Abstract
Introduction
Erectile dysfunction (ED) is more common in men with type 2 diabetes mellitus (T2DM), obesity, and/or the metabolic syndrome (MetS).
Aim
To investigate the associations among proxy measures of diabetic severity and the presence of metabolic syndrome (MetS) with erectile dysfunction (ED) in a nationally representative U.S. data sample.
Methods
We performed a cross-sectional analysis of adult participants in the 2001–2004 National Health and Nutrition Examination Survey (NHANES).
Main outcome measures
ED was ascertained by self-report. T2DM severity was defined by calculated measures of glycemic control and insulin resistance (IR). Insulin resistance was estimated using fasting plasma insulin (FPI) levels and the homeostasis model assessment of insulin resistance (HOMA-IR) definition. We classified glycemic control using HbA1c and fasting plasma glucose levels (FPG). Metabolic syndrome was defined by the American Heart Association and National Heart, Lung, and Blood Institute criteria. Logistic regression models, adjusted for sociodemographics, risk factors and comorbidities, were fitted for each measure of T2DM severity, MetS, and the presence of ED.
Results
Proxy measures of glycemic control and insulin resistance were associated with ED. Participants with FPG between 100–126mg/dL (5.6–7 mmol/L) and ≥126mg/dL (>7mmol/L) had higher odds of ED, OR 1.22 [CI, 0.83–1.80] and OR 2.68 [CI, 1.48–4.86], respectively. Participants with HbA1c 5.7–6.4% (38.8–46.4 mmol/mol) and ≥6.5% (47.5 mmol/mol) had higher odds of ED, (OR 1.73 [CI, 1.08–2.76] and 3.70 [CI, 2.19–6.27], respectively). When FPI and HOMA-IR were evaluated by tertiles, there was a graded relation among participants in the top tertile. In multivariable models, a strong association remained between HbA1c and ED (OR 3.19 [CI,1.13–9.01]). MetS was associated with >2.5-fold increased odds of self reported ED (OR 2.55 [CI, 1.85–3.52]).
Conclusions
Poor glycemic control, impaired insulin sensitivity and the MetS are associated with a heightened risk of ED.
Keywords: diabetes mellitus, diabetes severity, endothelial dysfunction, erectile dysfunction, insulin resistance, men’s health, metabolic syndrome
Introduction
Erectile dysfunction (ED) is an increasingly common condition, with population prevalence estimates between 30 and 50%[1–3]. It is well known that ED is more common in men with type 2 diabetes mellitus (T2DM), obesity, and/or the metabolic syndrome (MetS) [4]. Men diagnosed with T2DM have been found to have an accelerated onset of ED, with the diagnosis established 10–15 years earlier than men without T2DM [3]. Moreover, men with ED associated with T2DM have been shown to have higher levels of disease-specific health distress, increased rates of depression, poorer adaptation to their diabetes, and marked reductions in overall quality of life [5]. The pathogenesis of ED is multifactorial, with hormonal, neurologic, vascular, psychogenic and lifestyle contributors. Erectile dysfunction is considered an early and valid surrogate marker for systemic endothelial dysfunction [6] and subsequent macrovascular disease [7–8]. Insulin resistance, hyperglycemia and several associated metabolic derangements could contribute to the pathophysiologic cascade responsible for endothelial dysfunction via impairments in vascular nitric oxide (NO) synthesis, impaired vasodilation, and damage due to heightened states of inflammation and oxidative stress [9–10].
Type 2 diabetes mellitus is a leading cause of end-organ disease and death in the United States. Among persons with T2DM, poorer glycemic control and more pronounced insulin resistance are associated with increased risk of microvascular complications, macrovascular disease and all-cause mortality [11–12]. The metabolic syndrome is a constellation of physiologic derangements that includes abdominal obesity, dyslipidemia, elevated blood pressure, and impaired glucose tolerance. More than one-third of the adult U.S population meet criteria for MetS [13]. Several small cohort studies have demonstrated an inverse relation between glycemic control and degree of potency [14–15]. Other studies have additionally demonstrated MetS to be an independent risk factor for ED [16–19].
We sought to investigate the associations between the severity of T2DM, as measured by proxies of glycemic control and insulin resistance, and the presence of metabolic syndrome with ED in a nationally representative U.S. data sample. We hypothesized that biochemical markers of glycemic control and insulin resistance would be associated, in a graded fashion, with the odds of ED.
