Abstract
Aim
Although patterns are emerging for macroorganisms, we have limited understanding of the factors determining soil microbial community composition and productivity at large spatial extents. The overall objective of this study was to discern the drivers of microbial community composition at the extent of biogeographical provinces and regions. We hypothesized that factors associated with land use and climate would drive soil microbial community composition and biomass.
Location
Great Basin Province, Desert Province and California Floristic Province, California, USA.
Methods
Using phospholipid fatty acid analysis, we compared microbial communities across eight land-use types sampled throughout the State of California, USA (n = 1117).
Results
The main factor driving composition and microbial biomass was land-use type, especially as related to water availability and disturbance. Dry soils were more enriched in Gram-negative bacteria and fungi, and wetter soils were more enriched in Gram-positive, anaerobic and sulphate-reducing bacteria. Microbial biomass was lowest in ecosystems with the wettest and driest soils. Disturbed soils had less fungal and more Gram-positive bacterial biomass than wildland soils. However, some factors known to influence microbial communities, such as soil pH and specific plant taxa, were not important here.
Main conclusions
Distinct microbial communities were associated with land-use types and disturbance at the regional extent. Overall, soil water availability was an important determinant of soil microbial community composition. However, because of the inclusion of managed and irrigated agricultural ecosystems, the effect of precipitation was not significant. Effects of environmental and management factors, such as flooding, tillage and irrigation, suggest that agricultural management can have larger effects on soil microbial communities than elevation and precipitation gradients.
Keywords: California, disturbance, microbial biomass, PLFA, soil microbial community, water
INTRODUCTION
How do communities organize and differentiate themselves along geographical, climatic and environmental gradients? Classic studies of plant communities indicate the importance of environmental and elevation gradients in driving species composition and suggest that communities segregate according to optimal conditions for individual taxa along these gradients (Whittaker, 1956; Curtis, 1959). At very large spatial extents (e.g. regions, provinces and continents), climatic factors are the primary drivers of plant communities (Whittaker et al., 2001). Recent studies have indicated that precipitation gradients and their direct influence on soil water availability as well as the interaction between energy (e.g. evapotranspiration) and precipitation are driving factors structuring global and regional plant communities (O’Brien, 1998; Kreft & Jetz, 2007). Although soil microorganisms play important roles in many ecosystem functions, comparatively less is known about factors influencing microbial community composition at large spatial extents.
At small extents, biotic interactions alter the soil habitat in which microbes live and influence microbial composition. Plants add and remove mineral nutrients from soil environments, and these rates are species specific. Some researchers have detected differences among microbial communities based on plant species composition (e.g. Batten et al., 2006). Food web interactions among different groups of microorganisms (e.g. competition and predation), as well as between microorganisms and higher trophic level organisms, also influence soil communities (De Deyn et al., 2003; Wardle, 2006).
Abiotic factors such as soil fertility (Bardgett et al., 1999), substrate availability (Bååth et al., 1995), pH (Bååth et al., 1995), climate (Steinberger et al., 1999), soil temperature (Zogg et al., 1997) and moisture, as well as shifts in seasonality (Bardgett et al., 1999; Steenwerth et al., 2006), have an impact on soil microbial communities at small extents. For example, fungal- :bacterial ratios generally decrease with increased soil water saturation (e.g. Bossio & Scow, 1998), and organic carbon inputs often select for rapidly growing, heterotrophic microorganisms (Sylvia et al., 2005). Furthermore, management factors such as tillage (Calderón et al., 2001) and farming system (Petersen et al., 1997) also influence microbial community composition. Thus, at the site level, abiotic and biotic heterogeneity has been identified as an important driver of soil microbial communities. Whether these same abiotic and biotic factors are important at large spatial extents is relatively unknown.
Recent studies suggest that the composition of soil microbial communities, similarly to communities of macroorganisms, vary at large spatial extents (e.g. landscapes, regions and continents) because of environmental heterogeneity in factors such as soil texture, climate, plant community composition, availability of labile carbon and soil pH (Steenwerth et al., 2003; McCulley & Burke, 2004; Fierer & Jackson, 2006; Martiny et al., 2006). In one study, changes in bacterial composition across sites were more closely associated with environmental gradients than with spatial extent; environmentally similar areas were similar in bacterial composition, regardless of geographical location (Horner-Devine et al., 2004). Likewise, when stream-bed bacterial community structure was compared among three biomes (eastern deciduous, south-eastern coniferous and tropical evergreen forests), samples within a biome were more similar in composition than samples from different biomes that differed in multiple biological, physical and chemical components (Findlay et al., 2008).
Anthropogenic disturbance and land use influence microbial community composition at multiple spatial extents and are linked closely to soil environmental heterogeneity. Long-term agricultural management practices alter soil microbial community composition, even decades after that management practice has ceased (Buckley & Schmidt, 2003). Tillage influences multiple soil properties, including aeration and organic matter availability (Calderón et al., 2000), physically disrupts fungal hyphae (Evans & Miller, 1990) and alters microbial community composition (Calderón et al., 2001).
To address the roles of environmental heterogeneity and disturbance in determining composition at the regional extent, we compared soil microbial communities from a diverse set of ecosystems that differed in land use (i.e. land-use types such as coastal grasslands, inland grasslands, deserts, coniferous forests and freshwater wetlands, as well as perennial and annual agricultural fields) from California. The scale of the study (i.e. c. 400 km × 600 km) is of medium extent, with a small grain size (i.e. individual soil samples taken from various field sites). We included sites from all three major biogeographical provinces in California (i.e. the California Floristic Province, the Great Basin Province and the Desert Province). These encompassed a wide range of land-use types, annual precipitation and elevation, providing a relatively long disturbance and environmental gradient (cf. Vetaas & Ferrer-Castán, 2008). We hypothesized that land use (e.g. disturbance regime, dominant plant species and associated soil characteristics) would be the primary driver of microbial community composition and biomass, given its strong effect on the soil environment in which microbes exist. Alternatively, soil microbial community composition and biomass at large spatial extents (i.e. the major biogeographical provinces of California) were hypothesized to be driven by climatic gradients (e.g. temperature and precipitation).
METHODS
Description of dataset and soil sampling
A total of 1117 samples from eight land-use types and 17 California counties were included in the analysis (Table 1, Fig. 1). These sampling sites represent all three Californian biogeographical provinces and six of the ten regions within these provinces (i.e. Northwestern California, Sierra Nevada, Great Valley, Central Western California, East of Sierra Nevada and Mojave Desert) (see Appendix S1 in Supporting Information; sensu Hickman, 1993). Climate varies widely because of differences in proximity to coastal areas, changes in elevation and rainshadow effects. For example, because of the maritime effect coastal areas have cool summers and mild winters; these areas rarely experience frost or freezing. In contrast, sites in the Great Valley (the central portion of California) have very hot and dry summers and cool to moderately cold, wet winters. As elevation increases in both the Coast Range and the Sierra Nevada, average temperatures drop. Cold, freezing temperatures are common during the winter months in the Sierra Nevada. Sites to the east of the Sierra Nevada experience the rainshadow effect, receive little precipitation and have large annual and diurnal variations in temperature. Overall, mean annual precipitation among sites ranges from approximately 12–135 cm (http://www.cimis.water.ca.gov/cimis/data.jsp; http://cdec.water.ca.gov/) (Appendix S2).
Table 1.
