Abstract
Obstructive sleep apnea (OSA) is a condition characterized by upper airway muscle atonia with continued diaphragmatic efforts, resulting in repeated airway obstructions, periods of intermittent hypoxia, large thoracic pressure changes, and substantial shifts in arterial pressure with breathing cessation and resumption. The hypoxic exposure and hemodynamic changes likely induce the structural and functional deficits found in multiple brain areas, as shown by magnetic resonance imaging (MRI) procedures. Altered cerebral blood flow (CBF) may contribute to these localized deficits; thus, we examined regional CBF, using arterial spin labeling procedures, in 11 OSA (age, 49.1±12.2 years; 7 male) and 16 control subjects (42.3±10.2 years; 6 male) with a 3.0-Tesla MRI scanner. CBF maps were calculated, normalized to a common space, and regional CBF values across the brain quantified. Lowered CBF values emerged near multiple bilateral brain sites in OSA, including the corticospinal tracts, superior cerebellar peduncles, and pontocerebellar fibers. Lateralized, decreased CBF appeared near the left inferior cerebellar peduncles, left tapetum, left dorsal fornix/stria terminalis, right medial lemniscus, right red nucleus, right midbrain, and midline pons. Regional CBF values in OSA are significantly reduced in major sensory and motor fiber systems and motor regulatory sites, especially in structures mediating motor coordination; those reductions are often lateralized. The asymmetric CBF declines in motor regulatory areas may contribute to loss of coordination between upper airway and diaphragmatic musculature, and lead to further damage in the syndrome.
Keywords: Arterial spin labeling, Cerebral hemodynamics, Motor coordination, Sensory control, Hypoxemia
Introduction
Obstructive sleep apnea (OSA) is defined by interrupted breathing during sleep from repeated upper airway obstructions while diaphragmatic efforts continue, resulting in intermittent hypoxemia, repetitive arousals, and disruption of normal sleep architecture [10]. A principal OSA characteristic is a loss of coordination of the breathing musculature, with atonia of genioglossal and other upper airway muscles in the presence of active diaphragmatic movements.
Routine magnetic resonance imaging (MRI) in OSA, upon visual examination, shows no gross brain pathology, except white matter infarcts or cerebellar changes [9]. However, voxel-based morphometry and manual volumetric procedures using high-resolution T1-weighted images, T2-relaxometry, and diffusion tensor imaging-based indices, show gray and white matter changes in autonomic, breathing, mood and anxiety, and cognitive control sites [19, 20, 23]. Functional deficits within structurally-damaged areas, based on functional MRI procedures, also appear in OSA during autonomic and respiratory challenges in regions that control autonomic, breathing, and cognitive functions [15]. The processes contributing to brain injury and associated altered brain function are unknown, but may include aberrant local perfusion changes, induced by frequent hypoxic episodes, blood pressure surges, failure of cerebral autoregulation, or endothelial changes induced by epigenetic processes[ 17].
Reduced cerebral blood flow (CBF) appears in OSA, based on imaging procedures with relatively limited resolution. Decreased CBF values emerge in various brain areas of awake OSA subjects assessed by transcranial Doppler [34], and single photon emission computed tomography procedures [12], and during sleep, as well as wakefulness states, as evaluated by tracer-based computerized tomography [25]. However, precise assessment of localized CBF changes in areas damaged in OSA is lacking.
Arterial spin labeling (ASL) procedures are non-invasive MRI-based methods that quantify regional CBF values with greater sensitivity and specificity than Doppler techniques [4, 14, 32], and provide whole brain CBF values more rapidly than Doppler procedures. ASL-based CBF measurements take advantage of arterial water as a freely diffusible tracer, and do not require use of contrast agents [11]. The procedure has been used to quantify regional CBF changes in different disease conditions [3, 11], and to monitor disease progression [3], and treatment effects. The technique may assist precise examination of regional CBF changes in OSA subjects.
