliability in adolescent populations (Martin, Pollock, Bukstein, & Lynch, 2000) and has been used in several previous studies focusing on adolescent populations with schizophrenia spectrum disorders (Howes et al., 2009). Training of interviewers (who were advanced doctoral students) was conducted over a 2-month period, and inter-rater reliabilities exceeded the minimum study criterion of Kappa ≥ 80.
Actigraphy
The ActiGraph Monitor (Pensacola, FL) is a wrist-worn device containing a miniature tri-axial accelerometer that produces a digital integration of the amount and duration of movement. In our study, participants wore the wristwatch and total activity counts were recorded in 60-second intervals for a continuous period of five days. The ActiLife scoring program also provides validation data confirming how long the ActiGraph wristwatch was worn based on measurements of activity. Additionally, participants provided information about the timing and duration of sleep, and this information was verified utilizing the ActiLife software. We calculated the Total Physical Activity, defined as the cumulative activity counts while participants were awake and wearing the device divided by the net number of counts recorded for each participant (i.e., total time the watch was worn) (Walther et al., 2011; Walther et al., 2010). The software determines periods in which the watch was not worn (e.g., temporarily removing it for a shower), and these were excluded from analyses. There was no significant difference in the time the watch was not worn between the UHR and the control participants. The ActiLife version 5.10 scoring program provides estimates for the number of consecutive counts per minute (CPM) falling within different categories of activity (defined by cut points). The activity level variables include percentage of time in Sedentary (activities that do not increase energy expenditure higher than a resting level), Light (very low energy expenditure activities such as cooking food, walking slower than 2.0 miles per hour, washing dishes), Moderate (activity which noticeably accelerates the heart rate: e.g., brisk walking, dancing, gardening, housework, active involvement in sports, carrying moderate loads), and Vigorous activity (causing rapid breathing and a substantial increase in heart rate: e.g., running, fast cycling, aerobics, competitive sports, carrying and moving heavy loads).
Structural Imaging
Magnetic resonance imaging (MRI) of the brain was acquired on each subject using a Siemens 3-Tesla Magnetom TIM Trio MRI scanner (Siemens AG, Munich, Germany) with a 12-channel head coil. A T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (MPRAGE; sagittal plane; repetition time [TR] = 2530 ms; echo times [TE] = 1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms, 9.08 ms; GRAPPA parallel imaging factor of 2; 1 mm3 isomorphic voxels, 192 interleaved slices; FOV = 256mm; flip angle = 7°; time = 6:03 min) covering the whole brain was acquired for anatomic segmentation. A turbo spin echo proton density (PD)/ T2-weighted acquisition (TSE; axial oblique aligned with anterior commissure-posterior commissure line (AC-PC line); TR= 3720ms; TE=89ms; GRAPPA parallel imaging factor of 2; .9 × .9 mm voxels; FOV=240mm; flip angle: 120°; 77 interleaved 1.5mm slices; time = 5:14 min) was acquired to check for incidental pathology. The entire imaging protocol including localizing images, gradient echo field mapping, arterial spin labeling scan, and BOLD weighted resting state scan took approximately 30 minutes.
Processing
Target structures, consisting of the left and right hippocampi and parahippocampal gyri, were delineated automatically on the MPRAGE using the FreeSurfer suite of automated tools (Fischl et al., 2002). Figure 1 depicts the target regions of interest in this study. This approach has been validated and widely used in high impact studies (Tae et al., 2008; Rajendara et al., 2009; Kuhn et al., 2012). The processing stream involved motion correction, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al., 2002; Fischl et al., 2004), intensity normalization (Sled, Zijdenbos, & Evans, 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl, Liu, & Dale, 2001; Segonne, Pacheco, & Fischl, 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Fischl & Dale, 2000). FreeSurfer also calculates values for each participant's total intracranial volume (TICV) (i.e., the sum of whole-brain grey matter + white matter + cerebrospinal fluid), and each structure was divided by the TICV to control for whole brain volume.
Figure 1.