Description of land-use types, their county of origin, year and season sampled, and number of replicates.
| County | Land use | Notes | Year sampled | Season sampled | Replicates |
|---|---|---|---|---|---|
| Butte | Coniferous forest | Coniferous forest (site 1) | 1998 | Summer | 2 |
| Coniferous forest (site 2) | 1998 | Summer | 2 | ||
| Colusa | Inland grasslands | Serpentine soils | 2002 | Spring | 18 |
| Rice fields | Periodically flooded | 1994 | Winter | 32 | |
| 1994 | Spring | 16 | |||
| 1994 | Winter | 32 | |||
| 1994 | Spring | 32 | |||
| El Dorado | Coniferous forest | Coniferous forest (site 1) | 1998 | Summer | 1 |
| Coniferous forest (sampled across 16 sites) | 2000 | Summer | 50 | ||
| Fresno | Annual agriculture | Cotton fields (site 1) | 2000 | Autumn | 4 |
| 2001 | Autumn | 4 | |||
| Annual agriculture | Cotton fields (site 2) | 1995 | Autumn | 2 | |
| Coniferous forest | 2003 | Autumn | 9 | ||
| Perennial agriculture | Almond orchards | 2004 | Summer | 37 | |
| Kern | Annual agriculture | Cotton fields (site 1) | 1995 | Autumn | 8 |
| Annual agriculture | Cotton fields (site 2) | 2002 | Autumn | 8 | |
| Annual agriculture | Cotton fields (site 3) | 1995 | Autumn | 8 | |
| Desert | Mojave Desert | 2001 | Spring | 36 | |
| 2002 | Summer | 6 | |||
| 2002 | Winter | 6 | |||
| Perennial agriculture | Almond orchards | 1995 | Autumn | 16 | |
| Perennial agriculture | Fig orchards | 1995 | Autumn | 11 | |
| Kings | Annual agriculture | Cotton fields | 2000 | Autumn | 4 |
| 2001 | Autumn | 4 | |||
| Perennial agriculture | Walnut orchards | 1995 | Autumn | 10 | |
| Lake | Inland grasslands | Serpentine soils | 2001 | Spring | 36 |
| 2002 | Spring | 51 | |||
| Mendocino | Perennial agriculture | Vineyards (site 1) | 1998 | Summer | 3 |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 1 | |||
| Perennial agriculture | Vineyards (site 2) | 1998 | Summer | 3 | |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 3 | |||
| Perennial agriculture | Vineyards (site 3) | 1998 | Summer | 3 | |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 4 | |||
| Mono | Desert | Great Basin Desert | 2001 | Spring | 24 |
| Monterey | Annual agriculture | Dry-farmed hay fields | 1998 | Spring | 7 |
| Annual agriculture | Intensively farmed vegetable fields | 1998 | Spring | 5 | |
| Inland grasslands | Non-serpentine soils | 1998 | Spring | 25 | |
| Napa | Perennial agriculture | Vineyards (site 1) | 2003 | Summer | 58 |
| Perennial agriculture | Vineyards (site 2) | 1998 | Summer | 3 | |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 6 | |||
| Perennial agriculture | Vineyards (site 3) | 2003 | Summer | 16 | |
| Placer | Coniferous forest | Coniferous forest (sampled across 9 sites) | 2000 | Summer | 25 |
| Sacramento | Freshwater wetland | Periodically flooded | 2000 | Summer | 4 |
| 2000 | Autumn | 22 | |||
| 2001 | Summer | 22 | |||
| Sonoma | Coastal grasslands | Non-serpentine soils | 2003 | Spring | 32 |
| Perennial agriculture | Vineyards (site 1) | 1998 | Summer | 3 | |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 3 | |||
| Perennial agriculture | Vineyards (site 2) | 1998 | Summer | 3 | |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 3 | |||
| Perennial agriculture | Vineyards (site 3) | 1998 | Summer | 3 | |
| 2000 | Summer | 3 | |||
| 2001 | Summer | 3 | |||
| Perennial agriculture | Vineyards (site 4) | 1998 | Summer | 3 | |
| 2000 | Summer | 4 | |||
| Stanislaus | Perennial agriculture | Almond orchards | 1998 | Autumn | 143 |
| Perennial agriculture | Peach orchards | 2001 | Autumn | 41 | |
| Yolo | Annual agriculture | Bean fields | 1997 | Summer | 9 |
| Annual agriculture | Corn fields (site 1) | 2003 | Summer | 16 | |
| Annual agriculture | Corn fields (site 2) | 1995 | Summer | 9 | |
| 1997 | Summer | 9 | |||
| Annual agriculture | Safflower fields | 1997 | Summer | 9 | |
| Annual agriculture | Tomato fields (site 1) | 2003 | Summer | 18 | |
| Annual agriculture | Tomato fields (site 2) | 1995 | Spring | 49 | |
| 1995 | Summer | 24 | |||
| 1997 | Summer | 12 | |||
| Yuba | Coniferous forest | Coniferous forest (site 1) | 2001 | Spring | 4 |
| Coniferous forest | Coniferous forest (site 2) | 1998 | Summer | 2 | |
| Coniferous forest | Coniferous forest (site 3) | 2003 | Autumn | 15 | |
| Total number of replicates | 1117 |
Figure 1.
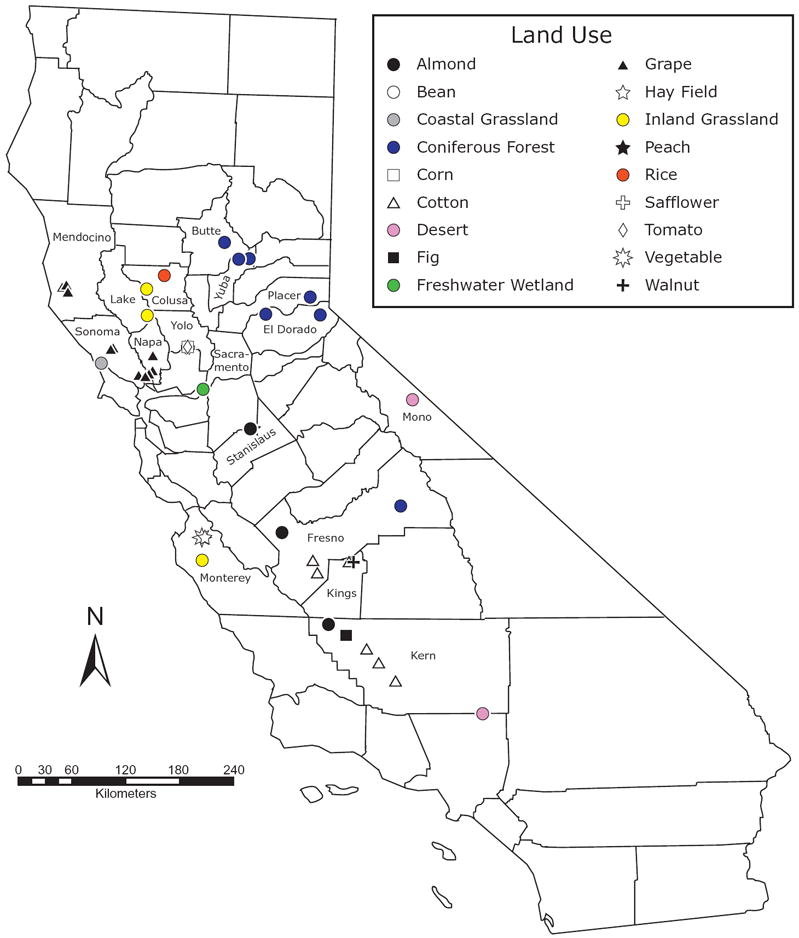
Map indicating the location of the 17 Californian counties in which soils were sampled for phospholipid fatty acid (PLFA) analysis. Land-use types sampled in each county are described in Table 1. Different symbols are used to indicate different crop types sampled within annual and perennial agriculture. Given the close proximity of fields sampled in Yolo County, symbols for each crop type are obscured; as indicated in Table 1, two sites were sampled in Yolo County. At these sites, bean, corn, safflower and tomato fields were sampled. Additionally, the eastern-most coniferous forest symbols in El Dorado and Placer counties represent an average location for samples taken from 16 and 9 transects, respectively. Similarly, the symbols in Monterey County were taken from 37 sites, representing 5 vegetable fields, 7 hayfields and 25 inland grasslands (see Steenwerth et al., 2003, for a detailed map).