Our aim was to assess localized CBF changes in OSA over control subjects using non-invasive ASL procedures. We hypothesized that regional CBF values would be altered in OSA, in brain sites that showed structural injury earlier in the condition.
Material and Methods
Subjects
We studied 11 OSA (age, 49.1±12.2 years; body-mass-index, 24.1±3.7 kg/m2; apnea-hypopnea-index, 32.9±16.6 events/hour; education, 18.5±2.4 years; 7 male) and 16 control subjects (age, 42.3±10.2 years; body-mass-index, 28.3±4.0 kg/m2; education, 16.9±4.4 years; 6 male). No participants were taking cardiovascular-altering medications, such as β-blockers, α-agonists, angiotensin-converting enzyme inhibitors, or vasodilators, or any mood changing drugs, such as selective serotonin reuptake inhibitors. Subjects with any history of heart failure, stroke, diagnosed cerebral conditions, metallic implants, or body weight more than 125 kg (scanner limitation) were excluded. Control subjects were healthy and without any medications that might alter brain structure or hemodynamics, and with no contraindications to the MRI scanner. All procedures were approved by the Institutional Review Board at University of California at Los Angeles, and each subject provided written informed consent prior to study.
Magnetic Resonance Imaging
Brain studies were performed on a 3.0-Tesla MRI scanner (Siemens, Magnetom, Tim-Trio, Erlangen, Germany). Subjects lay supine, awake, and breathed normally during scans. High-resolution T1-weighted images were collected using the magnetization-prepared-rapid-acquisition-gradient-echo pulse sequence [repetition time (TR)=2200 ms, echo-time (TE)=2.34 ms, inversion time=900 ms, flip-angle (FA)=9°, 320×320 matrix size, 230×230 mm field of view (FOV), 0.9 mm slice thickness, and 192 sagittal slices]. Proton-density and T2-weighted images were collected using a dual-echo turbo spin-echo pulse sequence in the axial plane (TR=10,000 ms, TE1,2=17, 134 ms, FA=130°, 256×256 matrix size, 230×230 mm FOV, 3.0 mm slice thickness, and ~56 slices). ASL imaging was performed using a pseudocontinuous ASL pulse sequence in the axial plane (TR=4000 ms, TE=11 ms, FA=90°, bandwidth=3004 Hz/pixel, label offset=90 mm, label-delay=1200 ms, 64×64 matrix size, 230×230 mm FOV, 3.5 mm slice thickness, 20% distance factor, 38 axial slices, and 100 repeats).
Data Processing and Analysis
T1-weighted, proton-density, and T2-weighted images were visually-assessed for any gross brain pathology, including infarcts, cystic lesions, and tumors, before data processing. ASL data were also examined for any potential motion or imaging artifacts.
Calculation of CBF maps
Using MATLAB-based custom software, whole-brain CBF maps were calculated. Labeled and non-labeled ASL brain volumes were realigned to remove any potential head-motion related artifacts; both non-labeled and labeled images were aligned to the first image. Using the realigned echo-planner-imaging (EPI) scans, perfusion images were calculated with simple subtraction from non-labeled to labeled images. Perfusion images were used to calculate CBF maps (units, ml/100g/min), based on a modified single-compartment ASL perfusion model [35]. The non-labeled EPI scans and CBF maps were averaged across the series to derive mean EPI scan and CBF map per individual subject.
Normalization of CBF maps and regional analyses
SPM8 software (www.fil.ion.ucl.ac.uk/spm) was used to strip skulls from the individual CBF maps using the corresponding EPI images. Subsequent normalization of CBF maps to a common space was performed using linear affine and non-linear transformation procedures (www.MriStudio.org). Both EPI images and CBF maps were first normalized to the JHU_MNI_SS_b0 “Eve” atlas, a single-subject template in the Montreal Neurological Institute (MNI) common space, using a linear affine transformation with trilinear interpolation. After linear transformation of EPI images and CBF maps to the MNI common space, a single-channel large deformation diffeomorphic metric mapping transformation approach was used for non-linear transformation to the MNI space. Non-linear transformed matrices were obtained with cascading alpha values, and then parameters from transformation matrices were applied to both mean EPI images and CBF maps [29]. The resulting transformed CBF maps were used to automatically quantify mean regional CBF values from whole-brain structures using region of interest (ROI) editor software.