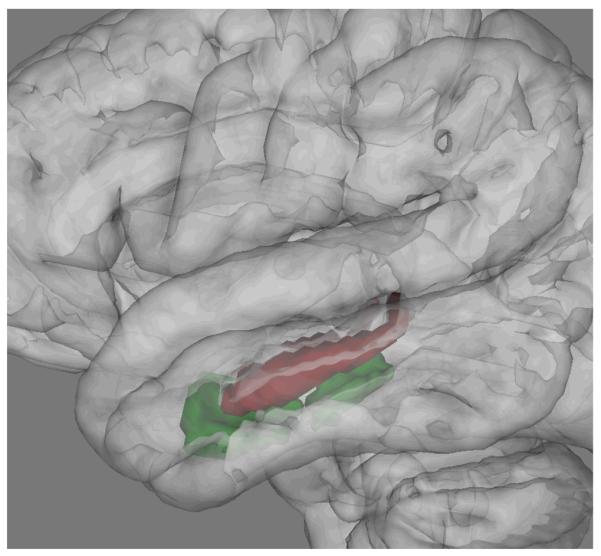
Targeted Medial Temporal Lobe Structures
Note: The volumes of the hippocampus (red) and parahippocampal gyrus (green) were hypothesized to be affected ultra high-risk youth, and to be positively associated with activity level.
Statistical Approach
Independent t-tests and chi-square tests were employed to examine differences between groups in respective continuous and categorical demographic variables. Independent t-tests were utilized to determine if any differences existed between those participants with and without missing data. Although there was not a significant group difference in age, there was a trend [t(54)=1.42, p = .16], and to control for any subtle age-based neurodevelopmental changes or age-based differences in activity level, all analyses controlled for age. Analyses of Covariance (ANCOVA) controlling for age were utilized to determine group differences in symptoms, hippocampal and parahippocampal volumes, and percentage of time spent in a category of activity level (Sedentary, Light, Moderate, Vigorous). Partial correlations controlling for age were utilized to determine associations between Total Physical Activity and both brain structures and symptoms.
Results
As noted, a total of 29 UHR and 27 control adolescents and young adults participated in the study and there were no differences in symptoms, brain volumes, or activity levels between those with or without imaging or actigraph data. There were no significant differences in demographic characteristics such as age, parental education, or gender between the UHR and Control groups. To rule out the possibility that the presence of negative symptoms might drive activity levels alone, analyses were conducted with negative symptoms treated as a covariate. The inclusion of negative symptoms did not alter the magnitude, direction, or significance of any of the group comparison or correlation analyses, and was omitted from the following analyses. One-sample Kolmogorov-Smirnov tests revealed that distributions for target hippocampal volumes, parahippocampal gyri volumes, Total Physical Activity, and activity category variables were normally distributed, meeting assumptions for parametric statistics.
Symptoms
ANCOVA analyses indicated elevated levels of symptoms in the UHR group in the positive F(1,53) = 67.9, p ≤ .01 and negative F(1,55) = 28.78 p ≤ .01 domains, as well as the Avolition scale F(1,53) = 24.93, p ≤ .01 and Occupational Functioning scale F(1,53) = 16.96, p ≤ .01.
Types of Activity
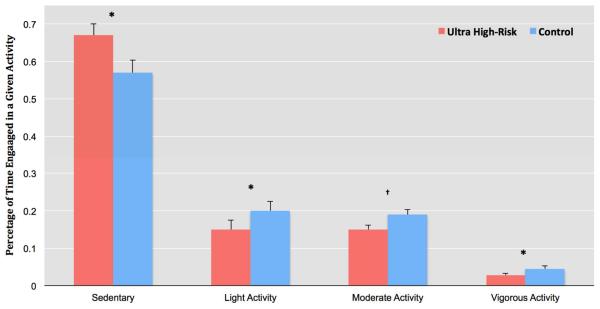
The first goal of the study was to determine group differences in type of activity. As noted, the percentage of time spent in a given activity during the waking period was divided into Sedentary, Light, Moderate, and Vigorous activity categories (based on CPM counts). ANCOVA analysis indicated that the UHR group exhibited a significantly greater proportion of Sedentary activity, F(1,40) = 8.18, p ≤ .01. In contrast, the control group showed significantly higher levels of Light activity F(1,40) = 9.06, p ≤ .01, a trend suggesting higher levels of Moderate activity F(1,39) = 1.47, p = .12, and significantly greater Vigorous activity F(1,40) = 4.79, p ≤.01 (See Figure 2 for means). ANCOVA analysis also indicated a strong trend to suggest that the control group showed elevated Total Physical Activity when compared to the UHR group, F(1,41) = 2.23, p ≤ .06 (see Table 1 for means).
