Abstract
Objective
Rheumatoid arthritis (RA) is associated with an increased risk of cardiovascular disease and mortality. Lipid-lowering therapy is reportedly underused in patients with RA. However, longitudinal cohort studies comparing the use of lipid-lowering medications in patients with RA vs the general population are lacking.
Methods
Cardiovascular risk factors, lipid measures and use of lipid-lowering agents were assessed in a population-based inception cohort of patients with RA and a cohort of non-RA subjects followed from 1/1/1988 to 12/31/2008. The National Cholesterol Education Program (NCEP) adult treatment panel III (ATP III) guidelines were assessed at the time of each lipid measure throughout follow-up. Time from meeting guidelines to initiation of lipid-lowering agents was assessed using Kaplan-Meier methods.
Results
The study population included 412 RA and 438 non-RA patients with ≥1 lipid measure during follow-up and no prior use of lipid lowering agents. Rates of lipid testing were lower among patients with RA compared to non-RA subjects. Among patients who met NCEP ATP III criteria for lipid-lowering therapy (n=106 RA and n=120 non-RA), only 27% of RA and 26% of non-RA subjects initiated lipid-lowering agents within 2 years of meeting guidelines for initiation.
Conclusions
There was substantial undertreatment in both the RA and non-RA cohorts who met NCEP ATP III criteria for initiation of lipid-lowering agents. Patients with RA did not have as frequent lipid testing as individuals in the general population.
Keywords: rheumatoid arthritis, lipids, lipid lowering therapy
Introduction
Hyperlipidemia is an important risk factor for coronary heart disease (CHD) in the general population (1, 2) with a continuous, graded increase, in cardiovascular (CV) risk with increasing serum cholesterol levels and a concomitant decline in CV risk with reductions in serum cholesterol (3, 4).
Research over the past decade has demonstrated increased CV risk in patients with rheumatoid arthritis (RA) versus non-RA subjects (5–7). However, the association between lipid levels and CV risk in RA appears to be more complex than in the general population, with systemic inflammation being a potential important contributor to changes in lipid profile (8). Growing evidence suggests that patients with active untreated RA have reduced total cholesterol (TC), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) (9–12). In contrast, declines in inflammatory activity may be accompanied by increases in serum lipid values (8, 13–17).
Lipid-lowering therapy is recommended for patients at risk for CV disease related to hyperlipidemia. The National Cholesterol Education Program (NCEP) has published clinical guidelines (Adult Treatment Panel III, ATPIII) for cholesterol testing and management in the general population (Table 1). The need for increased attention to CV risk reduction in RA is highlighted by recent studies reporting the underuse of lipid-lowering agents (LLA) in patients with RA during both primary (18) and secondary prevention (19). To address the need for CV risk management in RA, the European League Against Rheumatism (EULAR) has recommended that interventions for CV risk factor reduction, including the management of hyperlipidemia, should be undertaken according to national guidelines (20). To better assess CV risk management in RA, we performed a study of LLA use in patients with RA compared to patients without RA.
Table 1.
The National Cholesterol Education Program adult treatment panel III guidelines for initiation of lipid-lowering therapy
| Cardiovascular risk category | LDL level to consider lipid-lowering therapy |
|---|---|
| High risk: CHD or CHD risk equivalent (MI, PAD, AAA, DM, revascularization procedures) or 10-year Framingham risk score for hard CHD endpoints >20% | ≥130 mg/dl |
| Moderately high risk: ≥2 risk factors* AND 10-year Framingham risk score for hard CHD endpoints 10–20% | ≥130 mg/dl |
| Moderate risk: ≥2 risk factors* AND 10-year Framingham risk score for hard CHD endpoints <10% | ≥160 mg/dl |
| Low risk: 0–1 risk factor* | ≥190 mg/dl |
Risk factors include: current smoking, hypertension or anti-hypertensive medication use, high density lipoproteins < 40 mg/dl, family history of premature CHD, and age (≥45 years in men and ≥55 years in women)
AAA = abdominal aortic aneurysm; CHD = coronary heart disease; DM = diabetes mellitus; MI = myocardial infarction; PAD = peripheral arterial disease, LDL = low density lipoprotein.