Statistical analysis
The IBM statistical package for the social sciences (IBM SPSS Statistics 20) was used to assess demographics and regional CBF values. Demographic data were examined with independent samples t-tests and Chi-square. Regional CBF values were assessed with independent samples t-tests (statistical threshold of p≤0.05).
Results
Demographics
No significant differences were observed between groups in age (p=0.13), gender (p=0.18), or education (p=0.38). However, body-mass-indices were significantly increased in OSA over controls (p=0.01).
Regional CBF Changes in OSA
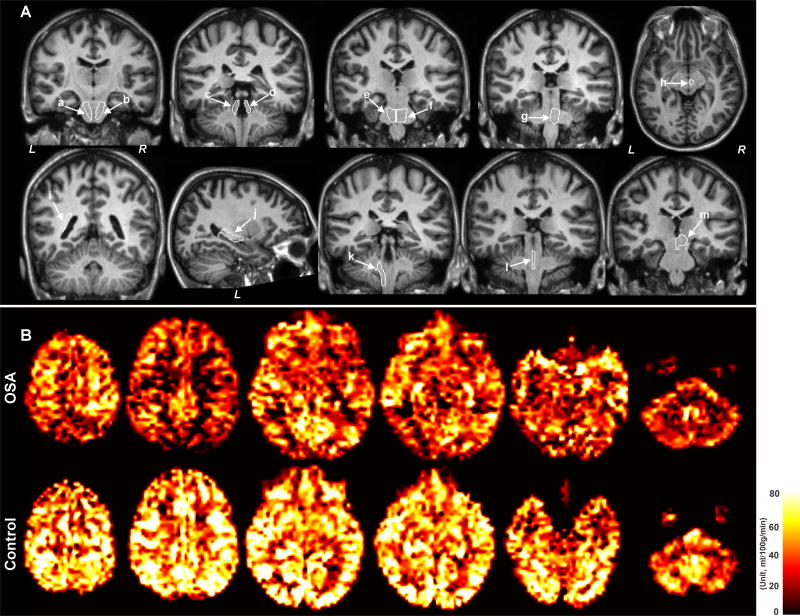
Significantly reduced regional CBF values appeared near multiple brain areas in OSA compared to control subjects (Table 1, Fig. 1A), but no sites showed significantly increased localized values in OSA over controls. Examples of whole-brain CBF maps from an OSA and a control subject are shown in Fig. 1B. Reduced CBF values in OSA appeared near the bilateral corticospinal tracts (right, p=0.009; left, p=0.04), superior cerebellar peduncles (right, p=0.02; left, p=0.03), and pontocerebellar fibers (right, p=0.01; left, p=0.05). However, unilateral reductions in CBF of OSA subjects appeared in the medial lemniscus (right, p=0.02), red nucleus (right, p=0.012), tapetum (left, p=0.04), dorsal fornix/stria terminalis (left, p=0.02), inferior cerebellar peduncle (left, p=0.02), and midbrain (right, p=0.05), compared to control subjects (Fig. 1A); reduced flow also appeared in the midline pons (p= 0.01)
Table 1.