Figure 2.
Group Differences in Activity Between Youth at Ultra High-Risk for Psychosis And Matched Controls.
Note: Activity represents the percentage of time spent in four levels of activity based on actigraphy counts per minute. * p ≤ .05; † Indicates a trend level difference p ≤ .15; Error bars represent standard error.
Brain Structure
ANCOVA analyses indicated that the UHR group showed significantly smaller right, F(1,51) = 10.17, p ≤ .01, and left, F(1,51) = 4.44, p ≤ .01, hippocampal volumes. Likewise, the UHR group showed a non-significant trend towards smaller left, F(1,51) = .99, p = .18, and significantly smaller right, F(1,51) = 2.87, p ≤ .05, parahippocampal gyral volumes (See Table 1).
Activity Level and Brain Structure
Partial correlations were employed to determine relationships between Total Physical Activity, symptoms, and the noted brain structures in the UHR group. There were no significant relationships between Total Physical Activity and hippocampal volumes. Significant moderate positive correlations were detected between Total Physical Activity and bilateral parahippocampal gyral volumes, indicating that higher levels of motor activity were associated with larger volumes, and conversely, that smaller volumes are associated with a lower activity (See Table 2). The correlation for the left parahippocampal gyrus continued to reach the strict significance threshold when using a Bonferroni correction but the association for the right hemisphere volume did not.
TABLE 2.
Associations Between Total Physical Activity and Medial Temporal Structure, and Symptoms in Youth at High Risk for Psychosis
| Structure/Domain | Total Physical Activity | |
|---|---|---|
| Correlation a | p-Value | |
| Hippocampus | ||
| Right | .10 | p = .33 |
| Left | .08 | p = .36 |
| Parahippocampal Gyrus | ||
| Right | .44 | p ≤ 0.05 |
| Left | .51 | p ≤ 0.01 |
| Symptoms | ||
| Positive | .02 | p = .46 |
| Negative | −.16 | p = .24 |
| Avolition | −.16 | p = .24 |
| Occupation | −.36 | p ≤ 0.05 |
Partial correlations controlling for age were employed. Positive and negative symptom domains as well as avolition and occupational functioning scales are scored utilizing the Structured Interview for Prodromal Syndromes (SIPS). When employing a Bonferroni correction, the association for the left parahippocampal gyrus continued to meet the strict significance threshold but the association for the right volume did not.
Partial correlations were also employed in exploratory analyses to determine relationships between Total Physical Activity and brain structures in the healthy control group. Although the correlations were in a direction that would suggest that larger volumes are linked to more physical activity (there were small effects for the hippocampus and the parahippocampal gyrus), the associations were not significant.
Relationships with Symptoms
Exploratory analyses were conducted to examine the relationships between Total Physical Activity and domains of positive and negative symptoms (as well as two negative symptom scales that are relevant to activity) in the UHR sample. There was no relationship between Total Physical Activity and positive symptoms. The negative symptom domain and Avolition were not significantly associated with Total Physical Activity although the direction of the relationship suggested an association between less activity and higher negative symptoms. There was a significant negative correlation between Total Physical Activity and poorer Occupational Functioning (e.g., difficulty performing role functions that were previously performed without problems) (See Table 2).
Discussion
The present investigation provides a unique view into the naturalistic activity levels of UHR adolescents and young adults in comparison to matched healthy control youth. In the first study to utilize actigraphy in a UHR sample, we report findings to suggest that a sedentary lifestyle may be deleterious for both brain development and emerging symptomatology. Findings that UHR patients show smaller medial temporal structures and less light-to-vigorous activity levels are important given the key role of the hippocampal formation in diathesis-stress conceptualizations and the broader literature suggesting the beneficial effects of exercise on brain health.