Methods
This retrospective population-based study was conducted using the unique medical records linkage system of the Rochester Epidemiology Project (REP) which allows access to the complete records from all health care providers in the area. The potential of the REP for population-based research has been well-established, and its capabilities for studies in patients with rheumatic diseases have been well-described (21, 22).
The study population included all Olmsted County, Minnesota residents age ≥18 years who fulfilled the 1987 American College of Rheumatology (ACR) criteria for RA between 1/1/1988 and 12/31/2007, and a comparison cohort of subjects without RA of similar age and sex from the same underlying community. RA incidence date was defined as the first day of fulfillment of four out of seven ACR criteria for RA. For each patient with RA, a comparator subject without RA with similar age and sex was randomly selected from the same underlying population. The index date for non-RA subjects corresponded to the RA incidence date of the corresponding RA patient. All subjects were followed longitudinally until death, migration from the county, or 12/31/2008.
The original and complete medical records of all subjects were reviewed longitudinally by trained nurse abstractors, supervised by the principal investigator. Information on CV risk factors (current cigarette smoking; blood pressure, diagnosis of hypertension/anti-hypertensive medication use; family history of premature CHD; personal history of myocardial infarction [MI], peripheral arterial disease, abdominal aortic aneurism, diabetes mellitus, revascularization procedures), the use of LLA (including statins, fibrates, bile acid sequestrants and niacin) and RA characteristics was abstracted.
All clinically measured lipid values, i.e., TC, LDL, HDL and triglycerides from incidence/index date to the last followup were abstracted. The NCEP/ATPIII guidelines were used to identify indications for initiation of LLA. Study subjects were classified into four CV risk categories (Table 1). In accordance with the NCEP/ATPIII guidelines, the Framingham risk score (FRS) was used to define the 10-year risk for hard CHD endpoints, namely MI/coronary death (1). The study protocol was approved by Mayo Clinic and Olmsted Medical Center institutional review boards.
Statistical Analysis
Several subgroups of patients were used to examine the rate of LLA initiation. First, patients in either cohort who were not taking LLA prior to RA incidence/index date were compared (n=536 RA and 544 non-RA). Second, patients in either cohort with ≥1 lipid measure were compared (n=412 RA and 438 non-RA). In these patients, the NCEP/ATPIII criteria were assessed using the first lipid test after RA incidence/index date. Finally, each patient’s lipid measures and other CV risk factors were examined chronologically to identify patients who met NCEP/ATPIII criteria at any time during follow-up (n=106 RA and 120 non-RA subjects). Kaplan-Meier methods were used to examine the rate of initiation of LLA; the log rank test was used to compare the cohorts. Cox proportional hazards models were used to compare the cohorts adjusting for age, sex, time since RA incidence/index date and CV risk level. Time to the next lipid test following a normal or an abnormal lipid test in RA vs. non-RA cohort was examined using mixed models with random effects for subject to account for variation within subjects.
Results
The study population included 650 RA patients and 650 non-RA subjects. There were 536 patients with RA and 544 non-RA subjects with no LLA use prior to RA incidence/index date. The mean age at RA incidence for these RA patients (53.8 years, standard deviation [SD] 15.6 years) was similar to that of the non-RA subjects at the index date (54.0±15.7 years; p=0.87). The proportion of females was similar in RA (70%) vs. non-RA (71%; p=0.82). The median followup was 7.4 years in RA and 8.4 years in non-RA cohort. There were 21% of patients with family history of CHD in RA vs 22% in non-RA cohort (p=0.59). There were similar proportions of current smokers in RA (20%) vs. non-RA (17%) cohorts (p=0.25). In the RA cohort, 374 (70%) patients were positive for rheumatoid factor or anti-citrullinated peptide antibodies, and 148 (28%) had radiographic joint erosions/destructive changes in the first year of RA. The baseline erythrocyte sedimentation rate was 23.1±19.0 mm/hr. During the follow-up, 336 (63%) of patients were exposed to methotrexate, 382 (71%) used other disease-modifying antirheumatic drugs (DMARDs), 110 (21%) received biologics, and 424 (79%) used systemic corticosteroids.