Methods
Study population
For this study, we used data from the National Health and Nutrition Examination Survey (NHANES) 2001–2002 and 2003–2004 survey cycles. These surveys are used to assess the health and nutritional status of adults and children in the U.S. The NHANES interview includes demographic, socioeconomic, and health-related questions. The examination component consists of medical and physiological measurements, as well as laboratory tests. The NHANES 2001–2004 survey is the most recent collection that included questions assessing erectile function. The analytic cohort included 3,306 male participants, 20 or more years of age, who underwent a physical examination as well as the fasting subsample laboratory draw, and responded to the ED questionnaire.
T2DM and Severity Definitions
We used calculated (laboratory) measures to define the presence of T2DM and insulin resistance. Insulin resistance was estimated using fasting plasma insulin levels and the homeostasis model assessment of insulin resistance (HOMA-IR) definition [fasting insulin (mg/dL) multiplied by fasting glucose (mg/dL) divided by a correction factor of 405]. The HOMA-IR is a method widely used in epidemiologic studies to quantify insulin resistance and beta-cell function. HOMA-IR has been shown to directly approximate measured insulin resistance, but can be limited in patients with severe hyperglycemia and in non-obese diabetic patients. To overcome the limitations of any one measure, we included both HOMA-IR and fasting insulin levels. For fasting insulin and HOMA-IR, these measures were grouped into tertiles due to the absence of clinically relevant cut-off values and their skewed distributions. We categorized glycemic control using hemoglobin-A1c (HbA1c) and fasting plasma glucose (FPG) levels as follows: FPG <100 mg/dL (<5.6 mmol/L), 100 to 125 mg/dL (5.6 to 6.9 mmol/L), and ≥126 mg/dL (7.0 mmol/L) and HbA1c <5.7% (< 38.8 mmol/mol), 5.7 to 6.4% (38.8 to 46.4 mmol/mol), ≥6.5% (47.5 mmol/mol) according to cutoffs recommended by the American Diabetes Association [20].
Metabolic syndrome
Participants were examined in the mobile examination center, where waist circumference and blood pressure measurements were obtained. We used the mean values of three or more consecutive blood pressure measurements for our analysis. High-density lipoprotein cholesterol (HDL-c) and triglyceride measurements were obtained after a 4 to 9 hour fast. We defined the the presence of MetS using the American Heart Association and National Heart, Lung, and Blood Institute criteria [21] as the presence of three or more of the following five traits: abdominal obesity (male waist circumference > 102 cm), elevated serum triglycerides (>150mg/dL), low HDL-c (<40mg/dL in men), elevated blood pressure (>130/85 mmHg), and impaired glucose tolerance (fasting glucose >100 mg/dL).
Erectile dysfunction
We defined ED using the participant response to the question: “Many men experience problems with sexual intercourse. How would you describe your ability to get and keep an erection adequate for satisfactory intercourse? Would you say that you are: Always or almost always able, usually able, sometimes able, or never able to get and keep an erection?” We categorized men who reported that they were “sometimes able” or “never able” to get and keep an erection as having ED as validated by O’Donnell et al [22]. We excluded 20 (0.6% [unweighted]) participants who refused to answer the question, 42 (1.3%) that responded “don’t know”, and 623 (15.6%) missing a response to the question.
Other clinical characteristics
Age, sex, race/ethnicity, highest level of education, marital status and household income were assessed by questionnaire. We used the racial/ethnic group variables reported by NHANES, and reclassified them as “non-Hispanic white”, “non-Hispanic black”, “Hispanic”, and “other/multiracial” according to the analytic guidelines. We categorized income strata as $0–19,999, $20,000–34,999, $35,000–74,999, and ≥$75 000. Education levels were classified as “Less than high school”, “High school/GED equivalent”, and “College or greater.” Marital status was categorized as “Never married”, “Married or living partner”, “Widowed”, and “Divorced or Separated.”
We calculated Quételet’s (body mass) index (BMI) as weight in kilograms divided by height in meters squared, and categorized using the World Health Organization (WHO) cutoffs, as lean or normal weight (<24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Participant comorbidities in addition to behavioral risk behaviors such as smoking and alcohol use were collected from the interview questionnaire. Physical activity was estimated by deriving a metabolic equivalent score for self-reported leisure and normal-time activities [23].