Over the course of a five-day period, the UHR group engaged in sedentary behavior for a significantly greater percentage of time (i.e., roughly 65%). Sedentary behavior has been widely associated with obesity and health problems in adolescent populations (Rosenberger et al., 2013), and in addition to contributing to poor emotional well-being and peer-support (Costigan, Barnett, Plotnikoff, & Lubans, 2013), it may also exacerbate the health related side-effects of pharmacological treatment (Maayan & Correll, 2010) and potentially lead to poorer compliance. In contrast, the healthy control adolescents and young adults in our study participated in light to vigorous activity a greater percentage of time. Participation in moderate activity and vigorous activity have been found to have ameliorative effects, such as reduced cardio metabolic risk factors, regardless of the amount of time spent engaging in sedentary activities (Ekelund et al., 2012). In this investigation, the UHR group showed a small percentage of time engaging in a beneficial level of activity.
Because negative symptoms appear long before the onset of formal psychosis (Strous et al., 2004), closely follow other stable features (e.g., cognition) (Harvey, Koren, Reichenberg, & Bowie, 2006), and remain relatively constant (i.e., not ebbing and flowing in a manner consistent with positive symptoms), many have argued that this domain is reflective of “core” pathology, lying more closely to the pathophysiology driving the illness (Gold et al., 2012; Horan & Blanchard, 2003). Indeed, new large scale efforts are underway to improve assessment and focus attention on this critical set of symptoms (Forbes et al., 2010) which have been found to contribute substantially to the disability seen in schizophrenia (Villalta-Gil et al., 2006), and characterizes UHR samples (Lencz et al., 2004). Findings that low levels of activity are correlated with smaller parahippocampal gyral volumes and Occupational Functioning (a prominent dimension of negative symptomology in the UHR conceptualization) extend the study of movement function in this group beyond striatal based dyskinesias (Mittal, Daley, O'Niell, Bearden, & Cannon, 2010; Mittal et al., 2011; Mittal, Neumann, Saczawa, & Walker, 2008; Mittal et al., 2007; Mittal & Walker, 2007; Mittal, Walker, et al., 2010), and provide new light on how sedentary behavior may potentially drive the onset of illness. Likewise, findings that an elevated activity level is tied to better functioning are consistent with a recent literature review of studies of patients with schizophrenia (Gorczynski & Faulkner, 2010).
Observations that activity level is tied to medial temporal lobe health are consistent with a body of literature that indicates that healthy exercise contributes to medial temporal health (Cotman & Berchtold, 2002). Animal models have been instrumental in establishing the effects of aerobic activity on medial temporal health, suggesting that aerobic activity promotes hippocampal growth and related function by promoting neurogenesis (a process by which neurons are generated from neural stem and progenitor cells) (Wolf, Melnik, & Kempermann, 2011) and cell proliferation (increase in the number of cells as a result of cell growth and cell division) (Koehl et al., 2008) while slowing apoptosis (programmed cell death) (Avula, Muthukumar, Zaman, McCarter, & Fernandes, 2001). Because the subgranular zone (SGZ) of the dentate gyrus (a part of the hippocampus) is a predominant region for neurogenesis in adulthood (one of only two major sites of adult neurogenesis in the brain), and a number of newborn cells become functionally integrated into proximal brain tissue (Eriksson et al., 1998), exercise research has focused attention on the hippocampus and other surrounding medial temporal structures. For example, in an animal study examining the effects of treadmill exercise on cell proliferation in the dentate gyrus, Sprague-Dawley rats were classified into controls, an easy exercise group, and a moderate exercise group (Kim et al., 2002). Results suggested a dose dependent response where easy and moderate exercise group rodents exhibited significant cell proliferation (increase in BrdU-positive cells) in the dentate gyrus compared to controls after 30 minutes of exercise a day for one week. With regard to the present sample, its is important to note that several animal models have suggested that an aerobic exercise program promotes neuroplastic changes in the hippocampal formation (e.g., significant increase of protein level in the hippocampal formation and PV-immunoreactive neurons in CA1 and CA2/CA3) during adolescent period (Gomes da Silva et al., 2010).