There was no statistically significant difference in the time from index date to the first lipid measure in RA vs non-RA subjects (log rank p=0.68). By 5 years after RA incidence/index date, 80.5% of RA patients and 77.4% of non-RA subjects had been tested. The rates of lipid testing were lower in RA than in non-RA subjects, with 2209 lipid tests during 4454 person-years (0.50 per patient per year; 95% confidence interval [CI] 0.48, 0.52) in RA patients and 2780 lipid tests during 5119 person-years (0.54 per patient per year; 95%CI 0.52, 0.56) in the non-RA subjects (p<0.001). While patients with lipid values within the normal range may be less likely to receive repeat lipid tests than those with abnormal lipid values, we found no differences when comparing RA vs non-RA patients in the time to next lipid test following a normal LDL test (defined as LDL<160 mg/dL; p=0.78) or in the time from an abnormal LDL test (LDL≥160 mg/dL) to the next lipid test (p=0.88).
Apart from lower rates of lipid testing among patients with RA, by 2 years after RA incidence/index date, only 3.3% of RA patients vs 4.7% of non-RA subjects had initiated LLA (log rank p=0.019). By 5 years the LLA initiation rates were 9.0% in RA vs 13.5% in non-RA cohort, by 10 years - 20.6% vs 27.6%, respectively. There was no apparent difference in LLA initiation in RA vs non-RA cohorts, adjusting for age, sex, TC and LDL (hazard ratio [HR] 0.92; 95%CI 0.70, 1.21).
In order to assess the NCEP/ATPIII criteria, a lipid measure was required. A total of 412 patients with RA and 438 non-RA subjects had ≥1 lipid measure after RA incidence/index date with no prior LLA use and were included in this analysis (Table 2). The age and sex distributions and time from the incidence/index to the first lipid test were similar in both groups. RA subjects had lower TC and LDL, and higher systolic blood pressure than non-RA subjects. The baseline prevalence of CV risk factors and the prevalence of CHD (or CHD risk equivalents) were similar in both groups (Table 2). FRS estimates and the initial spread of risk per the NCEP/ATPIII criteria were similar in RA vs non-RA subjects.
Table 2.
Baseline characteristics of patients with rheumatoid arthritis (RA) and non-RA subjects with at least one lipid test after RA incidence/index date with no prior lipid-lowering medication use
| Characteristic | RA (n=412) | Non-RA (n=438) | p-valueX |
|---|---|---|---|
|
| |||
| Age at lipid test*, years, mean ± SD | 56.2 ± 13.9 (range 21–90) | 55.8 ± 13.9 (range 22–94) | 0.60 |
|
| |||
| Sex, female | 287 (70%) | 314 (72%) | 0.52 |
|
| |||
| RA duration at lipid test*, years, mean ± SD | 2.1 ± 2.2 | 2.2 ± 2.3 | 0.52 |
|
| |||
| Length of follow-up from lipid test* to last follow-up, years, mean ± SD | 7.6 ± 4.8 | 8.3 ± 5.0 | -- |
|
| |||
| TC, mg/dL, mean ± SD | 195 ± 37 | 205 ± 43 | 0.002 |
|
| |||
| LDL, mg/dL, mean ± SD | 115 ± 33 | 122 ± 37 | 0.017 |
|
| |||
| HDL, mg/dL, mean ± SD | 55 ± 17 | 56 ± 17 | 0.19 |
|
| |||
| HDL < 40 mg/dl | 72 (17%) | 67 (15%) | 0.39 |
|
| |||
| TG, mg/dL, mean ± SD | 128 ± 78 | 136 ± 99 | 0.62 |
|
| |||
| Current smoker | 80 (19%) | 70 (16%) | 0.19 |
|
| |||
| Hypertension or anti-hypertensive medication use | 117 (28%) | 105 (24%) | 0.14 |
| Systolic blood pressure, mm Hg | 131 ± 19 | 129 ± 19 | 0.021 |
| Diastolic blood pressure, mm Hg | 77 ± 11 | 77 ± 11 | 0.89 |
|
| |||
| Family history of premature CHD | 84 (20%) | 104 (24%) | 0.24 |
|
| |||
| Personal history of CHD or CHD risk equivalent (MI, PAD, AAA, diabetes mellitus, revascularization procedures | 54 (13%) | 51 (12%) | 0.52 |
|
| |||
| Framingham risk score, % | 6.1% ± 7.4% | 5.8% ± 7.6% | 0.46 |
|
| |||
| Cardiovascular risk category: | 0.48 | ||
| High | 74 (18%) | 71 (16%) | |
| Moderately high | 42 (10%) | 34 (8%) | |
| Moderate | 85 (21%) | 101 (23%) | |
| Low | 211 (51%) | 232 (53%) | |
|
| |||
| NCEP ATP III criteria recommend initiation of lipid-lowering therapy | 56 (14%) | 65 (15%) | 0.60 |
“Lipid test” refers to the first lipid test after RA incidence/index date.
