Abstract
Objective
To describe the characteristics and health effects of adolescent (age 13–19 years) prescription drug abuse and misuse using the Researched Abuse Diversion and Addiction-Related Surveillance (RADARS®) System.
Method
Secondary analysis of data collected from RADARS System participating poison centers was performed. Data for all intentional exposures from 2007 through 2009 were used to describe adolescent prescription opioid (oxycodone, fentanyl, hydrocodone, hydromorphone, morphine, methadone, buprenorphine, and tramadol) and stimulant (methylphenidate and amphetamines) exposures.
Results
A total of 16,209 intentional adolescent exposures to prescription drugs were identified, 68% to opioids and 32% to stimulants. The mean age was 16.6 years (SD ± 1.7 years). Slightly more than half (52.4%) of drug mentions involved females. The five most frequently misused or abused drugs were hydrocodone (32%), amphetamines (18%), oxycodone (15%), methylphenidate (14%), and tramadol (11%). Of all exposures, 38%were classified as suspected suicidal. Of adolescents who intentionally exposed themselves to prescription drugs, 30% were treated in a health care facility, 2,792 of whom were admitted to the hospital, including 1,293 to the intensive care unit. A total of 17.2% of intentional exposures were associated with no effect, 38.9% minor effects, 23.3% moderate effects, 3.6% major effects, and 0.1% were associated with death. Oxycodone and methadone were associated with the most deaths. No deaths were associated with exposures to stimulants.
Conclusions
Prescription drug misuse and abuse poses an important health problem and results in thousands of hospitalizations of adolescents per year. Further work is needed to develop focused interventions and educational programs to prevent prescription drug abuse and misuse by adolescents.
Keywords: adolescent, prescription, drug abuse, drug misuse
Adolescent prescription drug abuse and misuse is a major public health problem. In the 2005 National Survey on Drug Use and Health, which examined data for 18,678 adolescents, 8.2% had misused a medication, and 3.0% had reported symptoms of a substance use disorder related to prescription medication misuse in the prior year.1 “Pharming” parties, where adolescents get together to exchange and misuse and abuse prescription drugs, are becoming more popular.2 Because of the increased use of stimulant drugs such as methylphenidate (Ritalin) and amphetamine (Adderall) for the treatment of attention-deficit/hyperactivity disorder (ADHD) and the availability of opioid pain medications,3 access to prescription drugs is at an all-time high.
In the 2006 Monitoring the Future study, only four specific substances showed evidence of increasing use by adolescents; two of these were the prescription opioids Vicodin (hydrocodone) and OxyContin (oxycodone).4 In 2009, the use of Vicodin and OxyContin were the highest observed so far.5 Vicodin showed some decrease in use in 2011, whereas amphetamines, OxyContin, and other narcotic drugs increased.6 In another survey published in 2009, the non-medical use of prescription drugs ranked fourth among most abused class of drugs by adolescents after alcohol, tobacco, and marijuana, respectively.7 In a 2005 survey of 1,086 high school–aged adolescents, 12% reported engaging in non-medical use of opioid pain medications, and 2% reported engaging in nonmedical use of stimulant medications in the last 12 months.8 In a large national survey published in 2008, 7%of 12- to 17-year-olds reported nonprescribed prescription pain reliever use in the last year, and 1% met DSM-IV criteria for past-year abuse or dependence.9 Adolescent nonmedical prescription drug users are five to seven times more likely than nonusers to report illicit drug use and drug abuse.10 Similarly, nonmedical prescription analgesic use at age 18 to 24 is associated with a threefold increase in the odds of developing a general substance or opioid use disorder 3 years later.11 Clearly, use of prescription opioids and stimulants is a prominent part of the drug abuse problem in the adolescent population.
In this study, we sought to determine the demographic characteristics, drug exposure profiles, and associated medical outcomes of adolescent prescription drug abusers. Adolescent exposures are likely to be different from adult exposures, as adolescents are less likely have extensive experience with the medications they are abusing. When compared with younger children (≤6 years of age), whose exposure to prescription opioids are nearly always accidental,12 adolescents are more likely to take these substances intentionally.
We used data from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS®) System Poison Center Program to describe the characteristics and health effects of adolescent adolescent prescription drug abuse and misuse. To achieve this, we conducted this study with four specific aims: to describe the characteristics of adolescent prescription drug abuse and misuse with respect to age, gender, intentionality of exposure, and number of substances involved in each case; to determine the most common locations for adolescent exposure to prescription drugs; to examine the relationship between reported adolescent exposure to prescription drugs and drug availability in the area (as measured by drug prescription rates in the individual’s three-digit ZIP code); and to measure the association between intentional exposures to specific prescription drugs and medical outcomes, including hospitalization, intensive care, or death.