Statistical methods
All statistical analyses were performed using SAS v9.3 (SAS Institute Inc, Cary, North Carolina) and incorporated the recommended NHANES sample weights, strata and cluster design variables. Graphics were constructed using R v2.15. We utilized the sub-sample fasting sampling weights in the glucose, insulin and cholesterol laboratory collection component in order to appropriately account for NHANES’ complex survey structure and produce estimates that are representative of the total, non-institutionalized civilian US population. We considered 2-tailed p-values <0.05 as statistically significant, without adjusting for multiple comparisons.
We compared clinical characteristics among participants with and without ED using the chi-square test. We calculated the odds of self-reported ED associated with each individual laboratory measure of diabetic severity using logistic regression. We constructed two multivariable regression models to analyze each “severity measure.” In model A, we adjusted for sociodemographic factors including age, race/ethnicity, education, and marital status. In model B we included sociodemographic factors from model A and added other known risk factors for ED: smoking history, alcohol use, and hypertension (defined as SBP>140mmHg or DBP> 90mmHg). We conducted a sensitivity analysis assigning participants who responded to the ED question as “refused” or “don’t know” as having ED. We conducted companion analyses stratified by BMI and self-reported physical activity, but did not include these factors in multivariable models given known associations among obesity, physical activity, glycemic control and insulin resistance. We also explored whether age and/or race/ethnicity modified the associations among markers of glycemic control and insulin resistance and ED by adding multiplicative interaction terms to the respective multivariable models. Finally, we examined whether a diagnosis of metabolic syndrome was associated with ED and whether age and/or race/ethnicity modified this association as well.
Results
Of the 3,991 men surveyed in this 2001–2004 survey cohort, 3,306 (83%) answered the interview question regarding ED. The weighted population prevalence of self reported ED was 19.9% ([CI], 18.7–21.2%). The weighted population prevalence of T2DM (diagnosis by HbA1c) was 7.0% ([CI], 5.2–8.8%) and metabolic syndrome was 17.6% ([CI], 16.5–18.7%).
Prevalence and correlates of erectile dysfunction and T2DM severity
Tables 1 shows sociodemographic correlates of ED in the NHANES population. The prevalence of ED was highest among Caucasians, married men 60 years and older, participants with less than a high school education, and participants in the lowest income brackets (<$35,000/year).
Table 1.
Prevalence rates of ED by demographic category
| Demographics | Prevalence rates of Self-reported history of ED (Weighted) | ||
|---|---|---|---|
| No % | Yes % | P-value | |
| Overall prevalence | 80.1 | 19.9 | <0.0001 |
|
| |||
| Age | |||
| 20–39 | 95.6 | 4.4 | <0.0001 |
| 40–59 | 83.7 | 16.3 | |
| 60–69 | 48.6 | 51.4 | |
| 70+ | 31.3 | 68.7 | |
|
| |||
| Marital status | |||
| Married/partner | 77.4 | 22.6 | <0.0001 |
| Never married | 93.1 | 6.9 | |
| Divorced/Separated | 81.2 | 18.8 | |
| Widowed | 25.3 | 74.7 | |
|
| |||
| Race | |||
| White, Non-Hispanic | 79.1 | 20.9 | 0.04 |
| Black, Non-Hispanic | 85.3 | 14.7 | |
| Hispanic | 79.9 | 20.1 | |
| Other/multiracial | 85.5 | 14.5 | |
|
| |||
| Household Income (USD) | |||
| $0 to $19,999 | 71.0 | 29.0 | 0.003 |
| $20,000 to $34,999 | 70.9 | 29.1 | |
| $35,000 to $74,999 | 81.8 | 18.2 | |
| > $75,000 | 87.4 | 12.6 | |
|
| |||
| Education | |||
| Less than High School | 68.4 | 31.6 | 0.002 |
| High School/GED | 83.6 | 16.4 | |
| College | 81.8 | 18.2 | |
OR = odds ratio; CI = confidence interval; USD = US dollars; GED = General Educational Development test.