Average CBF values (ml/100g/min) from different brain regions in OSA and control subjects.
| Brain Regions | OSA (n = 11) (Mean ± SD) | Controls (n = 16) (Mean ± SD) | P values |
|---|---|---|---|
| Right insula | 51.7±7.0 | 53.9±10.1 | 0.581 |
| Left insula | 48.3±10.7 | 55.9±10.2 | 0.074 |
| Right cerebellum | 39.9±12.8 | 47.6±8.5 | 0.076 |
| Left cerebellum | 38.9±12.6 | 46.5±8.7 | 0.075 |
| * Right corticospinal tract | 34.6±8.5 | 46.9±12.5 | 0.009 |
| * Left corticospinal tract | 35.1±9.9 | 44.4±11.2 | 0.038 |
| Right inferior cerebellar peduncle | 33.3±10.2 | 39.7±12.9 | 0.181 |
| * Left inferior cerebellar peduncle | 28.8±9.4 | 39.3±11.8 | 0.023 |
| * Right superior cerebellar peduncle | 32.2±10.3 | 44.8±13.6 | 0.016 |
| * Left superior cerebellar peduncle | 35.9±10.4 | 45.9±11.7 | 0.031 |
| * Right medial lemniscus | 36.9±8.1 | 50.5±16.2 | 0.017 |
| Left medial lemniscus | 37.8±10.6 | 47.2±16.2 | 0.100 |
| Right cingulum, cingulate gyrus | 30.9±2.7 | 34.5±6.2 | 0.056 |
| Left cingulum, cingulate gyrus | 28.8±4.01 | 29.9±5.8 | 0.574 |
| Right dorsal fornix/stria terminalis | 40.7±12.3 | 40.6±7.8 | 0.990 |
| * Left dorsal fornix/stria terminalis | 36.6±10.9 | 45.8±7.7 | 0.017 |
| * Right pontocerebellar fibers | 38.4±10.6 | 54.4±17 | 0.011 |
| * Left pontocerebellar fibers | 37.4±11.3 | 49.0±16 | 0.049 |
| * Right red nucleus | 36.6±11.7 | 52.3±16.2 | 0.012 |
| Left red nucleus | 35.3±15.6 | 46.5±15.5 | 0.077 |
| Right tapetum | 16.8±12 | 20.1±9.2 | 0.435 |
| * Left tapetum | 13.7±6.1 | 20.4±9.2 | 0.045 |
| * Right midbrain | 36.6±8.9 | 44.0±9.3 | 0.050 |
| Left midbrain | 36.9±9.2 | 43.8±9.0 | 0.064 |
| * Midline pons | 40.6±9.0 | 54.5±15.2 | 0.012 |
| Left fronto-orbital gyrus | 35.2±10.7 | 37.1±8.9 | 0.085 |
| Right fronto-orbital gyrus | 50.3±14.2 | 53.1±8.5 | 0.523 |
CBF, Cerebral blood flow; OSA, Obstructive sleep apnea; SD, Standard deviation;
significant at p≤0.05.
Fig. 1.
(A) Regions of interest (ROIs) from different brain sites, overlaid onto T1-weighted images that showed significant bilateral and unilateral reduction of CBF in OSA. The bilateral areas included the corticospinal tracts (coronal; a, b), superior cerebellar peduncles (coronal; c, d), pontocerebellar fibers (coronal; e, f), and regions of unilateral CBF reduction, which included the right medial lemniscus (coronal; g), right red nucleus (axial; h), left tapetum (coronal; i), left dorsal fornix/stria terminalis (sagittal; j), left inferior cerebellar peduncle (coronal; k), midline pons (coronal; l), and right midbrain (coronal; m). All brain images are displayed in neurological convention, with the left side of brain represented on the left side of the image. (L = Left, R = right). (B) Examples of whole-brain CBF maps in an OSA (age, 55.7 years; male), and a control subject (age, 54.9 years; male). Warm colors indicate corresponding regional CBF values.
Discussion
Overview
Significantly reduced regional CBF values appeared in the vasculature for multiple brain regions in OSA subjects, and many of these sites, including the red nucleus, corticospinal tracts, midline pons, and medial lemniscus, assist coordination of respiratory musculature, in addition to roles in other motoric functions. The cerebellar peduncles and pontocerebellar fibers similarly participate in motor coordination, as well as autonomic regulation; the tapetum and fornix fibers serve significant roles in memory and cognitive functions, and the stria terminalis is involved in stress and anxiety control.