METHOD
The RADARS System
We analyzed acute event data with short-term follow-up from the RADARS System. The RADARS System is composed of multiple programs that monitor prescription opioid and stimulant abuse and diversion across the spectrum of addiction disorders in a timely fashion and undergo a rigorous quality assurance process.13 The current study exclusively uses data from the Poison Center program.
The RADARS System collects exposure and short-term follow-up data prospectively and has been successfully used to detect the abuse of commonly prescribed analgesics,13 to determine trends in abuse and misuse of prescription opioids,14 and to characterize the otherwise under-recognized toll of prescription drug abuse in children.12 This is the first RADARS System study to characterize the misuse and abuse of prescription opioids and stimulants in the adolescent population.
Study Population and Variables of Interest
The study data included all intentional exposure calls for prescription opioids and stimulants managed by RADARS System participating poison centers for persons 13 through 19 years old during the initial 2.5-year study period, from the third quarter of 2007, when data on stimulant exposures was first collected, though the fourth quarter of 2009 (Supplement 1, available online.) A call to a poison center is otherwise referred to as an “exposure” or “case.” A case may involve multiple drugs or “mentions.” The analyses described in this study are based on drug mentions. Data were collected over a patient’s course and recorded in a standardized electronic database with fixed data fields. The American Association of Poison Control Centers’ National Poison Data System manual provides standard coding rules and definitions for all data fields. Variables of interest included: geographic location (three-digit ZIP code) of the residence of the exposed individual, location of the call itself, age, number of opioid and stimulants mentioned, intention of exposure (for example, to “get high” or to attempt suicide), number of substances, site of exposure (for example, residence, or school), and medical outcome of the case (no effect, mild, moderate, major, or death). Intentional misuse was defined as an exposure resulting from the intentional improper or incorrect use of a substance for reasons other than the pursuit of a psychotropic effect. This included deliberately increasing a dosage of a medication to enhance its therapeutic effect. Intentional abuse was defined as an exposure resulting from the intentional improper or incorrect use of a substance in which the victim was likely attempting to gain a high, euphoric effect, or some other psychotropic effect, including recreational use of a substance for any effect.
Poison centers employ nurses, pharmacists, physician assistants and physicians to collect data on a standard computerized form. Poison centers submit data weekly to the RADARS System database. Each case then undergoes a rigorous quality control process. There were 45 participating poison centers, which covered 45 states and encompassed 84% of all US poison centers during the period of study. Each poison center obtained institutional review board approval to participate. Additional information about the methods has been reported elsewhere.13
Drugs of interest in this study included the following: methadone, morphine, hydrocodone, hydromorphone, buprenorphine, fentanyl, oxycodone, oxymorphone, buprenorphine tramadol, methylphenidate, and amphetamine. The severity of outcomes was coded by specialists in poison information at local poison centers. Medical outcomes were determined for all intentional exposures. Outcomes were summarized by drug group (opioid versus stimulant) and by specific drug. Unintentional exposures included general unintentional, unintentional therapeutic error, or adverse reaction. Intentional exposures included suspected suicide, intentional misuse, intentional abuse, intentional unknown, or taking the drug to prevent or stop withdrawal symptoms. Smith et al. conducted a study that validated the poison center classifications of intentional exposure cases involving prescription opioids.15
Descriptive statistics were computed to characterize adolescent prescription drug abuse and misuse and to determine proportions of mild, moderate, major outcomes, and death by drug groups. A Spearman rank order correlation was used to determine whether adolescent exposure to prescription drugs related to drug availability in the area, specifically in the individual’s three- digit ZIP code. Spearman rank order was used, as data were not normally distributed. Both SAS version 9.1 and SPSS Version 17.0 statistical software were used.
Unique Recipients of Dispensed Drug Rates
To account for drug availability, we calculated Unique Recipients of Dispensed Drug (URDD) rates. URDD represents the number of unique persons filling a prescription for a particular drug, accounts for drug availability within a community, and can help describe drug-specific adverse consequences of medication availability. URDD rates were calculated for each specific drug as well as drug class. An URDD rate is shown as exposure/1,000 URDD/quarter. To determine URDD rates, prescription data were purchased from Surveillance Data Inc. (SDI) Health LLC.
RESULTS
Of a total of 636,812 mentions of prescription drug exposures reported to RADARS System poison centers between the third quarter 2007 and fourth quarter 2009, 3.3% (21,347) were adolescent exposures to the prescription drugs of interest. Of these adolescent exposures, 22% (4,815) were classified as unintentional and 77% (16,209) as intentional. Intentional unknown were 1% of the exposures, and use for withdrawal were less than 1% of all adolescent exposures. Of all exposures, 38% were classified as suspected suicidal.