statistically significant differences (p<0.05) are shown in bold
HDL = high density lipoproteins; LDL = low density lipoproteins; TC = total cholesterol; TG = triglycerides; SD = standard deviation; CHD = coronary heart disease; AAA = abdominal aortic aneurysm; DM = diabetes mellitus; MI = myocardial infarction; PAD = peripheral arterial disease; NCEP ATPIII = The National Cholesterol Education Program adult treatment panel III
The first lipid test result after incidence/index date met NCEP/ATPIII criteria for LLA initiation in 14% of RA patients and 15% of non-RA subjects. In this subset, the difference in LLA initiation between the two groups did not reach statistical significance (HR 0.84, 95%CI 0.52, 1.34). During the 2 years after the first lipid test, 24.5% of RA patients and 24.8% of non-RA subjects had initiated LLA (log rank p=0.61).
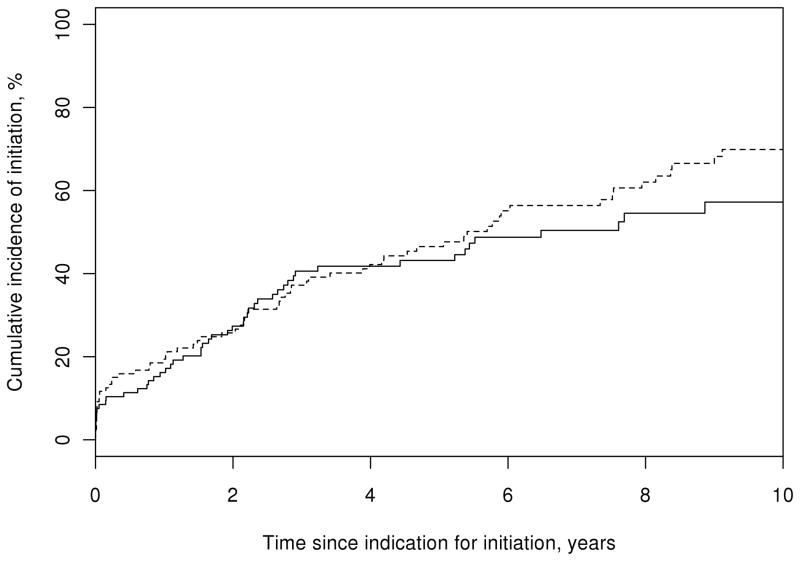
To further investigate the use of LLA in RA, we identified patients who had any lipid test that met NCEP/ATPIII criteria. During follow-up, there were 106 RA patients and 120 non-RA subjects with a lipid test that satisfied the criteria (Table 3). These patients were older with similar proportions of females in both groups. The prevalence of CHD and most CV risk factors were similar in both groups. However, FRS was higher in RA vs non-RA subjects (p=0.007), in spite of lower TC and LDL. Increased levels of systolic blood pressure in RA vs non-RA subjects (p=0.005) could contribute to the higher FRS in RA. There were a greater percentage of RA subjects categorized as high and moderately high risk based on their FRS compared to non-RA subjects. In the study subjects with any lipid test that met the NCEP/ATPIII criteria, the difference in initiation of LLA between RA patients and non-RA subjects did not reach statistical significance (log rank p=0.36; Figure 1). By 2 years after the lipid test, 27.3% of RA patients and 25.7% of non-RA subjects had initiated LLA. By 5 years, there were 43.2% of RA patients and 46.5% of non-RA subjects who had initiated LLA. By 10 years after the lipid test, 58.0% of RA patients and 69.9% of non-RA subjects had initiated LLA. After adjusting for age, sex, time since RA incidence/index date and CV risk category, patients with RA were somewhat less likely to receive LLA than non-RA subjects, but this difference did not reach statistical significance (HR 0.81; 95%CI 0.56, 1.16).