Soils were sampled in both agricultural and wildland ecosystems, as well as from a range of soil moisture and disturbance regimes. Annual and perennial agricultural soils included a diverse set of crops (Table 1). All agricultural soils were drip or flood irrigated, except for hay which was rain-fed only. All soils were sampled from the soil surface down to a depth of 10–15 cm. Moist soils were immediately frozen at −20 °C until analysis, to prevent changes in microbial community composition during storage and prior to extraction. Samples from dry soils (e.g. desert soils and some agricultural soils) were kept sealed and dry under ambient conditions.
Most soil types were sampled once (e.g. coniferous forests, most annual agricultural soils and some grassland soils), whereas other soil types were sampled annually (e.g. grapes and some grassland soils) or multiple times throughout a growing season (e.g. rice, tomato, Mojave Desert and freshwater wetland soils) (Bossio & Scow, 1998; Bossio et al., 1998; Kelley, 2003; Steenwerth et al., 2003; Drenovsky et al., 2005a,b; Roberts, 2005; Batten et al., 2006; Bossio et al., 2006). This sampling scheme allowed us to determine the influence of annual and/or seasonal variation on microbial communities at larger spatial scales.
Phospholipid fatty acid analysis
Phospholipid fatty acid (PLFA) analysis is a biochemical technique that exploits differences in cellular membrane composition between microbial groups to characterize microbial communities. Although many methods exist to describe microbial communities, PLFA has certain advantages over physiologically based methods, such as community-level physiological profiling (CLPP), and DNA fingerprinting methods, such as denaturing gradient gel electrophoresis and terminal restriction length polymorphism analysis. PLFA is a relatively rapid and inexpensive method for describing microbial communities based on standardized protocols (Pinkart et al., 2002), allowing for sites to be sampled intensively. PLFA profiles also are very reproducible, with high precision between replicate samples and thus reliable sample profile extraction (Drenovsky et al., 2004). In a literature review of studies comparing CLPP, PLFA and DNA fingerprinting methods, PLFA had the lowest type-II error rate (i.e. the lowest rate of failing to detect a difference among communities where one exists) (Ramsey et al., 2006). Given its greater statistical power, ease of measurement and ability to detect changes in community composition, PLFA is well suited to studies of microbial communities in soil environments.
Overall, PLFA provides three types of information: (1) a biochemical ‘fingerprint’ of the community (the pattern of fatty acids detected in each sample), (2) an estimate of total microbial biomass (the summed concentration of all fatty acids detected in each sample), and (3) biomarkers for selected groups of microorganisms. To test whether sample variation between land-use types was greater than seasonal or annual variation within a land-use type we included soils that had been sampled repeatedly either within a growing season or over multiple years at a given site in the analysis of community fingerprints.
Whole soil microbial communities were extracted from 8 g of soil (dry weight, DW) following Bossio & Scow (1998). When weighing soils for analysis, coarse roots and rocks (> 2 mm) were excluded. Following initial extraction, solvents of increasing polarity were used to separate the phospholipid fraction from the neutral lipid and glycolipid fractions using solid phase extraction columns (0.5 g Si; Supelco, Bellefonte, PA, USA). The phospholipid fraction then was dried under N2 gas, transesterified, and methylated. Following methylation, the samples were dried once again under N2 gas and redissolved in hexane containing a known concentration of the internal standard 19:0. Samples were then analysed by gas chromatography using bacterial fatty acid standards and MIDI peak identification software (Microbial ID, Newark, DE, USA).
Environmental characterization
Where available, the United States Department of Agriculture (USDA) Natural Resource Conservation Service Soil Surveys (Soil Survey Division, 2006) were used to determine soil order, soil texture and pH. Soil survey data were not available for the two desert soils but they were classified as aridisols. Previous laboratory analyses of 1:5 soil:water extracts were used to determine soil pH for these desert soils, and soil texture was determined by feel. Mean annual precipitation was based on 5–10-year averages from the California Irrigation Management Information system (CIMIS) and the Department of Water Resources’ California Data Exchange Center (DWR/CDEC). (http://www.cimis.water.ca.gov/cimis/data.jsp; http://cdec.water.ca.gov). Geographical information (latitude, longitude and elevation) was based on information from topographic maps (http://terraserver-usa.com).
Statistical analyses
Correspondence analysis (CA) was used to compare and visualize microbial fingerprints among samples (n = 1117) using canoco for Windows, version 4.5 (Microcomputer Power, Ithaca, NY, USA). CA is a unimodal technique that simultaneously ordinates samples and fatty acids; therefore, in the ordination plots, samples with more similar fingerprints plot more closely together than samples with less similar fingerprints. Additionally, samples plotting in similar locations to fatty acids have higher concentrations of those fatty acids and lower concentrations of fatty acids plotting further from that sample. Sequentially removing lipids allowed us to determine the influence of rarer lipids on sample ordination. Very similar ordinations were observed when 48, 45, 40 or 36 lipids were included in the analysis (data not shown). All reported analyses are based on ordinations including 36 lipids. Across the dataset, the minimum number of fatty acids detected per sample was 10; the maximum was 85. In total, three fatty acids were unique to only one sample in the dataset: 16:1 iso h; unknown fatty acid 12.112 (an unidentified fatty acid approximately 12 carbons long); and 9:0 3OH.
To elucidate relationships among microbial communities, environmental characteristics, land use and biogeographical location, PLFA samples and their associated descriptive characteristics were analysed using canonical correspondence analysis (CCA), a direct ordination technique. Samples plotting close to specific descriptive characteristics are associated with those variables. Three groups of explanatory variables were used in the analysis. The first group consisted of environmental variables, including soil order, soil texture, soil pH class (e.g. slightly acidic, acidic, etc.), total PLFA, precipitation, number of growing days, soil temperature regime (i.e. thermic, mesic, frigid) and elevation. The second group was composed of land management practices (i.e. annual agriculture, perennial agriculture, tillage, no tillage, irrigation, flooding, dryland farming). The third group consisted of spatial coordinates, in which latitudinal and longitudinal coordinates of each site (x and y, respectively) were used to calculate a cubic trend surface (x, y, x2, xy, y2, x3, x2y, xy2 and y3) (Legendre, 1990). Variation in microbial communities was partitioned among the three explanatory variable groups [environment (E), management (M) and spatial variables (S)] using partial regression analysis with CCA (Borcard et al., 1992; Heikkinen et al., 2004). Two CCA runs, using the forward selection option in canoco and the Monte Carlo permutation test (with 9999 permutations), were performed for each of the three predictor groups to exclude variables not contributing significantly to the explained variation (P < 0.05) (Borcard et al., 1992). With the forward selection option in canoco, partial Monte Carlo permutation tests were used to determine the marginal and conditional effects of predictor variables on the ordination (Lepš & Šmilauer, 2003). Some samples from the CA (a subset of the coniferous forest and annual agriculture samples) were omitted because of incomplete descriptive information (thus n = 1028). Continuous variables were plotted as vectors, and nominal variables were plotted as centroids.
Variation partitioning resulted in eight fractions: pure effect of environment (a); management (b); or spatial components (c); joint effects of environment and management (d); environment and spatial components (e); or management and spatial components (f); the three groups of explanatory variables (g); and unexplained variation (h). A complete explanation of these partitioning analyses can be found in Heikkinen et al. (2004).