In this study, ages were limited to 13 through 19 years. The mean age for intentional exposures was 16.6 years (SD = 1.7 years) and the median age was 17 years (interquartile ratio = 15–18). Males composed 47.5% of mentions and females 52.4% (Table 1). Overall, 68% (10,966 CI) of the intentional exposures were to prescription opioids and 32%(5,243 CI) to prescription stimulants. The mean age of the suspected suicides group was 16.6 (SD = 1.7) and they were predominately female (64.2%).
TABLE 1.
Drug Type Used, by Gender
| Gender | Opioid n (%) | Stimulant n (%) | Total n (%) |
|---|---|---|---|
| Male | 5,085 (46) | 2,618 (50) | 7,731 (48) |
| Female | 5,868 (54) | 2,620 (50) | 8,504 (52) |
| Unknown | 13 (<1) | 1 (<1) | 14 (<1) |
| Total | 10,966 (100) | 5,243 (100) | 16,209 (100) |
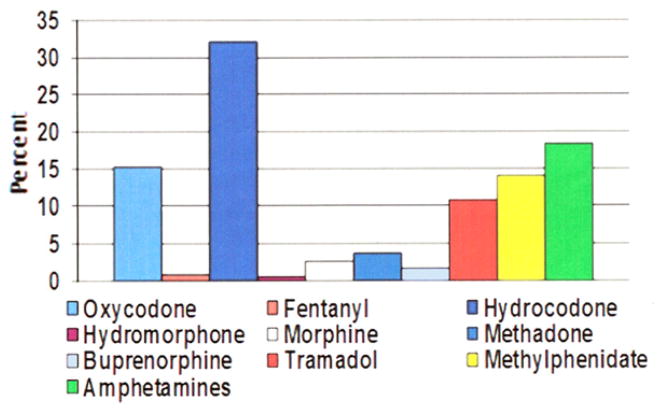
The five most frequently mentioned drugs, accounting for 90% of all intentional adolescent exposures, were hydrocodone (32%, n = 5,232), amphetamines (18%, n = 4,372), oxycodone (15%, n =2,471), methylphenidate (14%, n =2,269), and tramadol (11%, n = 1752) (Figure 1). In all, 46% (n =5,085) of opioid intentional exposures involved males, and 50%(n =2,618) of stimulant intentional exposures involved males (p < .001). The number of substances involved in each case ranged from 1 to 19 substances. A single substance was involved in 48.4% (n = 7,842) of adolescent intentional exposures, whereas more than one substance was involved in 51.6% (n = 8,360) of adolescent intentional exposure in this group.
FIGURE 1.
Proportion of adolescent intentional exposures, by specific drug
When analyzing the location of exposures, we found that the vast majority of intentional exposures occurred in the adolescent’s own home (86.3%, n = 14,032). Other, less common sites of intentional exposure include other residence (3.2%, n = 517) and school (3.5%, n = 355). Of adolescents who intentionally exposed themselves to prescription drugs, 4,830 (29.8%) were treated in a health care facility, nearly half (n = 2,792) of whom were admitted to the hospital, including 1,293 to the intensive care unit. Of the suspected suicidal intention case patients who were followed to completion, 28.5% (n = 918) were treated and released. Of the 2,036 suspected suicidal adolescents admitted to the hospital, 44.4% were admitted to the intensive care unit 20.3% to the medical floor, and 35.3% to a psychiatry service.
Adjusting for drug availability in the exposed individual’s three-digit ZIP code, we found the correlation illustrated in Figure 2. The correlation evaluated monotonic correlation of URDD, a surrogate of drug availability, and number of exposures in a given ZIP code. Other possibilities for denominators include total number of prescriptions or population. In this study, URDD was chosen because drug diversion and appropriate prescription medication storage and use are dependent on a unique individual’s actions. Using URDD as a denominator eliminates refills and repeated prescriptions to an individual, and best represents drug availability through the medical system. Using a Spearman correlation coefficient, there was a statistically significant correlation of 0.44, indicating a weak relationship for drug mentions explained by URDD rate.
FIGURE 2.