The processes underlying the altered regional CBF values to those sites in OSA are unclear. However, the asymmetric nature of the altered flow, and the preferentially-reduced CBF to motor regulatory and coordination areas, suggest that the reduced flow may contribute to the pathological respiratory motor coordination found in OSA, manifested as atonia of upper airway musculature occurring at times when activation prior to diaphragmatic muscle onset is required. Inadequate local perfusion would lead to failed or inadequate performance of sensory and motor coordination structures, here represented by the red nucleus, cerebellar pathway, corticospinal tracts, and medial lemniscus. The deficits could contribute to failed coordination of respiratory musculature.
OSA and cerebral hemodynamics
OSA subjects show profound overall CBF changes during apneas [12, 25, 34], and apnea-induced hypoxemia, combined with decreased cerebral perfusion, may lead to chronically altered CBF in OSA [2]. Resting arterial CBF is reduced in OSA, assessed by transcranial Doppler. We used advanced MRI-based ASL procedures that quantify regional CBF values with greater sensitivity and specificity than Doppler procedures [32]. Typically, Doppler procedures can provide 85% accuracy [14], while ASL techniques can provide up to 100% [4], relative to invasive radioactive tracer procedures. Also, Doppler procedures are operator-dependent, allow limited evaluation of only some brain vessels, e.g., middle cerebral arteries, are relatively low-resolution, and are very time-consuming over ASL techniques.
Other imaging procedures, including tracer-based computerized tomography, show decreased CBF in OSA in brainstem and cerebellar regions during wakefulness [25]. During sleep, OSA subjects exhibit decreased CBF in frontal, parietal, and occipital cortices, pons, and the cerebellum, based on single photon emission computed tomography procedures [12, 25]. We extend these findings by demonstrating more-localized CBF changes in OSA.
CBF reduction and brain tissue changes
Animal models which examine the relationship between CBF reduction and brain tissue injury show that a 25 ml/100g/min CBF reduction leads to tissue infarcts in those areas [26]. In this study, we observed regional CBF reduction in several brain areas that ranged from 7–16 ml/100g/min in resting conditions in OSA subjects. However, CBF reduction would be larger for those brain sites during sleep with apneic episodes, and of course, apneic periods occur hundreds of times during a night. We believe that lower CBF values in resting conditions, enhanced CBF reduction during apneic episodes, and multiple exposures to apnea would significantly contribute to tissue injury in OSA. Structural injury and functional deficits are now well-documented for various brain regions in OSA, and many of the brain areas with reduced regional CBF values found here overlap areas of injury. Since decreased flow will lead to brain injury, contributing to symptoms in the syndrome, interventions to address reduced CBF may be a useful target in OSA.
Altered brain functions and CBF changes
Coordination of the upper airway muscles with diaphragmatic activation is of special importance in breathing; effective airflow depends on activation of the genioglossal fibers of the tongue slightly before diaphragmatic descent to prevent airway collapse from excessive negative pressure. In OSA, that coordination is largely lost, with atonia of the upper airway muscles, followed by airway collapse. Atonia appears not to result from damage to the XII motor nucleus pool, since obstruction does not appear in wakefulness, and apparently reflects a sleep state influence on coordination between brainstem motor nuclei and the phrenic motor pool.
Coordination pathways and nuclei were especially affected in regional CBF declines. The right red nucleus, which projects contralaterally to the left cerebellum, and provides coordination for much of the automatic motor musculature, was affected, as were contralateral cerebellar projecting fibers. The corticospinal motor fibers were also impacted. In addition, areas within the midline pons, including the periaqueductal gray modulate respiratory and cardiovascular activity, demonstrated by single neuron discharge [27, 28], and electrical stimulation evidence [5]. The medial lemniscus, the major somatosensory pathway from body structures, plays a significant role in motor coordination, and was affected [16]. The tapetum, forming part of the splenium of the corpus callosum, carries information for cognitive, and memory processes [22]. The hippocampus projects to the mammillary bodies and diencephalic structures through the fornix. The mammillary bodies show substantial volume loss, especially on the left side in OSA [19]; the reduced CBF to the dorsal left fornix may have contributed to that injury. The fornix fibers play a significant role in memory processes, by bridging hippocampal and mammillary structures [13], and the stria terminalis serves an important role in anxiety and stress [7]; all of these processes are deficient in OSA. Collectively, these pathways serve significant roles for upper airway motor coordination, memory and cognitive functions [1, 19].