Accumulating evidence from a series of translational studies has indicated that the pattern of results seen in the animal research is also true for human populations as well. For example, aerobic exercise has been found to result in increased hippocampal blood volume in healthy individuals (Colcombe et al., 2004). Further, exercise-related brain plasticity has been shown in the increased temporal lobe connectivity between the bilateral parahippocampal gyrus and bilateral medial temporal gyrus (Voss et al., 2013). There have also been several avenues of research to suggest exercise counteracts declining medial temporal function in aging. There have also been important studies that suggest positive effects of activity on medial temporal health in patients with Alzheimer's disease (Erickson et al., 2011; Yuede et al., 2009). To date, there have been a small number of studies showing positive effects from exercise are also seen in schizophrenia patients. As discussed above, Pajonk and colleagues (2010) found that after a brief cardiovascular exercise trial (12 weeks) in patients diagnosed with schizophrenia, those in the exercise group showed a significant increase in hippocampal volume (12%) when compared to a control group of schizophrenia patients in a non-aerobic activity (1%). Further, the changes in volume were positively associated with improvement in aerobic fitness (measured by a change in maximum oxygen consumption) and improvement in verbal memory. As noted, while related investigations have predominantly focused on medial temporal structures (because the integral role that the region plays in promoting neurogenesis and synaptic plasticity), it is also quite possible that physical activity also affects additional neural structures and future research will be necessary for assessing specificity.
While correlations were in a direction to suggest elevated activity was associated with increases in medial temporal volumes for the healthy controls, there were not any significant relationships in this group. However, it is likely that a better-powered sample would detect significant correlations in this group as well. Further, although it is not possible to definitively infer causality with the present design, it is possible that because the healthy individuals are already naturalistically quite active, the effect that activity has on medial temporal volume may have been attenuated. In addition it is noteworthy that the medial temporal structures differ in psychosis spectrum individuals, who show neuronal atrophy and the apparent loss of neurons and presynaptic proteins (Young et al., 1998; Heckers & Konradi, 2002; Dickey, McCarley, & Shenton, 2002; Pantelis et al., 2003; Pantelis et al., 2007; Pajonk et al., 2010). It is possible that the affected structures in the UHR group may have allowed for a greater effect. Higher powered future studies and research utilizing other relevant measures of brain function and neuronal composition (i.e., functional neuroimaging, and magnetic resonance spectroscopy) are sorely needed to improve our understanding the effects of activity on the brain in healthy and UHR populations.
It is important to note that the current study does not show causal relationships; it is not possible to definitely determine the extent to which the level of activity is a reflection of symptoms, or conversely, if it is contributing to symptom levels. However, as noted, when negative symptom domain was included as a covariate, it did not influence the direction of the findings in this study. In addition, the present investigation did not detect a significant relationship with activity and the more direct Avolition dimension (although the correlation was in the predicted direction), suggesting that although physical activity is significantly tied to at least one dimension of negative symptoms (Occupational Functioning), it may also, in part, reflect a unique domain of the UHR phenotype. One tentative possibility is that more symptoms contribute to a sedentary lifestyle in patient populations, which may then have a deleterious effect on medial temporal lobe maintenance and development. In turn, the medial temporal abnormalities may further contribute to negative symptoms (Benoit et al., 2012). This outcome would yield a putative cascade in which increasingly sedentary activity and brain abnormalities drive one another in an escalating cycle, driving the onset of psychosis. This notion has important ramifications for stress and hippocampal-based etiological models (Corcoran et al., 2003; Lipska, 2002; Walker & Diforio, 1997), and for our understanding of the developing brains of UHR youth. Future longitudinal studies will be important for clarifying predictive relationships, and studies with more in-depth negative symptom questions will be important to determine if the relationship between Occupational Functioning and the actigraph variable is due to unique aspects of the dimension (which asks about a variety of other behaviors such as effort and school performance) or primarily due to topic overlap.
The present study held several important innovations, and current results are consistent with a broader literature on morphology in psychosis and physical activity. Although the analyses yielded significant, and in some cases moderate, correlations between activity, medial temporal structure, and symptoms, the results should be considered to be preliminary until replication is seen in larger UHR samples. As noted, the correlation between activity and the left parahippocampal gyrus remained significant after a Bonferroni correction was used but the correlation for the right hemisphere volume no longer met the strict threshold. In addition, activity level was viewed naturalistically and experimental paradigms manipulating levels of exercise are needed. Although the 5-day period in the present study is longer than other actigraphy studies in patients with schizophrenia (e.g., 20 hours; Farrow et al., 2005), this period may not be long enough to be considered truly naturalistic. Future studies examining biological and genetic factors specific to the inflammation response and methylation will also be important for understanding the effects of physical activity in UHR youth. Finally, it is important to note that an emerging literature suggest significant gender differences in normative adolescent medial temporal development (Hu et al., 2013), and while there were not group differences in gender in the presence study, it will be important for better-powered future investigations to formulate specific hypotheses and study design around questions of gender differences. Although the present design allowed for correlational data, the relationships speak toward a potentially important etiological mechanism, and also toward a viable point of intervention. Taken together, the present study provides a solid foundation for building experimental trial for at-risk youth.