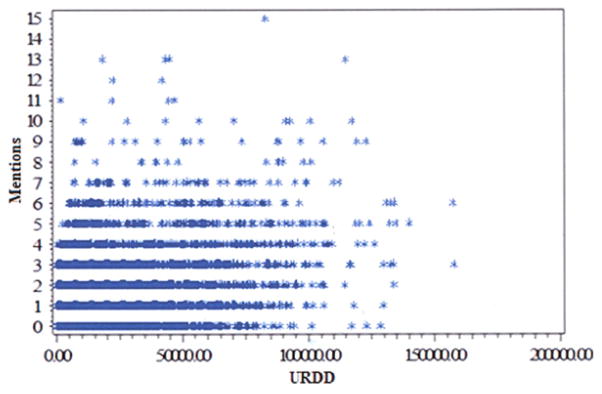
Unique Recipient of Dispensed Drug (URDD) versus drug mentions
When data are adjusted for availability, methadone had the highest URDD rate at 0.149 exposures per 1,000 URDD per quarter. However, we have access only to the number of methadone prescriptions dispensed through a pharmacy, and we do not have access to the methadone dispensed from opioid treatment programs. This is unique to methadone alone and likely falsely elevates the URDD rate for that specific drug. The other specific drugs with the highest URDD rates include methylphenidate at 0.108 exposures per 1,000 URDD per quarter, buprenorphine at 0.105, and amphetamines at 0.090. Fentanyl had the lowest URDD rate at 0.021 exposures per 1,000 URDD per quarter (Table 2).
TABLE 2.
Unique Recipient of Dispensed Drug (URDD) Rates for Specific Drugs
| Specific Drug | Mention Rate/1000 URDD/quarter | 95% CI |
|---|---|---|
| Oxycodone | 0.043 | 0.041–0.045 |
| Fentanyl | 0.021 | 0.017–0.024 |
| Hydrocodone | 0.034 | 0.033–0.035 |
| Hydromorphone | 0.036 | 0.029–0.043 |
| Morphine | 0.060 | 0.054–0.066 |
| Methadone | 0.149 | 0.138–0.161 |
| Buprenorphine | 0.105 | 0.093–0.119 |
| Tramadol | 0.052 | 0.050–0.055 |
| Methylphenidate | 0.108 | 0.104–0.113 |
| Amphetamines | 0.090 | 0.087–0.093 |
Analysis of health effects revealed, overall, the following: 16.9% (n = 2,750) of intentional exposures to prescription stimulants and opioids were not followed to case completion; 17.2%(n =2,785) were associated with no effect, 38.9% (n = 6,292) were associated with minor effects; 23.3% (n = 3,767) were associated with moderate effects, 3.6% (n = 584) were associated with major effects, and 0.1% (n = 20) were associated with death. Table 3 details the medical outcomes by drug group (opioids versus stimulants). Of the suspected suicidal intentional exposure group cases with known medical outcomes, 23.9% (n = 1,688) were associated with no effect, 40.9% (n = 2,891) with minor effects, 30.2% (n = 2,152) with moderate effects, 4.7%(n =336) with major effects, and 0.1% (n = 8) with death.
TABLE 3.
Medical Outcomes by Drug Group (Opioid Versus Stimulant)
| Medical Outcome | Opioid, n (%) | Stimulant, n (%) |
|---|---|---|
| No effect | 1,950 (22) | 835 (18) |
| Minor effect | 4,482 (51) | 1,810 (40) |
| Moderate effect | 1,952 (22) | 1,815 (40) |
| Major effect | 462 (5) | 122 (3) |
| Death | 20 (0.2) | 0 (0) |
| Total | 8,866 (100) | 4,582 (100) |
Note: “Minor effect” denotes that the patient exhibited some symptoms as a result of the exposure, but they were minimally bothersome to the patient. “Moderate effect” denotes that the patient exhibited symptoms as a result of the exposure, which are more pronounced, more prolonged, or more of a systemic nature than minor symptoms. Usually, some form of treatment is or would have been indicated. Symptoms were not life threatening. “Major effect” denotes that the patient has exhibited symptoms as a result of the exposure, which were life threatening or resulted in significant residual disability or disfigurement. “Death” denotes that the patient died as a result of the exposure or as a direct complication of the exposure, when the complication was unlikely to have occurred had the toxic exposure not preceded the complication.
When outcomes were analyzed for each specific drug, oxycodone (n =7, 35%) and methadone (n = 7, 35%) intentional exposures were associated with the most deaths, followed by buprenorphine (n = 3, 15%). No deaths were associated with tramadol, hydromorphone, morphine, methylphenidate, or amphetamine intentional exposures.
DISCUSSION
Characteristics of Adolescent Prescription Drug Abuse and Misuse with Respect to Age, Gender, Intentionality of Exposure, and Number of Substances Involved in Each Case
In terms of characterizing adolescent exposures to prescription drugs, there were several interesting and potentially useful findings that came out of this study. First, in our study of exposures reported to poison centers, more adolescents intentionally exposed themselves to prescription opioids than to prescription stimulants. In the prescription stimulant group, exposures to amphetamines were more common than exposures to methylphenidate.