Factors contributing to reduced CBF
Several mechanisms may contribute to the localized reduction in CBF values in OSA. The condition damages the insular, cingulate, and medial frontal cortices, as well as the raphe and ventrolateral medulla [20, 23], all essential brain areas for vascular regulation. The right insular, cingulate and medial frontal cortices exert significant influences on the sympathetic system [6], the raphe modulates vascular diameter [8], and the ventrolateral medulla is a final integrative site for sympathetic outflow [18]. Damage to these vascular regulatory sites may have contributed to the regional CBF deficits. The consequences of intermittent hypoxia to peripheral vascular regulation are especially apparent in developing animals, where injury to peripheral sympathetic ganglia appears [38].
Intermittent hypoxia affects both neural tissue and the vasculature, and the substantial hemodynamic alterations during each apneic event, shifting systolic and diastolic arterial pressure up to 240/130 mmHg [31], can also contribute to vascular injury. Cerebellar tissue changes appear in animal models of OSA with as few as 5 hours of 10% O2 intermittent hypoxic exposure [30]. The cerebellum exerts significant influences on blood pressure regulation, shows substantial injury in OSA [23], and may contribute to the flawed vascular regulation here.
Normally, PECO2 exerts a significant impact on the vasculature; vessels constrict and dilate in response to changes in PECO2 concentration [37]. The potential blunting of vasodilation to PECO2 in OSA is one more factor that may alter CBF. That consideration is made more complex by the injury to medullary and raphe systems in OSA, with raphe serotonergic neurons especially playing a significant role in mediation of CO2 effects on the vasculature [36]. We earlier showed in a human model of CO2 dysregulation, congenital central hypoventilation syndrome, that the vasculature was severely affected, particularly in the basilar arteries near the damaged raphe in those patients [21]. The medullary injury we previously reported for OSA may also interfere with CO2 dilatory effects [20], although that possibility is entirely speculative.
Intermittent hypoxic exposure also affects endothelial cells, resulting in abnormal vascular activity [33]. Hypoxic exposure retracts the lateral junctions between adjacent endothelial cells through reduction in adenyl cyclase activity, leading to increased space between cells [7], affecting the barrier properties of the endothelium. Such alterations in the microvasculature can result in compromised hemodynamic activity, leading to altered regional CBF.
Limitations
Limitations of this study include the small sample size, potential variability of regional CBF values in OSA between genders, restricted spatial resolution, and normalization accuracy of post processing. Although various brain sites demonstrated significant group differences between OSA and control subjects, showing sufficient power in those areas, the limited number of subjects may have restricted the power to show group differences in rostral brain sites that exhibit structural brain injury in OSA subjects [19, 20, 23]. That aspect may be especially the case for the left insula and right cingulate cortex, which show substantial injury in OSA [20, 23], but only showed a trend of lowered CBF here (p=0.07, p<0.056, respectively). Structural brain injury differences appear between genders in OSA subjects [24], but the small number of OSA subjects here restricted the ability to partition potential CBF differences induced by sex. The spatial resolution was limited due to technical limitations, and CBF values from smaller regional sites could have been contaminated from surrounding structures. The normalization process may introduce some inaccuracies, but CBF maps of all OSA and control subjects were visually inspected to verify only modest variation across subjects.