Descriptive statistics (means ± SE) for total PLFA and biomarker composition were calculated (sas version 8; SAS Institute, Cary, NY, USA). Given the structure of the data after pooling multiple studies, descriptive statistics were the most conservative means of data presentation. For those sites sampled multiple times in the same year (e.g. rice fields, tomato fields, freshwater wetlands and the Mojave Desert site), only one sampling time, which represented peak microbial biomass at that location, was included in the analysis. For those sites sampled in multiple years (e.g. some grape fields, rice fields, tomato fields, corn fields, inland grasslands, freshwater wetlands and the Mojave Desert site), one year was randomly selected to include in the analysis.
RESULTS
Soil microbial community variation among different ecosystem and land-use types
Soils from dry ecosystems (e.g. deserts) had very different microbial communities than soils from wetter ecosystems (e.g. rice fields and freshwater wetland soils) (Fig. 2a). These differences can be observed in the separation of the different land-use types along the first CA axis, which describes 27.2% of the variation in fatty acid composition. Land-use types along the left side of the first axis were more enriched in monounsaturated fatty acids (i.e. Gram-negative organisms; Wilkinson (1988) and 18:2ω6,9c (i.e. fungi; White et al., 1980) (Fig. 2b). Two fatty acids considered to be indicative of fungi, 18:1ω9c and 18:3ω6,9,12c (Nordby et al., 1981; Kourtev et al., 2003), also plotted along the left side of the first axis. In contrast, most saturated fatty acids (i.e. common in many organisms), branched fatty acids (i.e. Gram-positive organisms; O’Leary & Wilkinson, 1988), and 10Me branched fatty acids (i.e. actinomycetes; Kroppenstedt, 1985) plotted to the right along the first axis.
Figure 2.
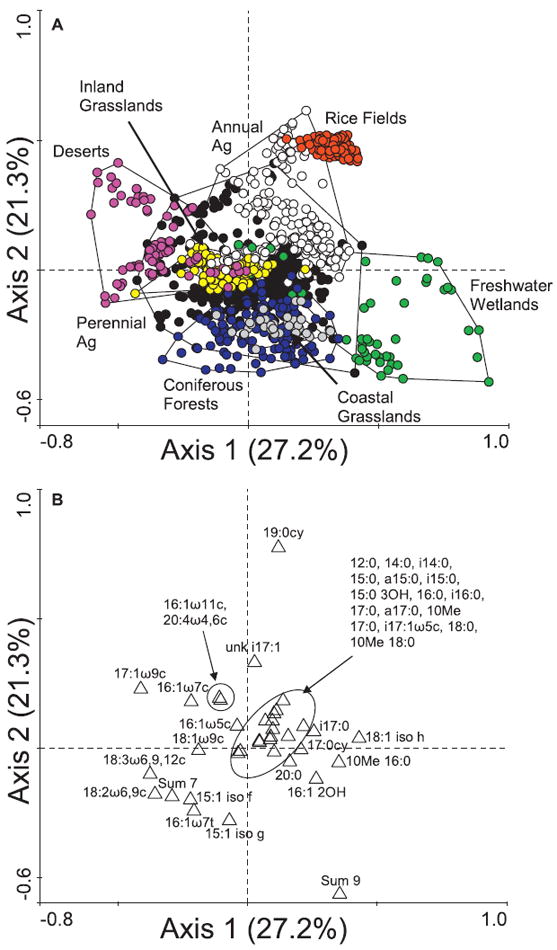
Ordination biplot of correspondence analysis (CA) results of all samples (a) and the 36 fatty acids included in the ordination (b) (n = 1117). Following ordination, ellipses were drawn around land-use types to aid in interpretation. Sum 7 indicates an unresolved peak potentially including the following fatty acids: 18:1ω7c; 18:1ω9t; and 18:1ω12t. Likewise, Sum 9 indicates an unresolved peak potentially including the following fatty acids: unknown 18.846 (a fatty acid approximately 18 carbons long); unknown 18.858 (a fatty acid approximately 18 carbons long); and 19:0cy ω10c. Key: deserts, pink; coastal grasslands, grey; inland grasslands, yellow; coniferous forests, blue; perennial agriculture, black; annual agriculture, white; rice fields, red; freshwater wetlands, green.
The second axis, which described 21.3% of the variation, reflects a disturbance gradient, with wildland areas tending to plot lower along this axis than agricultural areas (Fig. 2a). Perennial agriculture was positioned between annual agriculture and wildland areas. Fungal fatty acids were associated with samples from less disturbed areas (Fig. 2b). In contrast, most branched fatty acids plotted high along the second axis and were associated with more disturbed areas.
Axes 3 and 4 described an additional 12.5% and 6.6% of the variation in the data, respectively. Plotting the first three axes produced an ordination very similar to that of the first two axes (data not shown).
Inland grasslands were indistinguishable from one another (Fig. 2a), despite being sampled in two different biogeographical regions on two very different types of soil (serpentine versus non-serpentine). Coastal grasslands were more similar to coniferous forests than to inland grasslands. Although all desert samples plotted along the left side of the first axis, the two sampling locations were separated from one another along the second axis. The upper sample cluster included those from the Great Basin Desert, whereas the lower sample cluster included those from the Mojave Desert.
Rice field soils, despite being sampled throughout a single growing season, were very similar to one another, forming a tight cluster of points. Similarly, in those cases in which annual agriculture, perennial agriculture and grasslands had been sampled throughout a growing season or over multiple years, microbial communities showed higher fidelity to land-use type than to season or sampling year. Freshwater wetland soils were the most variable in composition, but this variation was associated with neither seasonal variation nor vegetation type.
Variation partitioning
Forward selection of the three groups of explanatory variables indicated that the microbial community was significantly related to the following variables (P < 0.002): (1) environment – soil texture (i.e. clay loam, loamy sand, sand, silty loam, loam, sandy loam, silty clay loam and gravelly loam), soil pH class (i.e. very strongly acid and slightly acid), soil type (i.e. aridisol, entisol, mollisol, vertisol and histosol), elevation, annual precipitation, climate zone, number of growing days, total PLFA and mesic soil temperature regime; (2) management – perennial agriculture, annual agriculture, irrigation, flooding, and tillage; and (3) location – spatial components of the cubic equation in the trend surface analysis (x, y, x3 and y3). Together, pure and joint effects of environment, management and spatial variables captured 66.7% of the total variation, respectively (Fig. 3). When this variation was decomposed, the largest fraction was accounted for by the environment (a + d + e + g: 60.9%), which included a strong overlap between environment and management (d + g: 29.4%). Without considering the variation explained by all three components (g), the joint effect of management and environment alone explained a greater percentage of the variation (d: 17.7%) than the remaining variance explained by spatial components (c + e + f: 7.7%) or management alone (b: 2.0%). This demonstrates that the microbial communities structured themselves largely in terms of environment followed by management practices but not with respect to any spatial components (Fig. 3).
Figure 3.