Stimulant abuse and misuse is on the rise. A self-administered Internet survey of 4,297 adults found a 4.3% past-year prevalence of nonmedical use of ADHD medications in the 18- to 25-year age group, most of whom did not report having their own prescription, meaning that the medications were not their own.16 Another large study, conducted by Wu et al. in 2003, included 24,409 subjects aged 16 to 25 years, and sought to determine gender differences in the prevalence and characteristics of misuse of prescription stimulants. The investigators found that overall prevalence of nonmedical use of prescription stimulants was 5.4% in the 16- to 17-year age group and 5.7% in the 18- to 19-year age group for males, and 5.5% in the 16- to 17-year age group and 6.6% in the 18- to 19-year age group for females. In that study, males who misused prescription stimulants were more likely than their female counterparts to misuse methylphenidate (78% versus 58%) but were less likely to misuse diet pills or amphetamines (14% versus 38%).17 Our study results were not consistent, as we found that intentional exposures to methylphenidate were equally distributed among male and female adolescents. Of our amphetamines misuse and abuse mentions, approximately half of the individuals involved were male. This is also inconsistent with the Wu et al. study, as they found that women misuse and abuse amphetamines at a rate more than twice that of males. It may be that fewer males take amphetamines but that those who do use these drugs take them at higher doses or co-ingest other substances, which may result in more frequent calls to poison centers, compared with females. In addition, their study population included older individuals than our study.
This study examined intentional adolescent exposures to prescription opioids and stimulants. Unintentional exposures, exposures such as therapeutic error (for example, accidentally doubling a dose or taking the wrong medication) comprised 22% of exposures. Unintentional exposures were not of primary interest, as this study focused primarily on misuse and abuse of prescription medications. Of intentional exposures to prescription medications, 49% were classified as suspected suicidal (38% of overall exposures were characterized as suicidal). The remainder were classified as intentional misuse, intentional abuse, intention unknown, or intentional withdrawal.
We chose to include suicide in the analysis of intentional drug exposures for two important reasons. First, it is possible that specialists in poison information who assign intent of exposure reason may overestimate the true suicidal intent. In a study aiming to validate poison center classifications of prescription opioid intentional exposure cases against clinical diagnostic criteria, specialists in poison information classified 50.24% of the intentional exposure cases as suicide, whereas a team of clinicians with expertise in the diagnosis and treatment of drug abuse and addiction classified only 27.84% of cases as suicidal intent. Clinicians were four times as likely to classify a case as “intention unknown” compared with specialists in poison information.14 Our second reason for including “suspected suicide” in our analysis of intentional adolescent exposures to prescription drugs is that suicide and drug abuse and misuse are often co-morbid conditions. In a large review of risk factors for youth suicide, substance abuse was associated with completed suicides (odds ratio [OR] = 5–13) in psychological autopsy studies. Substance abuse was a risk factor for suicide for all ages, but was more strongly associated in an older adolescent group than in a younger adolescent group.18 Suicide is the third leading cause of death among 15- to 24-year olds and accounts for 12.0% of all deaths annually in this age group.19 Furthermore, 6.9% of high school students reported making at least one suicide attempt in the previous 12 months, according to the Youth Risk Behavior Surveillance.20 Our findings of a female predominance (64%) of suicide attempts is consistent with other studies.21,22
Common Locations for Adolescent Exposure to Prescription Drugs
In this study, the site of exposure was the adolescent’s own home the majority of the time. This may have implications in preventing abuse and misuse of prescription drugs. Only rarely were prescription medications reportedly abused or misused at school or another residence. Where the individuals obtained the drugs is not included in our dataset, but it is clear that they are misusing and abusing them in their own homes. The most common means of acquisition of these medications is securing them from friends or family members, so perhaps adolescents are simply taking the substances where they are obtaining them. In addition, it may because there is no odor associated with the use of these substances, versus smoking marijuana or drinking alcohol, that adolescents feel comfortable taking these substances in their homes. However, an intoxicated adolescent found by his or her parents may be more likely to be brought to the attention of a poison center than an adolescent who abuses or misuses prescription medications while out of the home. For this reason, it is possible that the proportion of exposures at home is falsely elevated. It seems that educational efforts could be directed at parents to recognize warning signs of prescription drug abuse or misuse in the home. Parental education about locking up abuse-prone prescription medications can decrease their children’s access to these substances.
Relationship Between Reported Adolescent Exposure to Prescription Drugs and Drug Availability in the Area (as Measured by Drug Prescription Rates in the Individual’s Three-Digit ZIP Code)
We used unique recipient of dispensed drug (URDD; a surrogate for drug availability) as a denominator and drug mentions as a numerator to calculate URDD rate, a surrogate for drug availability in a given three-digit ZIP code. A Spearman correlation coefficient was used to determine whether drug mentions were explained by URDD rate and revealed a weak relationship. URDD rates are based on drug mentions, so it is possible that an individual is included several times, which may inflate the apparent correlation.