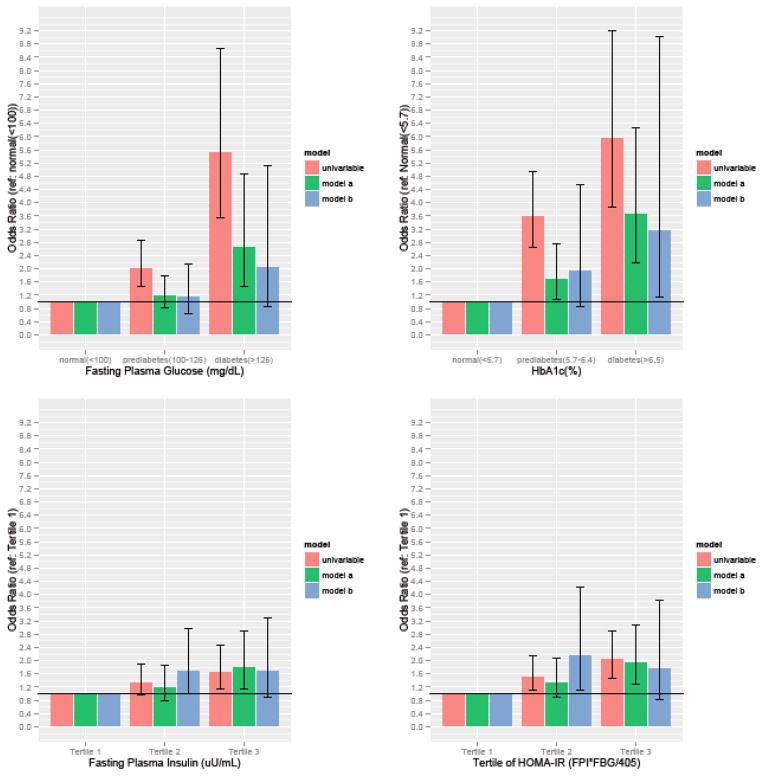
In multivariable model A, measures of both glycemic control (FPG and HbA1c) and insulin resistance (FPI and HOMA-IR) were directly associated with ED. Participants with FPG between 100–126mg/dL and ≥126mg/DL had higher odds of ED, OR 1.22 [CI, 0.83–1.80] and OR 2.68 [CI, 1.48–4.86], respectively. Participants with elevated HbA1c levels of 5.7–6.4% and ≥6.5% had higher odds of ED, (OR of 1.73 [CI, 1.08–2.76] and 3.70 [CI, 2.19–6.27], respectively). When fasting plasma insulin levels and HOMA-IR were evaluated by tertiles, there appeared to be a graded relation that reached statistical significance for participants in the top tertile. (Figure 1)
Figure 1. Odds ratios of ED by biochemical measures of T2DM severity.
OR = odds ratio; CI = confidence interval; T2DM = type 2 diabetes mellitus; FPG = fasting plasma glucose; FPI = fasting plasma insulin; HbA1c = glycosylated hemoglobin A1c; HOMA-IR = homeostasis model assessment of insulin resistance. *
*Model A: adjusted for sociodemographic factors; age, race/ethnicity, education, and marital status.
**Model B: adjusted for patients factors in model A as well as for additional ED risk factors; smoking history, alcohol use, and hypertension (defined as SBP>140mmHg or DBP> 90mmHg)