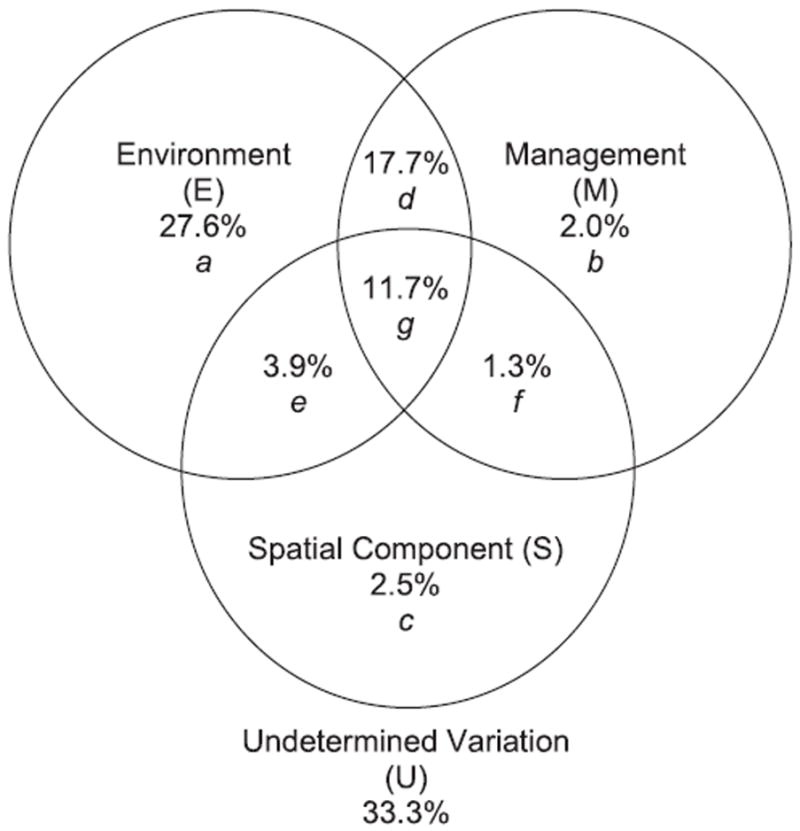
Variance partitioning of environmental, management and spatial components. Partitions are labelled a to g.
Relationships among soil properties, microbial communities and land use
As observed in the CA (see previous section ‘Soil microbial community variation among ecosystem and land-use types’), the CCA reflects similar associations among microbial communities according to land-use type, although these trends are represented by different axes in the CCA biplot (Fig. 4a,b). Soil water regime (i.e. flooded versus dryland or rain-fed sites), agricultural type (perennial versus annual agriculture) and the associated management practices (i.e. irrigation and tillage) were associated with the first axis, thus explaining the distribution of microbial communities along this axis. Soil type (i.e. vertisol versus histosol) was important in distinguishing differences between flooded soil microbial communities (i.e. rice soils versus freshwater wetland soils) along the second axis.
Figure 4.
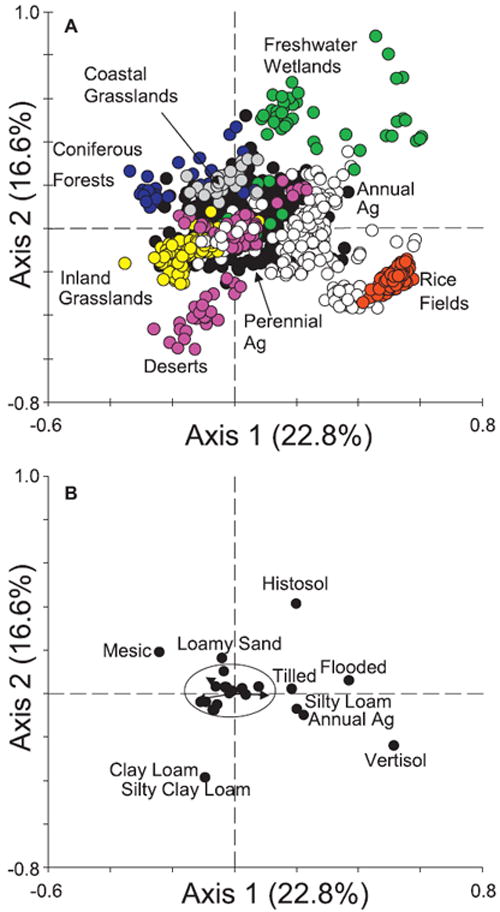
Canonical correspondence analysis (CCA) biplot of all samples (a) and the nominal and continuous descriptive characteristics (b), in which spatial components were run as covariates and environmental and management variables constrained the ordination (n = 1028). Only those descriptive characteristics deemed significant by the Monte Carlo permutation test were included in this ordination plot. Descriptive characteristics plotting within the ellipse near the origin include: very strongly acidic; slightly acidic; precipitation; elevation; perennial agriculture; total phospholipid fatty acid (PLFA); sand; loamy sand; gravelly loam; loam; mollisol; aridisol; climate zone; and growing days. Key: deserts, pink; coastal grasslands, grey; inland grasslands, yellow; coniferous forests, blue; perennial agriculture, black; annual agriculture, white; rice fields, red; freshwater wetlands, green.
Soil pH was not a major driver of microbial community composition. Most pH classes were not significant, and the two that were significant (slightly acidic soils and very strongly acidic soils) plotted near the origin, indicating they were not a major driver of community composition. Elevation also plotted near the origin and thus did not have a strong effect on structuring microbial communities.
Biomass and microbial group composition
Mean microbial biomass, based on total PLFA, varied 3.6-fold across land-use types (Fig. 5a). Total PLFA varied up to 166-fold (i.e. the highest total PLFA sample versus the lowest total PLFA sample). Lowest total PLFA was observed for one of the desert samples (c. 1.3 nmol g−1 soil DW), and highest total PLFA was observed for one of the almond orchard samples (c. 210.9 nmol g−1 soil DW). Grasslands (both inland and coastal) had higher microbial biomass than all other land-use types. Microbial biomass was lowest in rice fields and also tended to be low in freshwater wetland and desert soils. Coniferous forests, perennial agriculture and annual agriculture had intermediate values.
Figure 5.
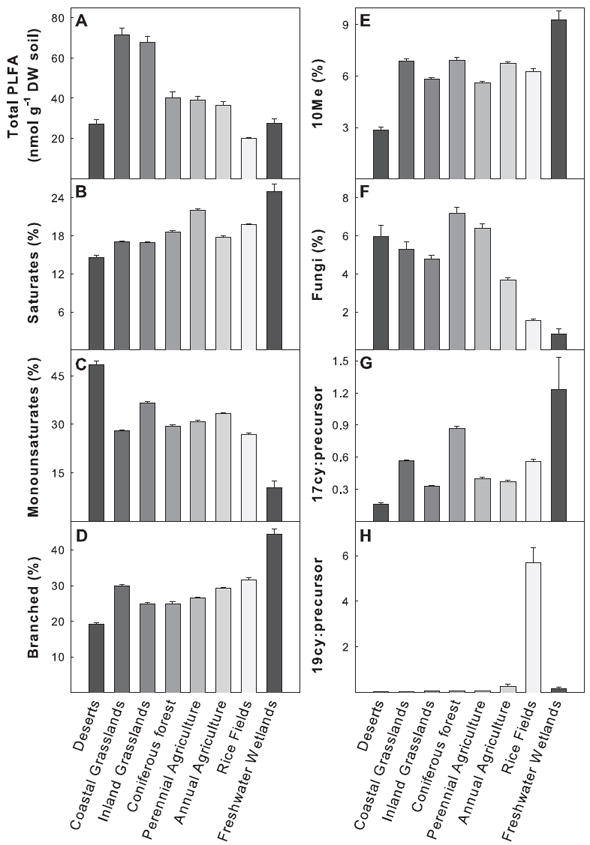
(a)–(h) Biomass (Total PLFA) and biomarker values for all land-use types. Biomarkers represent selected groups of microorganisms: Saturates, common in many bacteria; Monosaturates, Gram (−) bacteria; Branched, Gram (+) bacteria; 10Me, actinomycetes or sulfate-reducing bacteria (see Results). The ratios 17cy:precursor and 19cy:precursor indicate physiological stress. Note that the scaling of the axes is not the same in all panels. Data are means ± SE (n = 32–358).