The URDD rate for methadone may be artificially high. The URDD is based on pharmacy payment information. All of the prescription drugs of interest are exclusively dispensed by pharmacies with the exception of methadone, which is dispensed both by pharmacies and by opioid treatment programs. Many methadone maintenance treatment program patients are given take-home doses. No data on the number of these patients receiving doses to take home are widely reported. Therefore, there is more availability than that reported, which would artificially decrease the denominator and thus artificially may increase the URDD rate.23
Setlik et al. examined the American Association of Poison Control Data System from 1998 to 2005 and found a sharp increase in stimulant calls, which suggested a rising problem with ADHD stimulant medication abuse (up 76% over the study period). Case severity increased over time, which may be because abuse is rising but may also have been associated with an increase in the prescribing of amphetamine over the study period.24 This suggests that adolescent abuse and misuse of these drugs is continuing to increase over time, likely because of increasing drug availability.
A recent survey study sheds light on the lack of availability of preferred illicit opioids leading to purchase and use of diverted prescription buprenorphine/naloxone tablets. Researchers found two reasons that individuals addicted to opioids report using this substance: first, the inability to obtain the preferred illicit opioid of abuse from a trusted source, and second, lack of sufficient funds to purchase a preferred illicit opioid of abuse.25 In other words, decreased availability of an illicit opioid of choice and the high cost of that substance may drive an individual addicted to opioids to abuse or misuse a less desirable prescription opioid.
Association Between Intentional Exposures to Specific Prescription Drugs and Medical Outcomes, Including Hospitalization, Intensive Care, or Death
We found that prescription drug intentional exposure in the adolescent population resulted in thousands of hospitalizations during the study period. Others have found that persons who abuse or misuse prescription opioids incur higher health care costs and use health resources more than those who do not abuse or misuse prescription opioids.26 Our data suggest that hospitalization and health resource use associated with intentional prescription drug exposure may begin as early as adolescence. Major outcomes and deaths were disproportionately associated with the misuse and abuse of opioids compared with stimulants. Exposures to stimulants were not associated with any deaths in our study.
When one considers that that 68% of exposures in this study were to opioids and 32% were to stimulants, the finding that a large percentage (79%) of major outcomes and all of the deaths were associated with opioid exposures is disproportionate. In the no-effect and minor-effect medical associated outcome groups, the drug type (opioid or stimulant) is proportional to the proportion of exposures. No deaths were associated with tramadol, hydromorphone, morphine, methylphenidate, or amphetamine intentional exposures. In this population, prescription opioids seem much more dangerous, leading to more major associated medical outcomes and deaths when abused or misused than stimulants. There are several possible reasons for this observation. Although the adverse effects associated with stimulants may be severe, the potential for respiratory depression as result of opioid exposure is immediately life threatening. In addition, the opioids most often associated with death in this study are methadone and oxycodone, both of which come in high-dose formulations and thus may increase the risk of respiratory depression. We did not have access to dosing information.
This study is not without limitations. Some limitations are inherent to using poison center data. First, miscoding and missing data may be a problem. This is minimized by thoroughly training poison information specialists and subjecting the data to a rigorous quality control process. Poison center data are limited to voluntary reporting by patients or healthcare providers, and therefore are a likely underrepresentation of the extent of the problem of prescription drug abuse or misuse. Health care providers may be more likely to call for medical direction if the patient is very sick or intoxicated than if the patient appears well. Many recreational exposures to prescription drugs may not cause effects that the patient perceives as immediately harmful, and thus would not seek medical attention or advice. Adolescents may be less likely to call local poison centers for fear of repercussions. We cannot disregard the possibility of diagnostic bias in this study. Case outcomes are assigned in real time by the specialist in poison information during the management of the case. There may be a preconceived idea about what outcome is associated with a particular drug or class of substances. However, the difference in numbers of major effects between opioid and stimulant classes is large, even accounting for opioids comprising 68% of mentions. In addition, in a case of death, inarguably the most important and devastating outcome, there is little to no possibility of a misclassification of outcome.
Future Directions
We are in agreement that “the phenomenon of prescription opioid [and stimulant] abuse requires informed and effective interventions so that the benefits of prescription opioid use continue to outweigh its harms in the long run.”27 The personal cost to an individual that develops a substance abuse disorder is immeasurable. Adolescents with opioid use disorders in treatment have high rates of past-year criminal behaviors and high rates of psychiatric disorders.28 These are problems that can have long-reaching effects on the remainder of a young life. The economic burden of prescription opioid misuse and abuse is large with per-capita annual direct health care costs of nearly nine times more for abusers when compared with non-abusers, and presents a significant societal burden.25,29 In this particular study, we were unable to collect data to assess extent of drug problem of individual adolescent, for example, data on school performance and family behavior.