Table 3.
Characteristics of patients for whom a lipid test* during followup met the National Cholesterol Education Program adult treatment panel III criteria for initiation of lipid-lowering therapy.
| Characteristic | RA (n=106) | Non-RA (n=120) | p-valueX |
|---|---|---|---|
|
| |||
| Age at lipid test, years, mean ± SD | 63.4 ± 12.2 (min 36, max 90) | 62.8 ± 11.7 (min 36, max 94) | 0.70 |
|
| |||
| Sex, female | 60 (57%) | 81 (68%) | 0.09 |
|
| |||
| RA duration at lipid test, years, mean ± SD | 4.1 ± 3.6 | 3.8 ± 3.9 | 0.26 |
|
| |||
| Length of follow-up from lipid test to last follow-up, years, mean ± SD | 7.6 ± 4.7 | 8.1 ± 5.1 | -- |
|
| |||
| TC, mg/dL, mean ± SD | 240 ± 31 | 251 ± 35 | 0.017 |
|
| |||
| LDL, mg/dL, mean ± SD | 159 ± 24 | 166 ± 26 | 0.032 |
|
| |||
| HDL, mg/dL, mean ± SD | 49 ± 13 | 52 ± 15 | 0.18 |
|
| |||
| HDL<40 mg/dl | 29 (27%) | 28 (23%) | 0.49 |
|
| |||
| TG, mg/dL, mean ± SD | 160 ± 75 | 169 ± 79 | 0.34 |
|
| |||
| Current smoker | 29 (27%) | 23 (19%) | 0.14 |
|
| |||
| Hypertension or anti-hypertensive medication use | 54 (51%) | 52 (43%) | 0.25 |
| Systolic blood pressure, mm Hg | 142 ± 19 | 136 ± 23 | 0.005 |
| Diastolic blood pressure, mm Hg | 84 ± 10 | 81 ± 13 | 0.29 |
|
| |||
| Family history of premature CHD | 29 (27%) | 44 (37%) | 0.14 |
|
| |||
| Personal history of CHD or CHD risk equivalent (MI, PAD, AAA, diabetes mellitus, revascularization procedures | 31 (29%) | 30 (25%) | 0.47 |
|
| |||
| Framingham risk score, % | 12.9% ± 7.8% | 10.4% ± 8.3% | 0.007 |
|
| |||
| Cardiovascular risk category: | 0.018 | ||
| High | 48 (45%) | 39 (32%) | |
| Moderately high | 37 (35%) | 35 (29%) | |
| Moderate | 13 (12%) | 33 (28%) | |
| Low | 8 (8%) | 13 (11%) | |
“Lipid test” refers to the first lipid test after rheumatoid arthritis (RA) incidence/index date that meets criteria for initiation of lipid-lowering therapy;
statistically significant differences (p<0.05) are shown in bold
HDL = high density lipoproteins; LDL = low density lipoproteins; SD = standard deviation; TC = total cholesterol; TG = triglycerides; CHD = coronary heart disease; AAA = abdominal aortic aneurysm; DM = diabetes mellitus; MI = myocardial infarction; PAD = peripheral arterial disease
Figure 1.
Cumulative incidence of initiation of lipid-lowering therapy among 106 patients with rheumatoid arthritis (RA; solid line) and 120 non-RA subjects (dotted line) who meet The National Cholesterol Education Program Adult Treatment Panel III criteria for initiation of lipid-lowering therapy
Considering that the NCEP/ATPIII criteria were published in 2001 and could not have been used in clinical practice prior to their publication, we performed a sensitivity analysis of a subset of patients who met NCEP/ATPIII criteria in 2001 or after (n=63 RA and 79 non-RA). In this subset the results were similar to those in all the patients who met NCEP/ATPIII criteria, with no significant difference in LLA initiation in RA vs non-RA subjects (logrank p=0.81), and no significant difference in the likelihood of using LLA in RA vs non-RA, adjusting for age, sex, time since incidence/index date and CV risk category (HR 0.86; 95%CI 0.54, 1.36).