Perennial agriculture and freshwater wetland soils had the highest proportions of saturated fatty acids, which are present in many microbial groups. Deserts, with the lowest proportion of saturated fatty acids, had 1.7-fold fewer saturated fatty acids than freshwater wetland samples (Fig. 5b). In contrast, deserts had the highest proportions of monounsaturated fatty acids (indicative of Gram-negative organisms); this proportion was 4.7-fold greater than that observed in freshwater wetlands, which had the lowest proportions of this biomarker (Fig. 5c). Branched fatty acids (indicative of Gram-positive organisms) displayed an opposite trend, with freshwater wetlands having the greatest proportions and deserts having the lowest (Fig. 5d). Overall, there was a 2.3-fold difference in the proportion of branched fatty acids across all land-use types. Freshwater wetland soils also had the highest proportions of 10Me fatty acids, having 3.3-fold greater proportions of 10Me branched fatty acids than deserts, which had the lowest proportions (Fig. 5e).
Biomarker interpretation, especially for 10Me branched fatty acids, can depend on environmental conditions. In well-drained soils, 10Me fatty acids are assumed to be associated with actinomycetes (Kroppenstedt, 1985). In contrast, in wet soils (e.g. rice fields and freshwater wetlands) 10Me fatty acids also are prevalent but in this case are dominated by 10Me16:0 which is primarily found in the sulphate-reducing Desulfobacter bacteria (Dowling et al., 1986). Overall, perennial agriculture and coniferous forest soils had the highest proportions of the fungal marker, 18:2ω6,9c, and rice fields and freshwater wetland soils had the lowest proportions of 18:2ω6,9c (Fig. 5f). Across land-use types, the proportion of fungal fatty acids varied 8.4-fold.
PLFA biomarkers can also be used to interpret levels of anaerobic and nutritional stress between sites or samples comparing the ratio of cyclopropyl branched fatty acid to its precursor (Kieft et al., 1997). The ratio of 17cy:precursor varied 7.7-fold across land-use types, with highest values detected in freshwater wetland soils and the lowest values observed in desert soils (Fig. 5g). Rice fields had very high values of 19cy:precursor; their values were 186-fold higher than in desert soils, which had the lowest 19cy:precursor ratios (Fig. 5h). Excluding rice field soils, 19cy:precursor varied 5.6-fold among the other seven land-use types.
DISCUSSION
Influence of land use, disturbance and resource gradients
Land-use type and level of disturbance are important factors for the composition and structure of microbial communities across the State of California. Multivariate analysis and variance partitioning show that PLFA fingerprints from irrigated and/or flooded sites are typically associated with agricultural management practices such as tillage. Grasslands, forests, deserts and perennial agriculture have microbial communities that are distinctly different from those in highly managed soils. Local environment and the joint effects of local environment and management have more influence on microbial communities than spatial location.
We suggest that the land-use gradient is driven to some extent by water availability. Determinants of water availability in our study included both natural and anthropogenic inputs: natural climatic variation in precipitation (e.g. deserts); irrigation (e.g. perennial and annual agriculture); and natural and managed flooding regimes (e.g. freshwater wetlands and rice fields). In the CA ordination, samples from the driest sites, the deserts, plotted furthest to the left along the first axis, and samples from flooded sites (rice fields and freshwater wetlands) plotted furthest to the right along the first axis (Fig. 2). As soils become saturated, oxygen availability decreases, changing soil redox potential and creating an environment favourable for facultative or obligate anaerobic bacteria. Microbial communities are resource-limited in dry soils, in part because of low availability of labile carbon (Sylvia et al., 2005). Decreased proportions of fungal and Gram-negative bacterial fatty acids and increased proportions of Gram-positive, sulphate-reducing, anaerobic and general bacterial fatty acids were associated with ecosystems with higher water availability. Similarly, in a landscape-level comparison of California salt marsh sediments, soil moisture saturation was the most important factor structuring microbial communities, with monounsaturated:saturated fatty acid ratios and proportions of fungal fatty acids decreasing as soil saturation increased (Córdova-Kreylos et al., 2006).
Increases in cyclopropyl:monoenoic fatty acid ratios have been associated with anaerobic conditions and nutrient stress (Kieft et al., 1997). Freshwater wetland and rice field microbial communities exhibited high ratios for cy17:precursor and cy19:precursor, respectively, supporting the idea that anaerobic conditions in these flooded environments created physiological stress for soil microorganisms. Furthermore, the different responses of these two markers among land-use types (e.g. cy17:precursor is high while cy19:precursor is low for freshwater wetland soils) suggest that the suite of conditions in each land-use type favours not only different compositions but also distinct physiological responses in the soil microbial community.
One common aspect of annual cropping systems is tillage, a physical disturbance that influences soil physical, chemical and biological properties. In the short term, tillage: breaks down water-stable aggregates, exposing previously protected soil organic matter (Calderón et al., 2000); leads to increased soil CO2 efflux, N mineralization and denitrification (Jackson et al., 2003); and causes changes in microbial community composition, probably because of increased environmental stress (Calderón et al., 2000, 2001). Fresh residues and fertilizer are often incorporated during tillage, providing labile substrates for soil microorganisms that are quickly utilized (Jackson et al., 2003). In the long-term, repeated tillage decreases soil organic matter, reduces N availability and disrupts soil structure (Lal, 2002). These changes in soil properties can have lasting effects on soil microbial communities. For example, microbial communities in historically tilled soils in Michigan were more similar to currently tilled soils than nearby wildland areas, even many years after tillage ceased (Buckley & Schmidt, 2003). These changes in the soil microbes’ physical environment related to management practices may partly explain why environment and management factors alone described 47.3% of the variation in the CCA ordination (see Fig. 3, a + b + d + g).
Most samples from wildland habitats (e.g. coniferous forests, coastal grasslands, inland grasslands and some desert soils) plotted separately from agricultural samples. Disturbance was also a major factor distinguishing grassland and agricultural soil microbial communities in the Central Coast of California (Steenwerth et al., 2003). Factors that were important in distinguishing microbial communities in intensively managed annual agricultural soils from grassland soils include herbicide application, tillage, irrigation, fertilization and increases in soil pH associated with liming (Steenwerth et al., 2003).
Overall, the ordination of desert soils was driven by soil water availability. However, within this land use, soils from the Great Basin Desert and Mojave Desert separated from one another along the second axis. There are multiple potential reasons for this differentiation, including differences in disturbance regimes, number of growing days, and above-ground plant communities. Unlike the Mojave Desert soils, we know that some of the Great Basin soils are regularly subjected to significant sand deposition (Brown, 1995), and this entire site is grazed on a semi-annual basis (R. E. Drenovsky, pers. comm.). However, further research is required to determine whether disturbance or other factors drive differences in the microbial community between these soils.
For freshwater wetland soils there were large variations in microbial communities. Some of these soils are drained every year and planted with crops, whereas others are permanently flooded and planted with native species (for a description of sites see Bossio et al., 2006). Unlike what was observed for other land-use types, the composition of the freshwater wetland samples from intensively managed agricultural areas were similar to communities in undisturbed wildland soils. It is unclear why the freshwater wetland soils were less influenced by disturbance regimes than other soils or why they were the most variable in composition, overall.
Disturbed soils (e.g. annual agriculture) tended to have higher proportions of Gram-positive biomarkers and lower proportions of fungal biomarkers. The ability of Gram-positive organisms to sporulate may allow them to withstand tillage or other anthropogenic disturbance. Conventionally tilled wheat fields in Washington had higher proportions of Gram-positive bacteria compared with paired no-till wheat fields (Kennedy & Schillinger, 2006). Similarly, Gram-positive bacteria were more closely associated with more intensively farmed agricultural fields in a survey of six North Carolina agroecosystems (Zhang et al., 2005). In contrast, fungi can be very sensitive to disturbance, and their abundance frequently decreases in response to tillage (Zhang et al., 2005; but see Calderón et al., 2000, 2001).