Conclusions
Regional CBF values were lower in unilateral and some bilateral brain sites in OSA, including major sensory and motor fiber systems and motor regulatory regions; the declines were especially apparent in fiber and nuclear systems responsible for motor coordination. The asymmetric nature of the reduced CBF in selected motor areas, affecting the right, but not left red nucleus, and the left cerebellar peduncles, has the potential to contribute to impaired coordination of the respiratory musculature. The origins of the altered regional CBF values remain unclear, but may result from early injury of vascular regulatory areas in OSA from intermittent hypoxia, or from the substantial hemodynamic changes that accompany the large thoracic pressure alterations during apneic periods. By failing to provide adequate CBF to these affected areas, the potential for even further brain tissue damage exists. Moreover, since coordination by cerebellar and other motor areas is essential for maintaining timely activation of the upper airway musculature relative to diaphragmatic discharge, reduction in CBF of those structures may worsen apnea characteristics.
Highlights.
Regional cerebral blood flow (CBF) was examined in obstructive sleep apnea.
Values were significantly reduced, principally in major sensory and motor fiber systems.
Reduced CBF may contribute to upper airway and diaphragm coordination loss.
The reduced regional flow may lead to further neural damage in the syndrome.
Acknowledgments
Authors thank Ms. Rebecca Harper and Dr. Jenifer Ogren for assistance with data collection. This research was supported by the National Institutes of Health R01 HL113251 and R01 NR013693. HLR was supported by NHMRC CJ Martin Fellowship 606770.
Abbreviations
- OSA
Obstructive sleep apnea
- CBF
cerebral blood flow
- ASL
arterial spin labeling
- MRI
magnetic resonance imaging
- TR
repetition time
- TE
echo-time
- FOV
field of view
- FA
flip-angle
- EPI
echo-planner-imaging
Footnotes
Conflict of interest:
All authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aminoff MJ, Sears TA. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971;215:557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Am J Respir Crit Care Med. 1994;150:1587–1591. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- 3.Binnewijzend MA, Kuijer JP, Benedictus MR, van der Flier WM, Wink AM, Wattjes MP, van Berckel BN, Scheltens P, Barkhof F. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology. 2013;267:221–230. doi: 10.1148/radiol.12120928. [DOI] [PubMed] [Google Scholar]
- 4.Brown GG, Clark C, Liu TT. Measurement of cerebral perfusion with arterial spin labeling: Part 2. Applications. J Int Neuropsychol Soc. 2007;13:526–538. doi: 10.1017/S1355617707070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrive P, Bandler R, Dampney RA. Anatomical evidence that hypertension associated with the defence reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Res. 1988;460:339–345. doi: 10.1016/0006-8993(88)90378-2. [DOI] [PubMed] [Google Scholar]
- 6.Cechetto DF, Chen SJ. Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol. 1990;258:R245–255. doi: 10.1152/ajpregu.1990.258.1.R245. [DOI] [PubMed] [Google Scholar]
- 7.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cudennec A, Bonvento G, Duverger D, Lacombe P, Seylaz J, MacKenzie ET. Effects of dorsal raphe nucleus stimulation on cerebral blood flow and flow-metabolism coupling in the conscious rat. Neuroscience. 1993;55:395–401. doi: 10.1016/0306-4522(93)90508-d. [DOI] [PubMed] [Google Scholar]
- 9.Davies CW, Crosby JH, Mullins RL, Traill ZC, Anslow P, Davies RJ, Stradling JR. Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep. 2001;24:715–720. doi: 10.1093/sleep/24.6.715. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998;50:633–641. doi: 10.1212/wnl.50.3.633. [DOI] [PubMed] [Google Scholar]
- 12.Ficker JH, Feistel H, Moller C, Merkl M, Dertinger S, Siegfried W, Hahn EG. Changes in regional CNS perfusion in obstructive sleep apnea syndrome: initial SPECT studies with injected nocturnal 99mTc-HMPAO. Pneumologie. 1997;51:926–930. [PubMed] [Google Scholar]