The rate of subsequent improvement of lipid profile without initiation of LLA was also examined. Improvement was defined as no longer meeting the NCEP/ATPIII criteria at the time of a subsequent lipid measurement in a patient who had not initiated LLA. No difference in improvement was found in RA vs non-RA subjects (log rank p=0.26). By 2 years after the lipid test, 80.6% of RA patients and 82.5% of non-RA subjects had a continuing indication for LLA per the NCEP/ATPIII criteria. By 10 years after the lipid test, the estimates were 65.7% in RA vs 73.7% in non-RA subjects. After adjusting for age, sex, time since incidence/index date and CV risk category, patients with RA, who met NCEP/ATPIII criteria for LLA initiation but did not start this therapy, were somewhat less likely to need LLA in the future (HR for subsequently achieving improved lipids without therapy: 1.51; 95%CI 0.91, 2.52) than non RA subjects.
We examined the type of LLA used at initiation of lipid-lowering therapy. Among patients with RA who met NCEP/ATPIII criteria for LLA initiation and started LLA (n=54) the majority (85%) were started on statins, 2 patients used fibrates (Gemfibrozil), 2 patients used bile acid sequestrants (Cholestyramine and Colesevelam), and 4 patients used Niacin. Likewise, of 72 non-RA subjects who met NCEP/ATPIII criteria for LLA initiation and started LLA the vast majority (94%) was initiated on statins, 2 subjects used fibrates (Gemfibrozil) and 2 subjects used Niacin. Only 2 RA and 2 non-RA subjects used LLA before the publication of the Scandinavian Simvastatin Survival Study (4S) in April, 1994 (23), suggesting that the majority of lipid lowering treatment in our study was initiated after this landmark publication.
DISCUSSION
The results of this study provide compelling evidence that there is substantial undertreatment of adverse lipid profiles in patients with RA, as well as the general population. However, among individuals who met the NCEP/ATPIII criteria for LLA, the difference in likelihood of initiating LLA between the RA and non-RA subjects did not reach statistical significance. Over the time period of the study, there was substantial undertreatment in both patients with RA and non-RA subjects, with only 26–27% of patients with indications for LLA in either group having initiated therapy within 2 years after meeting criteria for initiation.
We found that the rates of lipid testing were significantly lower in RA patients vs non-RA subjects. This is in keeping with previous findings from our cohort and others reporting less than optimal preventive screening of individuals with RA (24, 25). It could be suggested that initial lower lipid levels in RA vs non-RA subjects may lead to the lower likelihood of subsequent reassessment of lipid levels in RA. However, our finding of similar time to next lipid test in RA vs non-RA subjects with LDL<160 mg/dL, as well as in those with LDL≥160 mg/dL suggests that lower lipid levels may not be the major reason for decreased likelihood of lipid reassessment in RA.
Interestingly, by 2 years after meeting NCEP/ATPIII criteria for LLA initiation, only 27% of RA patients and 26% of non-RA subjects had initiated LLA. Other investigators using cross-sectional data have also reported undertreatment along these lines (18, 26). Toms et al., found that only 5.2% of RA patients who were eligible for statin therapy per the NCEP criteria received LLA (18). That study, unlike our study, excluded patients with established CV disease and diabetes, and lipids were measured in all participants which allowed the identification of patients meeting the criteria who had not yet come to clinical attention. This likely explains why more undertreatment in RA was reported in that study than in our study. Furthermore, that study did not include a comparison cohort and thus low rates of statin use in RA may be due to the general undertreatment of subjects in the population at large. In a recent study Lindhardsen et al. using a very large cohort of patients with incident MI found that RA patients were ~30% less likely to initiate a statin within 30 days after MI and had decreased adherence to statin use compared to the non-RA subjects (19). Taken together with our findings, these results suggest that, among RA patients, undertreatment with lipid-lowering medications may be more apparent in patients with a history of CV disease.
The observed undertreatment may have serious implications, as RA patients are less likely to report symptoms of angina and more likely to experience unrecognized MI and sudden cardiac death than non-RA subjects (6). In our study, a substantial proportion of patients with RA who met criteria for initiation of LLA (Table 3) were already in the advanced CV risk groups (high and moderately high CV risk). This may be related to the less frequent lipid testing, a phenomenon also noted by others (27, 28), suggesting that RA patients were identified as meeting NCEP/ATPIII criteria much later than non-RA subjects, but after meeting criteria they had the same chance of receiving LLA as the non-RA cohort.