***HOMA IR is calculated as (FPI x FPG) / 405.
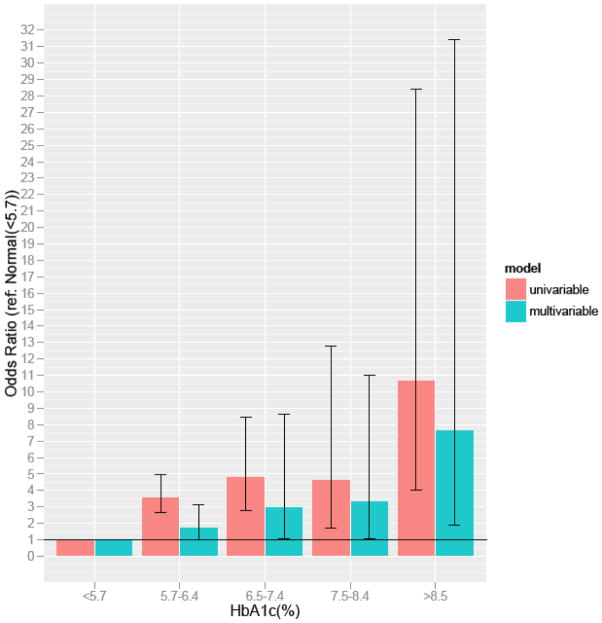
In model B, when adjusting for additional behavioral risk factors (smoking and alcohol use) and comorbid conditions (hypertension) a strong association remained between HbA1c and self-reported ED (OR 3.19 [CI,1.13–9.01]). Even after adjustment for patient demographics, behavioral risk factors and comorbidities, there was a significant association between ED and glycemic control, with a markedly increased odds of ED (as high as 7.70, [CI, 1.88–31.46]) in persons with HbA1c >7.5% relative to persons with normal HgA1c (Figure 2). When examining results across the spectrum of age, the relative odds of ED with elevated HbA1c was nominally higher for younger persons, although the interaction (age x HbA1c) was not statistically significant. Sensitivity analyses categorizing patients that “refused to answer” or “didn’t know” as having ED did not materially change the estimates (data not shown).
Figure 2.
Odds ratio for self-reported ED stratified by ranges of glycemic control
Correlates of erectile dysfunction and the metabolic syndrome
The manifestation of MetS (defined by the presence of ≥3 traits) was associated with more than a 2.5-fold increased odds of self reported ED (OR 2.55 [CI, 1.85–3.52]). When stratifying our results by age and race, we found that black men and those aged 20–39 had the highest odds of ED (OR 3.03 [CI, 1.12–8.22] and OR 5.51 [CI, 1.78–17.02] respectively). However, the multiplicative interaction terms were not significant for these parameters (p-value for interaction 0.5).
Impact of BMI and physical activity
We also conducted companion analyses of our markers of diabetic severity, stratified by BMI and self-reported physical activity. Results were qualitatively similar across the range of BMI, although the associations between T2DM severity metrics and ED were slightly more pronounced among persons with lean/normal BMI compared with persons who were overweight or obese. In our physical activity stratified analysis, results were similar across the spectrum of estimated physical activity (Supplementary tables 2–3).
Discussion
In this cross-sectional study of a representative sample of the U.S. male population, we noted significant associations among individual measures of glycemic control (HbA1c and FPG), insulin resistance (FPI and HOMA-IR) and self-reported ED.
First, we found the highest odds of ED in those patients with the poorest glycemic control (HbA1c >7.5%) and the most severe insulin resistance. Importantly, we also found elevated risk for men without strict criteria for T2DM but with abnormal glycemic control. There are multiple, often overlapping mechanisms that account for the development of ED in the context of T2DM. Insulin resistance and chronic hyperglycemia are both responsible for inhibition and reduction of NO synthase, an essential mediator of penile vaso-relaxation. Studies have demonstrated a reduction in the penile expression of both endothelial and neuronal NO synthetase (eNOS, nNOS) in an experimentally induced diabetic rat model [24]. Insulin resistant states are additionally associated with increased levels of cavernosal endothelin-1, a potent arterial and venous vasoconstrictor [25]. Hyperglycemia is responsible for the glycation of elastic fibers, impaired cavernosal relaxation, the formation of advanced glycation end-products (AGEs), and increased reactive oxidative damage all of which can lead to the development of peripheral neuropathy and endothelial injury [10].
An association between metabolic syndrome and ED was also found, which was more pronounced among younger NHANES participants. Indeed, a quintupling of the odds of ED for men younger than 40 with MetS may provide additional incentive for young men to improve lifestyle habits [26]. It is noteworthy that components of the MetS have been implicated in the pathophysiology of ED. For example, hypertension and low HDL-c are associated with accelerated atherosclerosis, which could compromise penile blood flow [27]. Insulin resistance and centripetal obesity have also been linked to lower testosterone concentrations, which could hamper libido, as well as adversely affect NOS-associated erectile function [28].
The rising prevalence of T2DM and metabolic syndrome in the U.S. population has been considered one of the most critical public health threats of the 21st century [29]. Our study demonstrates that glycemic control and metabolic syndrome are strongly associated with ED, even more so among younger men. Scuteri et al. found that the clustering of metabolic syndrome traits amplifies age-associated increases in arterial stiffness [30]. Our findings also imply that the components of the MetS complex may accelerate the age-associated vascular changes that are responsible for vasculogenic erectile impairment [31–33].