Acknowledgements
This work was supported by National Institutes of Health Grant R01MH094650 (Mittal).
Footnotes
There are no potential conflicts of interest to report.
References
- Allen P, Seal ML, Valli I, Fusar-Poli P, Perlini C, Day F, McGuire PK. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophrenia Bulletin. 2011;37(4):746–756. doi: 10.1093/schbul/sbp113. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, Wible CG, McCarley RW, Jakab M, Kasai K, Shenton ME. An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophrenia Research. 2002;58(2–3):123–134. doi: 10.1016/s0920-9964(01)00372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula CP, Muthukumar AR, Zaman K, McCarter R, Fernandes G. Inhibitory effects of voluntary wheel exercise on apoptosis in splenic lymphocyte subsets of C57BL/6 mice. J Appl Physiol. 2001;91(6):2546–2552. doi: 10.1152/jappl.2001.91.6.2546. [DOI] [PubMed] [Google Scholar]
- Benoit A, Bodnar M, Malla AK, Joober R, Lepage M. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Frontiers in Psychiatry. 2012;3:42. doi: 10.3389/fpsyt.2012.00042. doi: 10.3389/fpsyt.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berle JO, Hauge ER, Oedegaard KJ, Holsten F, Fasmer OB. Actigraphic registration of motor activity reveals a more structured behavioural pattern in schizophrenia than in major depression. BMC Research Notes. 2010;3:149. doi: 10.1186/1756-0500-3-149. doi: 10.1186/1756-0500-3-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, First MB, Cornblatt B. The psychosis risk syndrome and its proposed inclusion in the DSM-V: A risk-benefit analysis. Schizophrenia Research. 2010;120(1–3):16–22. doi: 10.1016/j.schres.2010.03.018. doi: S0920-9964(10)01188-6 [pii]10.1016/j.schres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal VA, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophrenia Bulletin. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Costigan SA, Barnett L, Plotnikoff RC, Lubans DR. The Health Indicators Associated With Screen-Based Sedentary Behavior Among Adolescent Girls: A Systematic Review. Journal of Adolescscent Health. 2013;54(4):382–392. doi: 10.1016/j.jadohealth.2012.07.018. doi: 10.1016/j.jadohealth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. doi: 10.1196/annals.1308.001 1021/1/1 [pii] [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Shenton ME. The brain in schizotypal personality disorder: a review of structural MRI and CT findings. Harvard Review of Psychiatry. 2002;10(1):1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Shenton ME. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biological Psychiatry. 1999;45(11):1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A, International Children's Accelerometry Database, Collaborators Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. Journal of the American Medical Association. 2012;307(7):704–712. doi: 10.1001/jama.2012.156. doi: 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn A, Nordbord C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Hunter MD, Wilkinson ID, Green RD, Spence SA. Structural brain correlates of unconstrained motor activity in people with schizophrenia. British Journal of Psychiatry. 2005;187:481–482. doi: 10.1192/bjp.187.5.481. doi: 10.1192/bjp.187.5.481. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I) Patient Edition American Psychiatric Press; Washington DC: 1995. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Science USA. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions in Medical Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. American Journal of Psychiatry. 2009;166(7):805–811. doi: 10.1176/appi.ajp.2009.08050757. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C, Blanchard JJ, Bennett M, Horan WP, Kring A, Gur R. Initial development and preliminary validation of a new negative symptom measure: the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophrenia Research. 2010;124(1–3):36–42. doi: 10.1016/j.schres.2010.08.039. doi: 10.1016/j.schres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton, Matthew J, Lawrie S, Sacchetti E. Neuroanatomy of vulnerability to psychosis: A voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(5):1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli Paolo, Perez Jorge, Broome Matthew, Borgwardt Stefan, Placentino Anna, Caverzasi Eduardo, McGuire Philip. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2007;31(4):465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Frank MJ. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Archives of General Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes da Silva S, Dona F, da Silva Fernandes MJ, Scorza FA, Cavalheiro EA, Arida RM. Physical exercise during the adolescent period of life increases hippocampal parvalbumin expression. Brain Dev. 2010;32(2):137–142. doi: 10.1016/j.braindev.2008.12.012. doi: 10.1016/j.braindev.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Schizophrenia Bulletin. 2010;36(4):665–666. doi: 10.1093/schbul/sbq049. doi: 10.1093/schbul/sbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophrenia Bulletin. 2006;32(2):250–258. doi: 10.1093/schbul/sbj011. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal neurons in schizophrenia. Journal of Neural Transmission. 2002;109(5–6):891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K, Wolbers T, Roder B, Hotting K. Cardiovascular fitness modulates brain activation associated with spatial learning. Neuroimage. 2012;59(3):3003–3014. doi: 10.1016/j.neuroimage.2011.10.021. doi: 10.1016/j.neuroimage.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ. Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophrenia Research. 2003;65(2–3):125–137. doi: 10.1016/s0920-9964(02)00410-3. [DOI] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of General Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. doi: 66/1/13 [pii]10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Hu S, Pruessner JC, Coupe P, Collins DL. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage. 2013;74(1):276–287. doi: 10.1016/j.neuroimage.2013.02.032. doi: 10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.06.011. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Janse Van Rensburg K, Taylor A, Hodgson T, Benattayallah A. Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: an fMRI study. Psychopharmacology. 2009;203(3):589–598. doi: 10.1007/s00213-008-1405-3. doi: 10.1007/s00213-008-1405-3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim HB, Jang MH, Lim BV, Kim YJ, Kim YP, Kim CJ. Treadmill exercise increases cell proliferation without altering of apoptosis in dentate gyrus of Sprague-Dawley rats. Life Science. 2002;71(11):1331–1340. doi: 10.1016/s0024-3205(02)01849-0. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Weinstein AA, Deuster PA, Whittaker KS, Tracy RP. Inflammatory markers and negative mood symptoms following exercise withdrawal. Brain, Behavior, and Immunity. 2008;22(8):1190–1196. doi: 10.1016/j.bbi.2008.05.011. doi: 10.1016/j.bbi.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Kühn S, Musso F, Mobascher A, Warbrick T, Winterer G, Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: First evidence from brain morphometry. Translational Psychiatry. 2012;2:e127. doi: 10.1038/tp.2012.51. doi:10.1038/tp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophrenia Research. 2004;68(1):37–48. doi: 10.1016/S0920-9964(03)00214-7. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- Lipska BK. Neonatal disconnection of the rat hippocampus: a neurodevelopmental model of schizophrenia. Dialogues Clin Neurosci. 2002;4(4):361–367. doi: 10.31887/DCNS.2002.4.4/blipska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Correll CU. Management of antipsychotic-related weight gain. Expert Review of Neurotheraputics. 2010;10(7):1175–1200. doi: 10.1586/ern.10.85. doi: 10.1586/ern.10.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Dependence. 2000;59(3):173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatry Quarterly. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Daley M, O'Niell J, Bearden CE, Cannon T. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophrenia Research. 2010;123:68–70. doi: 10.1016/j.schres.2010.08.002. doi: 10.1016/j.schres.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Jalbrzikowski M, Daley M, Roman C, Bearden CE, Cannon TD. Abnormal movements are associated with poor psychosocial functioning in adolescents at high risk for psychosis. Schizophrenia Research. 2011;130(1–3):164–169. doi: 10.1016/j.schres.2011.05.007. doi: 10.1016/j.schres.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Archives of General Psychiatry. 2008;65(2):165–171. doi: 10.1001/archgenpsychiatry.2007.23. doi: 65/2/165 [pii]10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, Trottman HD, Esterberg M, Dhrub SH, Simeonova DI, Walker EF. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. Journal of Abnormal Psychology. 2007;116(2):260–267. doi: 10.1037/0021-843X.116.2.260. doi: 2007-06673-004 [pii]10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. Journal of Abnormal Psychology. 2007;116(4):796–803. doi: 10.1037/0021-843X.116.4.796. doi: 2007-17062-012 [pii]10.1037/0021-843X.116.4.796. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Walker EF. Minor physical anomalies and vulnerability in prodromal youth. Schizophrenia Research. 2011;129(2–3):116–121. doi: 10.1016/j.schres.2011.02.022. doi: 10.1016/j.schres.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, Cannon TD. Markers of Basal Ganglia Dysfunction and Conversion to Psychosis: Neurocognitive Deficits and Dyskinesias in the Prodromal Period. Biological Psychiatry. 2010;68:93–99. doi: 10.1016/j.biopsych.2010.01.021. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. doi: S0140-6736(03)12323-9 [pii] 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, Wood SJ, Yucel M, Yung AR, Phillips LJ, McGorry PD. Neuroimaging and emerging psychotic disorders: the Melbourne ultra-high risk studies. International Review of Psychiatry. 2007;19(4):371–381. doi: 10.1080/09540260701512079. doi: 781049941 [pii]10.1080/09540260701512079. [DOI] [PubMed] [Google Scholar]
- Parker BA, Thompson PD, Jordan KC, Grimaldi AS, Assaf M, Jagannathan K, Pearlson GD. Effect of exercise training on hippocampal volume in humans: a pilot study. Research Quarterly in Exercise and Sport. 2011;82(3):585–591. doi: 10.1080/02701367.2011.10599793. [DOI] [PubMed] [Google Scholar]
- Pelletier AL, Mittal VA. Negative symptom measurement in individuals at-risk for psychosis. Psychiatry Research. 2013;205(1–2):181–182. doi: 10.1016/j.psychres.2012.08.020. doi: 10.1016/j.psychres.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating Activity and Sedentary Behavior from an Accelerometer on the Hip or Wrist. Medecine and Science in Sports and Exercise. 2013;4(5):964–975. doi: 10.1249/MSS.0b013e31827f0d9c. doi: 10.1249/MSS.0b013e31827f0d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Seidman Larry J, Giuliano Anthony J, Meyer Eric C, Addington Jean, Cadenhead Kristin S, Cannon Tyrone D, Group, North American Prodrome Longitudinal Study (NAPLS) Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. doi:10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions in Medecine and Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Annals of the New York Academy of Science. 2004;1021:23–26. doi: 10.1196/annals.1308.002. doi: 10.1196/annals.1308.002 1021/1/23 [pii] [DOI] [PubMed] [Google Scholar]
- Strous RD, Alvir JM, Robinson D, Gal G, Sheitman B, Chakos M, Lieberman JA. Premorbid functioning in schizophrenia: relation to baseline symptoms, treatment response, and medication side effects. Schizophrenia Bulletin. 2004;30(2):265–278. doi: 10.1093/oxfordjournals.schbul.a007077. [DOI] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam E, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50(7):569–581. doi: 10.1007/s00234-008-0383-9. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Villalta-Gil V, Vilaplana M, Ochoa S, Haro JM, Dolz M, Usall J, Group, Nedena Neurocognitive performance and negative symptoms: are they equal in explaining disability in schizophrenia outpatients? Schizophrenia Research. 2006;87(1–3):246–253. doi: 10.1016/j.schres.2006.06.013. doi:10.1016/j.schres.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychological Review. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal VA, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psycholology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T, Muller TJ. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011;42(3):276–283. doi: 10.1016/j.nbd.2011.01.017. doi: 10.1016/j.nbd.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Wiest R, Dierks T, Strik W, Muller TJ. White matter integrity associated with volitional motor activity. Neuroreport. 2010;21(5):381–385. doi: 10.1097/WNR.0b013e328337ca29. doi: 10.1097/WNR.0b013e328337ca29. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW. Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Archives of General Psychiatry. 1995;52(4):279–288. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Melnik A, Kempermann G. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain and Behavior Immunology. 2011;25(5):971–980. doi: 10.1016/j.bbi.2010.10.014. doi: 10.1016/j.bbi.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG. SNAP-25 deficitv and hippocampal connectivity in schizophrenia. Cerebral Cortex. 1998;3:261–268. doi: 10.1093/cercor/8.3.261. [DOI] [PubMed] [Google Scholar]
- Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, Csernansky JG. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiology of Disease. 2009;35(3):426–432. doi: 10.1016/j.nbd.2009.06.002. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]