Effects of plant species in croplands
Annual agriculture samples clustered together despite the different crops that they represented (e.g. tomatoes, corn, cotton and broccoli, among others) and a similar pattern was observed in perennial agriculture samples (e.g. peaches, figs, walnuts, grapes and almonds). Given that these plants are grown in monocultures, a clear effect of dominant plant type on microbial community composition should be evident. This was not the case, however, as microbial community composition was more related to differences in land use (e.g. tillage and irrigation regimes) than plant type, despite large variations in soil texture, soil order and soil pH. Comparable results were observed in studies of Michigan agricultural fields, with similar microbial rRNA patterns observed in areas planted with different crops (Buckley & Schmidt, 2003). Likewise, catabolic response patterns of soil microbial communities sampled throughout New Zealand were more strongly influenced by disturbance regime (e.g. pasture, indigenous forest or plantation Pinus forest) than by geographical location, soil properties or plant species (Stevenson et al., 2004).
CONCLUSIONS
We demonstrate that, at this regional extent, environmental factors and management practices strongly influenced the composition of microbial communities. Factors commonly associated with the geographical distribution of macroorganisms (e.g. patterns in elevation, temperature class and precipitation) did not show a strong relationship with the distribution of microbial communities. Likewise, spatial components, which may serve as a proxy for underlying effects of precipitation and temperature for long gradients, did not influence microbial community composition. Instead, factors such as soil water availability and disturbance were far more important. Soil water availability appears to be a better indicator of microbial community composition than precipitation, as it incorporates both anthropogenic water inputs and precipitation. Taken together, these findings suggest that soil microbial communities were divorced from factors influencing geographical distributions of other organisms because of the inclusion of managed agricultural systems in this study. Surprisingly, seasonal and annual variation had little impact on structuring these microbial communities. Despite being sampled in different seasons and different years, microbial communities sampled on the same land-use type were relatively similar. With the increasing focus on how microbial community function varies across landscapes, regions and continents (Green et al., 2008), the next step is to elucidate linkages between microbial function and composition in order to better understand how distribution patterns are ultimately related to ecological processes.
Supplementary Material
Description of land-use types, their county of origin, as well as the biogeographical province and region of origin.
Description of land-use types, their county of origin, and associated environmental characteristics.
Acknowledgments
We thank K. Batten, K. Baumgartner, D. Bossio, R. Duncan, R. Heizen, E. Kelley, B. Roberts, B. Sanden and S. Strauss for contributed data; K. Graham and M. Edwards for technical assistance; E. Carlisle and C. Sheil for illustration of Fig. 1; and the many Scow Lab undergraduates involved in sample processing and analysis. The comments of three referees significantly improved the manuscript. Partial support was provided by grant number 5 P42 ES04699 from the National Institute of Environmental Health Sciences, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Biography
K.M.S. conceived the concept, R.E.D. compiled the dataset, R.E.D. and K.L.S. analysed the contributed data, R.E.D. and K.L.S. jointly led the writing, and L.E.J. and K.M.S. provided thoughtful discussion and critique.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Bååth E, Frostegård Å, Pennanen T, Fritze H. Microbial community structure and pH response in relation to soil organic matter quality in wood-ash fertilized, clear-cut or burned coniferous forest soils. Soil Biology and Biochemistry. 1995;27:229–240. [Google Scholar]
- Bardgett RD, Lovell RD, Hobbs PJ, Jarvis SC. Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biology and Biochemistry. 1999;31:1021–1030. [Google Scholar]
- Batten KM, Scow KM, Davies KF, Harrison SP. Two invasive plants alter soil microbial community composition in serpentine grasslands. Biological Invasions. 2006;8:217–230. [Google Scholar]
- Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. [Google Scholar]
- Bossio DA, Scow KM. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microbial Ecology. 1998;35:265–278. doi: 10.1007/s002489900082. [DOI] [PubMed] [Google Scholar]
- Bossio DA, Scow KM, Gunapala N, Graham KJ. Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microbial Ecology. 1998;36:1–12. doi: 10.1007/s002489900087. [DOI] [PubMed] [Google Scholar]
- Bossio DA, Fleck JA, Scow KM, Fujii R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biology and Biochemistry. 2006;38:1223–1233. [Google Scholar]
- Brown JF. PhD Dissertation. University of California; Davis: 1995. Sand movement and vegetation interactions at Mono Lake, CA. [Google Scholar]
- Buckley DH, Schmidt TM. Diversity and dynamics of microbial communities in soils from agro-ecosystems. Environmental Microbiology. 2003;5:441–452. doi: 10.1046/j.1462-2920.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Calderón FJ, Jackson LE, Scow KM, Rolston DE. Microbial responses to simulated tillage in cultivated and uncultivated soils. Soil Biology and Biochemistry. 2000;32:1547–1559. [Google Scholar]
- Calderón FJ, Jackson LE, Scow KM, Rolston DE. Short-term dynamics of nitrogen, microbial activity, and phospholipid fatty acids after tillage. Soil Science Society of America Journal. 2001;65:118–126. [Google Scholar]
- Córdova-Kreylos AL, Cao YP, Green PG, Hwang HM, Kuivila KM, LaMontagne MG, Van De Werfhorst LC, Holden PA, Scow KM. Diversity, composition, and geographical distribution of microbial communities in California salt marsh sediments. Applied and Environmental Microbiology. 2006;72:3357–3366. doi: 10.1128/AEM.72.5.3357-3366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT. The vegetation of Wisconsin: an ordination of plant communities. University of Wisconsin Press; Madison, WI: 1959. [Google Scholar]
- De Deyn G, Raaijmakers C, Zoomer H, Berg M, De Ruiter P, Verhoef H, Bezemer T, Van der Putten W. Soil invertebrate fauna enhances grassland succession and diversity. Nature. 2003;422:711–713. doi: 10.1038/nature01548. [DOI] [PubMed] [Google Scholar]
- Dowling NJE, Widdel F, White DC. Phospholipid ester-linked fatty acid biomarkers of acetate-oxidizing sulfate-reducers and other sulfide-forming bacteria. Journal of General Microbiology. 1986;132:1815–1826. [Google Scholar]
- Drenovsky RE, Elliott GN, Graham KJ, Scow KM. Comparison of phospholipid fatty acid (PLFA) and total soil fatty acid methyl esters (TSFAME) for characterizing soil microbial communities. Soil Biology and Biochemistry. 2004;36:1793–1800. [Google Scholar]
- Drenovsky RE, Duncan RA, Scow KM. Soil sterilization and organic carbon, but not microbial inoculants, change microbial communities in replanted peach orchards. California Agriculture. 2005a;59:176–181. [Google Scholar]
- Drenovsky RE, Edwards MP, Gardner LE, Scow KM, Maroney AL, Loveless LK. Comparison of soil microbial communities in Pinot noir vineyards in Anderson, Carneros, and Russian River Appellations. In: Christensen LP, Smart DR, editors. Proceedings of the Soil Environment and Vine Mineral Nutrition Symposium; Davis, CA: American Society of Enology and Viticulture; 2005b. pp. 39–47. [Google Scholar]
- Evans DG, Miller MH. The role of the external mycelial network in the effect of soil-disturbance upon the vesicular – arbuscular mycorrhizal colonization of maize. New Phytologist. 1990;114:65–71. doi: 10.1111/j.1469-8137.1990.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences USA. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay RH, Yeates C, Hullar MAJ, Stahl DA, Kaplan LA. Biome-level biogeography of streambed microbiota. Applied and Environmental Microbiology. 2008;74:3014–3021. doi: 10.1128/AEM.01809-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Bohannan BJM, Whitaker RJ. Microbial biogeography: from taxonomy to traits. Science. 2008;320:1039–1043. doi: 10.1126/science.1153475. [DOI] [PubMed] [Google Scholar]