Strengths of our analyses include the cross-sectional analysis of a nationally representative sample including a broad age range, several racial/ethnic groups and multiple socioeconomic backgrounds. As such, our analysis provides a portrait of ED risk factors in the U.S. male population. The response rate to the ED questionnaire was also relatively high (83%), considering the sensitive topic. The availability of laboratory measures of fasting plasma glucose, fasting plasma insulin, and HbA1c allowed us to assess a graded association among these measures and ED. While previous studies have linked ED with T2DM [14–15, 34–35], obesity [4], hypertension [36], dyslipidemia [37], metabolic syndrome [16–19], our study is unique in its inclusion of these factors in parallel and the use of insulin resistance laboratory parameters in our examination of ED risk factors.
This study has several important limitations that should be noted. Data on ED were based on a single-question definition of ED, which limits a more granular assessment of the various domains of the disease. However, the single-question assessment of ED has been previously validated [22] and has also been used in prior studies that have used NHANES data [38]. Additionally, data on ED derived from self-report and may be subject to recall or misclassification bias. Furthermore, the sensitivity of the topic may have additionally caused participants to underreport the presence of ED, and the single-question ED survey limits evaluation of participants currently receiving treatment for the disease. Consequently, while we did not observe this in sensitivity analyses, our model may underestimate the true prevalence and risk effects. Finally, the cross-sectional nature of NHANES limits inferences regarding the causality of our associations. Nevertheless, our study establishes a link between glycemic control, insulin resistance, and ED in a nationally representative cohort with the ability to account for key sociodemographic and lifestyle factors.
Conclusions
Self-reported ED is associated with metabolic syndrome, insulin resistance, and glycemic control. Public health efforts to reduce the prevalence of obesity and to improve management of T2DM may help lessen the considerable burden of ED in the middle-aged and older male population. Conversely, educating the public about the ED – metabolic syndrome – T2DM associations may help to motivate men to implement lifestyle changes that might yield extensive health benefits.
Supplementary Material
Acknowledgments
Funding
Dr. Leppert is supported by K23 DK089086 grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH). Dr. Chertow is supported by K24 DK085446.
References
- 1.Laumann EO, Waite LJ. Sexual dysfunction among older adults: prevalence and risk factors from a nationally representative U.S. probability sample of men and women 57–85 years of age. J Sex Med. 2008;10:2300–11. doi: 10.1111/j.1743-6109.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 3.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res. 2005;17:391–8. doi: 10.1038/sj.ijir.3901333. [DOI] [PubMed] [Google Scholar]
- 5.De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care. 2002;25:284–91. doi: 10.2337/diacare.25.2.284. [DOI] [PubMed] [Google Scholar]
- 6.de Araña Rosaínz MJ, Ojeda MO, Acosta JR, Elías-Calles LC, González NO, Herrera OT, et al. Imbalanced low-grade inflammation and endothelial activation in patients with type 2 diabetes mellitus and erectile dysfunction. J Sex Med. 2011;8:2017–30. doi: 10.1111/j.1743-6109.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- 7.Shin D, Pregenzer G, Jr, Gardin JM. Erectile dysfunction: a disease marker for cardiovascular disease. Cardiol Rev. 2011;19:5–11. doi: 10.1097/CRD.0b013e3181fb7eb8. [DOI] [PubMed] [Google Scholar]
- 8.Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, Lieber MM, et al. A population- based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108–13. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trussell JC, Legro RS. Erectile dysfunction: does insulin resistance play a part? Fertil. Steril. 2007;88:771–8. doi: 10.1016/j.fertnstert.2007.01.116. [DOI] [PubMed] [Google Scholar]
- 10.Morano S. Pathophysiology of diabetic sexual dysfunction. J Endocrinol Invest. 2003;26:65–9. [PubMed] [Google Scholar]