- 13.Gaffan D. Loss of recognition memory in rats with lesions of the fornix. Neuropsychologia. 1972;10:327–341. doi: 10.1016/0028-3932(72)90025-5. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: a combination of transcranial doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:1101–1112. doi: 10.3171/JNS-07/12/1101. [DOI] [PubMed] [Google Scholar]
- 15.Henderson LA, Woo MA, Macey PM, Macey KE, Frysinger RC, Alger JR, Yan-Go F, Harper RM. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J Appl Physiol. 2003;94:1063–1074. doi: 10.1152/japplphysiol.00702.2002. [DOI] [PubMed] [Google Scholar]
- 16.Johansson RS, Cole KJ. Sensory-motor coordination during grasping and manipulative actions. Curr Opin Neurobiol. 1992;2:815–823. doi: 10.1016/0959-4388(92)90139-c. [DOI] [PubMed] [Google Scholar]
- 17.Kheirandish-Gozal L, Khalyfa A, Gozal D, Bhattacharjee R, Wang Y. Endothelial dysfunction in children with obstructive sleep apnea is associated with epigenetic changes in the eNOS gene. Chest. 2013;143:971–977. doi: 10.1378/chest.12-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res. 1993;609:174–184. doi: 10.1016/0006-8993(93)90871-j. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008;438:330–334. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–2052. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Dilated basilar arteries in patients with congenital central hypoventilation syndrome. Neurosci Lett. 2009;467:139–143. doi: 10.1016/j.neulet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letovsky SI, Whitehead SH, Paik CH, Miller GA, Gerber J, Herskovits EH, Fulton TK, Bryan RN. A brain image database for structure/function analysis. Am J Neuroradiol. 1998;19:1869–1877. [PMC free article] [PubMed] [Google Scholar]
- 23.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–977. [PMC free article] [PubMed] [Google Scholar]
- 24.Macey PM, Kumar R, Yan-Go FL, Woo MA, Harper RM. Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep. 2012;35:1603–1613. doi: 10.5665/sleep.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer JS, Ishikawa Y, Hata T, Karacan I. Cerebral blood flow in normal and abnormal sleep and dreaming. Brain Cogn. 1987;6:266–294. doi: 10.1016/0278-2626(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 26.Murphy BD, Chen X, Lee TY. Serial changes in CT cerebral blood volume and flow after 4 hours of middle cerebral occlusion in an animal model of embolic cerebral ischemia. Am J Neuroradiol. 2007;28:743–749. [PMC free article] [PubMed] [Google Scholar]
- 27.Ni HF, Zhang JX, Harper RM. Cardiovascular-related discharge of periaqueductal gray neurons during sleep-waking states. Brain Res. 1990;532:242–248. doi: 10.1016/0006-8993(90)91766-a. [DOI] [PubMed] [Google Scholar]
- 28.Ni HF, Zhang JX, Harper RM. Respiratory-related discharge of periaqueductal gray neurons during sleep-waking states. Brain Res. 1990;511:319–325. doi: 10.1016/0006-8993(90)90177-d. [DOI] [PubMed] [Google Scholar]
- 29.Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuro Image. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–128. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- 31.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strouse JJ, Cox CS, Melhem ER, Lu H, Kraut MA, Razumovsky A, Yohay K, van Zijl PC, Casella JF. Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA) Blood. 2006;108:379–381. doi: 10.1182/blood-2005-10-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care. 2002;8:242–250. doi: 10.1097/00075198-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol. 2008;105:1852–1857. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(o) and P(CO2) J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita K, Kobayashi S, Yamaguchi S, Kitani M, Tsunematsu T. Effect of smoking on regional cerebral blood flow in the normal aged volunteers. Gerontology. 1988;34:199–204. doi: 10.1159/000212953. [DOI] [PubMed] [Google Scholar]
- 38.Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng ZJ. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguous of F344 rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R299–308. doi: 10.1152/ajpregu.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]