- Heikkinen RK, Luoto M, Virkkala R, Rainio K. Effects of habitat cover, landscape structure and spatial variables on the abundance of birds in an agricultural-forest mosaic. Journal of Applied Ecology. 2004;41:824–835. [Google Scholar]
- Horner-Devine MC, Lage M, Hughes JB, Bohannan BJM. A taxa–area relationship for bacteria. Nature. 2004;432:750–753. doi: 10.1038/nature03073. [DOI] [PubMed] [Google Scholar]
- Hickman JC, editor. The Jepson manual: higher plants of California. University of California Press; Berkeley, CA: 1993. [Google Scholar]
- Jackson LE, Calderón FJ, Steenwerth KL, Scow KM, Rolston DE. Responses of soil microbial processes and community structure to tillage events and implications for soil quality. Geoderma. 2003;114:305–317. [Google Scholar]
- Kelley E. PhD Dissertation. University of California; Davis: 2003. Differences in vegetation structure, species composition, and soil microbial communities among forests of different successional ages and types in the Lake Tahoe Basin. [Google Scholar]
- Kennedy AC, Schillinger WF. Soil quality and water intake in traditional-till vs. no-till paired farms in Washington’s Palouse region. Soil Science Society of America Journal. 2006;70:940–949. [Google Scholar]
- Kieft TL, Wilch E, O’Connor K, Ringelberg DB, White DC. Survival and phospholipid fatty acid profiles of surface and subsurface bacteria in natural sediment microcosms. Applied and Environmental Microbiology. 1997;63:1531–1542. doi: 10.1128/aem.63.4.1531-1542.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtev PS, Ehrenfeld JG, Häggblom M. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry. 2003;35:895–905. [Google Scholar]
- Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proceedings of the National Academy of Sciences USA. 2007;104:5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroppenstedt RM. Fatty acid and menaquinone analysis of actinomycetes and related organisms. In: Goodfellow M, Minnikin DE, editors. Bacterial systematics. Academic Press; London: 1985. pp. 173–199. [Google Scholar]
- Lal R. Soil carbon dynamics in cropland and rangeland. Environmental Pollution. 2002;116:353–362. doi: 10.1016/s0269-7491(01)00211-1. [DOI] [PubMed] [Google Scholar]
- Legendre P. Quantitative methods and biogeographic analysis. In: Garbary DJ, South GR, editors. Evolutionary biogeography of the marine algae of the North Atlantic. Springer-Verlag; New York: 1990. pp. 9–34. [Google Scholar]
- Lepš J, Šmilauer P. Multivariate analysis of ecological data using canoco. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- McCulley RL, Burke IC. Microbial community composition across the Great Plains: landscape versus regional variability. Soil Science Society of America Journal. 2004;68:106–115. [Google Scholar]
- Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL, Horner-Devine MC, Kane M, Krumins JA, Kuske CR, Morin PJ, Naeem S, Øvreås L, Reysenbach AL, Smith VH, Staley JT. Microbial biogeography: putting microorganisms on the map. Nature Reviews Microbiology. 2006;4:102–112. doi: 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- Nordby HE, Nemec S, Nagy S. Fatty acids and sterols associated with citrus root mycorrhizae. Journal of Agricultural and Food Chemistry. 1981;29:396–401. [Google Scholar]
- O’Brien EM. Water–energy dynamics, climate, and prediction of woody plant species richness: an interim general model. Journal of Biogeography. 1998;25:379–398. [Google Scholar]
- O’Leary WM, Wilkinson SG. Gram-positive bacteria. In: Ratledge C, Wilkinson SG, editors. Microbial lipids. Academic Press; London: 1988. pp. 117–201. [Google Scholar]
- Petersen SO, Debosz K, Schjonning P, Christensen BT, Elmholt S. Phospholipid fatty acid profiles and C availability in wet-stable macro-aggregates from conventionally and organically farmed soils. Geoderma. 1997;78:181–196. [Google Scholar]
- Pinkart HC, Ringelberg DB, Piceno YM, Macnaughton SJ, White DC. Biochemical approaches to biomass measurements and community structure analysis. In: Hurst CJ, Crawford RL, Knudsen GR, McInerney MJ, Stetzenbach LD, editors. Manual of environmental microbiology. 2. American Society of Microbiology Press; Washington, DC: 2002. pp. 101–113. [Google Scholar]
- Ramsey PW, Rillig MC, Feris KP, Holben WE, Gannon JE. Choice of methods for soil microbial community analysis: PLFA maximizes power compared to CLPP and PCR-based approaches. Pedobiologia. 2006;50:275–280. [Google Scholar]
- Roberts BA. PhD Dissertation. University of California; Davis: 2005. The role of soil organic matter in a cotton-based cropping system. [Google Scholar]
- Soil Survey Division. USDA Soil Survey Division official soil series descriptions. USDA Natural Resources Conservation Service; Washington, DC: 2006. [24 July 2009]. Available at: http://soils.usda.gov/technical/classification/osd/index.html. [Google Scholar]
- Steenwerth KL, Jackson LE, Calderón FJ, Stromber MR, Scow KM. Soil microbial community composition and land use history in cultivated and grassland ecosystems of coastal California. Soil Biology and Biochemistry. 2003;35:489–500. [Google Scholar]
- Steenwerth KL, Jackson LE, Carlisle EA, Scow KM. Microbial communities of a native perennial bunchgrass do not respond consistently across a gradient of land-use intensification. Soil Biology and Biochemistry. 2006;38:1797–1811. [Google Scholar]
- Steinberger Y, Zelles L, Bai QY, von Lutzow M, Munch JC. Phospholipid fatty acid profiles as indicators for the microbial community structure in soils along a climatic transect in the Judean Desert. Biology and Fertility of Soils. 1999;28:292–300. [Google Scholar]
- Stevenson BA, Sparling GP, Schipper LA, Degens BP, Duncan LC. Pasture and forest soil microbial communities show distinct patterns in their catabolic respiration responses at a landscape scale. Soil Biology and Biochemistry. 2004;36:49–55. [Google Scholar]
- Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA, editors. Principles and applications of soil microbiology. 2. Pearson Prentice Hall; Upper Saddle River, NJ: 2005. [Google Scholar]
- Vetaas OR, Ferrer-Castán D. Patterns of woody plant species richness in the Iberian Peninsula: environmental range and spatial scale. Journal of Biogeography. 2008;35:1863–1878. [Google Scholar]
- Wardle DA. The influence of biotic interactions on soil biodiversity. Ecology Letters. 2006;9:870–886. doi: 10.1111/j.1461-0248.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- White DC, Bobbie RJ, Nickels JS, Fazio SD, Davis WM. Nonselective biochemical methods for the determination of fungal mass and community structure in estuarine detrital microflora. Botanica Marina. 1980;23:239–250. [Google Scholar]
- Whittaker RH. Vegetation of the Great Smoky Mountains. Ecological Monographs. 1956;23:41–78. [Google Scholar]
- Whittaker RJ, Willis KJ, Field R. Scale and species richness: towards a general, hierarchical theory of species diversity. Journal of Biogeography. 2001;28:453–470. [Google Scholar]
- Wilkinson SG. Gram-negative bacteria. In: Ratledge C, Wilkinson SG, editors. Microbial lipids. Academic Press; London: 1988. pp. 299–488. [Google Scholar]
- Zhang WJ, Rui WY, Tu C, Diab HG, Louws FJ, Mueller JP, Creamer N, Bell M, Wagger MG, Hu S. Responses of soil microbial community structure and diversity to agricultural deintensification. Pedosphere. 2005;15:440–447. [Google Scholar]
- Zogg GP, Zak DR, Ringelberg DB, Macdonald NW, Pregitzer KS, White DC. Compositional and functional shifts in microbial communities because of soil warming. Soil Science Society of America Journal. 1997;61:475–481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of land-use types, their county of origin, as well as the biogeographical province and region of origin.
Description of land-use types, their county of origin, and associated environmental characteristics.