- 11.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EAM, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–9. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 14.Awad H, Salem A, Gadalla A, El Wafa NA, Mohamed OA. Erectile function in men with diabetes type 2: correlation with glycemic control. Int J Impot Res. 2010;22:36–9. doi: 10.1038/ijir.2009.39. [DOI] [PubMed] [Google Scholar]
- 15.Romeo JH, Seftel AD, Madhun ZT, Aron DC. Sexual function in men with diabetes type 2: association with glycemic control. J Urol. 2000;163:788–91. [PubMed] [Google Scholar]
- 16.Heidler S, Temml C, Broessner C, Mock K, Rauchenwald M, Madersbacher S, et al. Is the metabolic syndrome an independent risk factor for erectile dysfunction? J Urol. 2007;177:651–4. doi: 10.1016/j.juro.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Mi H, Gao Y, Tan A, Lu Z, Wu C, et al. Metabolic syndrome: a potential and independent risk factor for erectile dysfunction in the chinese male population. Urology. 2012;80:1287–92. doi: 10.1016/j.urology.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Bal K, Oder M, Sahin AS, Karataş CT, Demir O, Can E, et al. Prevalence of metabolic syndrome and its association with erectile dysfunction among urologic patients: metabolic backgrounds of erectile dysfunction. Urology. 2007;69:356–60. doi: 10.1016/j.urology.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 19.Bansal TC, Guay AT, Jacobson J, Woods BO, Nesto RW. Incidence of metabolic syndrome and insulin resistance in a population with organic erectile dysfunction. J Sex Med. 2005;2:96–103. doi: 10.1111/j.1743-6109.2005.20120.x. [DOI] [PubMed] [Google Scholar]
- 20.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57:e1–e47. doi: 10.1373/clinchem.2010.161596. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Intern Med. 2005;20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 24.Akingba AG, Burnett AL. Endothelial nitric oxide synthase protein expression, localization, and activity in the penis of the alloxan-induced diabetic rat. Mol Urol. 2001;5:189–97. doi: 10.1089/10915360152745885. [DOI] [PubMed] [Google Scholar]
- 25.Francavilla S, Properzi G, Bellini C, Marino G, Ferri C, Santucci A. Endothelin-1 in diabetic and nondiabetic men with erectile dysfunction. J Urol. 1997;158:1770–4. doi: 10.1016/s0022-5347(01)64125-9. [DOI] [PubMed] [Google Scholar]
- 26.Glina S, Sharlip ID, Hellstrom WJ. Modifying risk factors to prevent and treat erectile dysfunction. J Sex Med. 2013;10:115–9. doi: 10.1111/j.1743-6109.2012.02816.x. [DOI] [PubMed] [Google Scholar]
- 27.Stehouwer CDA, Henry RMA, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–39. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 28.Gore JL, Swerdloff RS, Rajfer J. Androgen deficiency in the etiology and treatment of erectile dysfunction. Urol Clin North Am. 2005;32:457–468. vi–vii. doi: 10.1016/j.ucl.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 30.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–95. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 31.Chew KK, Finn J, Stuckey B, Gibson N, Sanfilippo F, Bremner A, et al. Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J Sex Med. 2010;7:192–202. doi: 10.1111/j.1743-6109.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- 32.Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systematic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6:99–109. doi: 10.1161/CIRCOUTCOMES.112.966903. [DOI] [PubMed] [Google Scholar]
- 33.Lin JW, Lee JK, Wu CK, Caffrey JL, Chang MH, Hwang JJ, et al. Metabolic syndrome, testosterone, and cardiovascular mortality in men. J Sex Med. 2011;8:2350–60. doi: 10.1111/j.1743-6109.2011.02343.x. [DOI] [PubMed] [Google Scholar]
- 34.Schouten BW, Bohnen AM, Dohle GR, Groeneveld FP, Willemsen S, Thomas S, et al. Risk factors for deterioration of erectile function: the Krimpen study. Int J Androl. 2009;32:166–7. doi: 10.1111/j.1365-2605.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 35.Grover SA, Lowensteyn I, Kaouache M, Marchand S, Coupal L, DeCarolis E, et al. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–9. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 36.Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000;164:1188–91. [PubMed] [Google Scholar]
- 37.Mulhall J, Teloken P, Brock G, Kim E. Obesity, dyslipidemias and erectile dysfunction: a report of a subcommittee of the sexual medicine society of North America. J Sex Med. 2006;3:778–86. doi: 10.1111/j.1743-6109.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 38.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207–12. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.