In the general population, underuse of LLA remains widespread despite published management guidelines (1). It has been estimated that based on the NCEP/ATPIII guidelines 65 million Americans would be eligible for LLA, of which only 50% have had their lipids assessed and <25% are treated to their NCEP/ATPIII target LDL levels (29). Concordant with these observations we found substantial undertreatment of adverse lipid profiles in the non-RA subjects and the rates of LLA use seen in our non-RA population are consistent with these numbers.
Reasons for the undertreatment in the RA cohort and the general population were not further explored, but may relate to the lack of systematic screening, lack of adherence to the NCEP/ATPIII criteria for clinical decision making, and lack of clarity regarding which of a patient’s physicians should be responsible for managing CV risk. Patient preferences regarding statin use and insufficient awareness among physicians and patients regarding the CV impact of chronic inflammatory diseases such as RA, drug cost, and concerns about polypharmacy may also contribute. Due to the fluctuations of lipid levels with changes of inflammatory activity and the decrease in lipid levels in patients with active RA, the prevalence of hyperlipidemia in RA may vary and the true extent of undertreatment may be easily underestimated, suggesting the need for more thorough CV risk screening in patients with severe RA. However, these considerations are purely speculative and require further investigation.
A majority (65.7%) of patients with RA and the non-RA subjects (73.7%) who met the NCEP/ATPIII criteria for starting LLA but did not do so were found to still satisfy the criteria 10 years later. Reasons for spontaneous reversal of the adverse lipid profile might have included improvement in modifiable CV risk factors such as smoking, body weight, lifestyle and others, which could not be well explored in our study.
This study is among the first population-based studies to examine the differences in use of LLA in RA vs general population. It has the advantage of longitudinal followup of a population-based RA incidence cohort and a non-RA cohort from the same community. A comprehensive review of the entire (inpatient and outpatient) medical records of all subjects was performed. The availability of extensive data on CV risk factors and LLA use is another strength of the study. We rigorously applied the NCEP/ATPIII criteria to determine indications for LLA.
The results of this study should be interpreted in light of the fact that the population of Olmsted County, Minnesota during the calendar years under investigation was >95% white. With the exception of a higher proportion of the working population employed in the health care industry, and correspondingly higher education levels, the population is socioeconomically similar to American whites (21). Thus, our results may not be generalizable to more diverse populations. We applied the NCEP/ATPIII criteria to identify indications for LLA in RA and the non-RA cohort. These criteria were published in 2001, so could not have been used for clinical decision making prior to 2001. However, a sensitivity analysis in the subset of patients who met NCEP/ATPIII criteria in 2001 or after yielded results that were similar to those for all patients.
As with any longitudinal study, there is a possibility that changes in the assessment of CV risk factors may have occurred during the study time. However, all subjects in both the RA and non-RA cohorts received their medical care from similar health care facilities in the area and any changes in the risk factor assessment during the study time would equally affect both groups. As in any retrospective study, only information about medications recorded in the medical record was available. Data regarding medication adherence were not available, and the effect of any recommendations about lifestyle modifications examined. Though we did not study non pharmacologic interventions or their effects, the majority of the patients in our study had a continuing indication for LLA at 10 years. Finally, the sample size of our study resulted in limited statistical power for some comparisons.
This study provides compelling evidence that there is substantial undertreatment of adverse lipid profiles in patients with RA, as well as the general population. These findings have important implications for the detection and prevention of CV comorbidity in RA. As findings from our study demonstrate, patients with RA, in spite of having lower lipid levels, tend to have higher Framingham risk scores, putting them at a greater CV risk. Physicians who care for these patients should be aware of the higher risk of CV disease already present at the time of initial diagnosis of RA and should actively monitor patients for it and pursue risk modification. It remains a fact that patients with RA do not have lipid testing as often as individuals in the general population. Perhaps a greater awareness of guidelines regarding LLA could improve treatment initiation overall. Co-management between primary care and rheumatologists may improve the delivery of preventive care for patients with arthritis (25).