Prescription Drug Monitoring Programs (PDMPs) have been implemented in several states, with many states planning to implement them in the near future. These programs were created to aid health care providers in ensuring adequate pain treatment for patients while decreasing the epidemic of prescription drug abuse and misuse. PDMPs may decrease the availability of prescription opioids available for diversion. In a preliminary study, intentional exposures to prescription opioids reported to poison centers increased at a significantly lower rate in states with an operating PDMP. It will be important to determine whether implementation of these state programs decreases the abuse and misuse rate in the adolescent population.30
It is our hope that the results of this study will aid in designing focused interventions and educational programs to prevent further prescription drug abuse in this population, which may include school-based screening, legal implications, and Drug Enforcement Administration (DEA) policy. Dr. Schinke31 has successfully determined risk and protective factors for substance use among early adolescent girls and then has used his results to develop a successful computer-delivered, parent-involvement intervention to prevent substance abuse in that subpopulation.32 This model of targeting interventions in adolescents aged 13 through 19 years at prevention and early intervention may save invaluable young lives, as well as lessening the burden of prescription opioid and stimulant abuse and misuse on society. Other potential interventions include educating the individuals receiving prescriptions for opioid and stimulant medications. Labeling the medication, in the package insert or preferably on the bottle itself, with precautions of abuse and misuse, specifically by adolescents in the home, can raise awareness of this problem.
This study identified adolescent intentional exposure to prescription opioids and stimulants associated with thousands of hospitalizations over our study period. Prescription opioid exposures are associated with a greater proportion of deaths and other major outcomes than are stimulant exposures in our adolescent population. It is our hope that the results of this study will aid in designing focused interventions and educational programs to prevent further prescription drug abuse in this population. Research on motivation for abusing and misusing prescription drugs in this population, as well as the development of screening tools, is needed. In addition, limiting availability may help in decreasing abuse and misuse of these substances in the adolescent population.
Acknowledgments
This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, the National Institutes of Health (NIH), through grant UL1RR031973. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Dr. Bartelson served as the statistical expert for this research.
Denver Health is a public non-profit organization providing data to industry, regulatory agencies, and researchers through the Researched Abuse Diversion and Addiction-Related Surveillance (RADARS®) System.
Disclosure: Drs. Zosel, Bartelson, Lowenstein, and Dart, and Ms. Bailey report no biomedical financial interests or potential conflicts of interest.
References
- 1.Schepis TS, Krishnan-Sarin S. Characterizing adolescent prescription misusers: a population-based study. J Am Acad Child Adolesc Psychiatry. 2008;47:745–754. doi: 10.1097/CHI.0b013e318172ef0ld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleeby J. Pharming Parties. 2007 Jan; Available at: http://www.jivemagazine.com/column.php?pid=3999.
- 3.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic Use. Abuse and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13:401–435. [PubMed] [Google Scholar]
- 4.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH publication 07-6202. Bethesda, MD: National Institute on Drug Abuse; May, 2007. Monitoring the Future: National Results on Adolescent Drug Use: Overview of Key Findings, 2006. [Google Scholar]
- 5.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No 10-7583. Bethesda, MD: National Institute on Drug Abuse; May, 2009. Monitoring the Future: National Results on Adolescent Drug Use: Overview of Key Findings, 2008. [Google Scholar]
- 6.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National Results on Adolescent Drug Use: Overview of Key Findings, 2011. Bethesda, MD: National Institute of Drug Abuse; Feb, 2012. [Google Scholar]