Acknowledgments
Funding: This work was funded by a grant from Pfizer and was made possible by a grant from the National Institutes of Health (R01 AR46849 from NIAMS) and by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging). This project was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 3.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–72. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–8. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 5.Georgiadis AN, Papavasiliou EC, Lourida ES, Alamanos Y, Kostara C, Tselepis AD, et al. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment--a prospective, controlled study. Arthritis Res Ther. 2006;8:R82. doi: 10.1186/ar1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–11. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–12. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–9. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 9.Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:842–5. doi: 10.1136/ard.62.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi HK, Seeger JD. Lipid profiles among US elderly with untreated rheumatoid arthritis--the Third National Health and Nutrition Examination Survey. J Rheumatol. 2005;32:2311–6. [PubMed] [Google Scholar]
- 11.Park YB, Lee SK, Lee WK, Suh CH, Lee CW, Lee CH, et al. Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol. 1999;26:1701–4. [PubMed] [Google Scholar]
- 12.van Halm VP, Nielen MM, Nurmohamed MT, van Schaardenburg D, Reesink HW, Voskuyl AE, et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann Rheum Dis. 2007;66:184–8. doi: 10.1136/ard.2006.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters MJ, Vis M, van Halm VP, Wolbink GJ, Voskuyl AE, Lems WF, et al. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann Rheum Dis. 2007;66:958–61. doi: 10.1136/ard.2006.059691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popa C, van den Hoogen FH, Radstake TR, Netea MG, Eijsbouts AE, den Heijer M, et al. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann Rheum Dis. 2007;66:1503–7. doi: 10.1136/ard.2006.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner G, Urowitz MB. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin Arthritis Rheum. 2009;38:372–81. doi: 10.1016/j.semarthrit.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 16.van Sijl AM, Peters MJ, Knol DL, de Vet RH, Sattar N, Dijkmans BA, et al. The effect of TNF-alpha blocking therapy on lipid levels in rheumatoid arthritis: a meta-analysis. Semin Arthritis Rheum. 2011;41:393–400. doi: 10.1016/j.semarthrit.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–8. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- 18.Toms TE, Panoulas VF, Douglas KM, Griffiths H, Sattar N, Smith JP, et al. Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: evidence for substantial undertreatment of lipid-associated cardiovascular risk? Ann Rheum Dis. 2010;69:683–8. doi: 10.1136/ard.2009.115717. [DOI] [PubMed] [Google Scholar]
- 19.Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Torp-Pedersen C, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis. 2012;71:1496–501. doi: 10.1136/annrheumdis-2011-200806. [DOI] [PubMed] [Google Scholar]
- 20.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 22.Kremers HM, Myasoedova E, Crowson CS, Savova G, Gabriel SE, Matteson EL. The Rochester Epidemiology Project: exploiting the capabilities for population-based research in rheumatic diseases. Rheumatology (Oxford) 2011;50:6–15. doi: 10.1093/rheumatology/keq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 24.Kremers HM, Bidaut-Russell M, Scott CG, Reinalda MS, Zinsmeister AR, Gabriel SE. Preventive medical services among patients with rheumatoid arthritis. J Rheumatol. 2003;30:1940–7. [PubMed] [Google Scholar]
- 25.Curtis JR, Arora T, Narongroeknawin P, Taylor A, Bingham CO, 3rd, Cush J, et al. The delivery of evidence-based preventive care for older Americans with arthritis. Arthritis Res Ther. 2010;12:R144. doi: 10.1186/ar3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soubrier M, Zerkak D, Dougados M. Indications for lowering LDL cholesterol in rheumatoid arthritis: an unrecognized problem. J Rheumatol. 2006;33:1766–9. [PubMed] [Google Scholar]
- 27.Strandberg TE, Kovanen PT, Eklund KK. Is the “lipid paradox” in rheumatoid arthritis really a paradox? Arthritis Rheum. 2011;63:3644–5. doi: 10.1002/art.30578. [DOI] [PubMed] [Google Scholar]
- 28.Bartels CM, Everett C, McBride P, Smith M, Kind A, Mell M. Reply to letter to the editor entitled “Is the “lipid paradox” in rheumatoid arthritis really a paradox?” by Strandberg et al. Arthritis Rheum. 2011;63:3645–6. [Google Scholar]
- 29.Clearfield MB. Underidentification and undertreatment of dyslipidemia. J Am Osteopath Assoc. 2003;103 (1 Suppl 1):S5–8. [PubMed] [Google Scholar]