- 7.Howard MM, Weiler RM, Haddox JD. Development and reliability of items measuring the nonmedical use of prescription drugs for the Youth Risk Behavior Survey: results from an initial pilot test. J School Health. 2009;79:554–560. doi: 10.1111/j.1746-1561.2009.00448.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyd CJ, McCabe SE, Cranford JA, Young A. Adolescents’ motivations to abuse prescription medications. Pediatrics. 2006;11:2472–2480. doi: 10.1542/peds.2006-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu LT, Ringwalt CL, Mannelli P, Patkar AA. Prescription pain reliever abuse and dependence among adolescents: a nationally representative study. J Am Acad Child Adolesc Psychiatry. 2008;47:1020–1029. doi: 10.1097/CHI.0b013e31817eed4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCabe SE, Boyd CJ, Young A. Medical and nonmedical use of prescription drugs among secondary school students. J Adolesc Health. 2007;40:76–83. doi: 10.1016/j.jadohealth.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd CJ, Teter CJ, West BT, Morales M, McCabe SE. Non-medical use of prescription analgesics: a three-year national longitudinal study. J Addict Dis. 2009;28:232–242. doi: 10.1080/10550880903028452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey JE, Campagna E, Dart RC. The underrecognized toll of prescription opioid abuse on young children. Ann Emerg Medicine. 2009;53:419–424. doi: 10.1016/j.annemergmed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Cicero TJ, Dart RC, Indiardi JA, Woody GE, Schnoll S, Muñoz A. The development of a comprehensive risk-management program for prescription opioid analgesics: researched Abuse, Diversion and Addiction-Related Surveillance (RADARS®) Pain Med. 2007;8:157–170. doi: 10.1111/j.1526-4637.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Spiller H, Lorenz DJ, Bailey EJ, Dart RC. Epidemiological trends in abuse and misuse of prescription opioids. J Addict Dis. 2009;28:130–136. doi: 10.1080/10550880902772431. [DOI] [PubMed] [Google Scholar]
- 15.Smith MY, Dart R, Hughes A, et al. Clinical validation of poison control center intentional exposure cases involving prescription opioids. Am J Drug Alcohol Abuse. 2006;32:465–478. doi: 10.1080/00952990600753982. [DOI] [PubMed] [Google Scholar]
- 16.Novak SP, Kroutil LA, Williams RL, Van Brunt DL. The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy. 2007;2:32. doi: 10.1186/1747-597X-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu LT, Pilowsky DJ, Schlenger WE, Galvin DM. Misuse of methamphetamine and prescription stimulants among youths and young adults in the community. Drug Alcohol Depend. 2007;89:195–205. doi: 10.1016/j.drugalcdep.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelkonen M, Marttunen M. Child and adolescent suicide: epidemiology, risk factors, and approaches to prevention. Paediatr Drugs. 2003;5:243–265. doi: 10.2165/00128072-200305040-00004. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Web-based Injury Statistics Query and Reporting System (WISQARS) National Center for Injury Prevention and Control, CDC; 2007. Available at: www.cdc.gov/injury/wisqars/index.html. [Google Scholar]
- 20.Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance—United States, 2007. MMWR Surveill Summ. 2008;57:1–131. [PubMed] [Google Scholar]
- 21.Fried LE, Williams S, Cabral H, Hacker K. Differences in risk factors for suicide attempts among 9th and 11th grade youth: a longitudinal perspective [published online September 24] J Sch Nurs. 2012 doi: 10.1177/1059840512461010. [DOI] [PubMed] [Google Scholar]
- 22.Nrugham L, Holen A, Sund AM. Suicide attempters and repeaters: depression and coping: a prospective study of early adolescents followed up as young adults. J Nerv Ment Dis. 2012;200:197–203. doi: 10.1097/NMD.0b013e318247c914. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta N, Bailey EJ, Cicero T, et al. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010;11:1078–1091. doi: 10.1111/j.1526-4637.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- 24.Setlik J, Bond GR, Ho M. Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics. 2009;124:875–880. doi: 10.1542/peds.2008-0931. [DOI] [PubMed] [Google Scholar]
- 25.Monte AA, Mandell T, Wilford BB, Tennyson J, Boyer EW. Diversion of buprenorphine/naloxone co-formulated tablets in a region with high prescribing prevalence. J Addict Dis. 2009;28:226–231. doi: 10.1080/10550880903014767. [DOI] [PubMed] [Google Scholar]
- 26.Strassels S. Economic burden of prescription opioid misuse and abuse. J Managed care pharmacy. 2009 Sep;15:556–562. doi: 10.18553/jmcp.2009.15.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer B, Rehm J. Illicit opioid use in the 21st century: witnessing a paradigm shift? Addiction. 2007;102:499–501. doi: 10.1111/j.1360-0443.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam GA, Stitzer MA. Clinical characteristics of treatment-seeking prescription opioid versus heroin using adolescents with opioid use disorder. Drug Alcohol Depend. 2009;101:13–19. doi: 10.1016/j.drugalcdep.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruetsch C. Empirical view of opioid dependence. J Manage Care Pharm. 2010;16:S9–S13. doi: 10.18553/jmcp.2010.16.S1-B.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434–442. doi: 10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- 31.Schinke SP, Fang L, Cole KC. Substance use among early adolescent girls: risk and protective factors. J Adolesc Health. 2008;43:191–194. doi: 10.1016/j.jadohealth.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schinke SP, Fang L. Computer-delivered KC parent-involvement intervention to prevent substance use among adolescent girls. Prev Med. 2009;495:429–435. doi: 10.1016/j.ypmed.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]