Abstract
Excess production of hyaluronan (hyaluronic acid [HA]) in the retro-orbital space is a major component of Graves' ophthalmopathy, and regulation of HA production by orbital cells is a major research area. In most previous studies, HA was measured by ELISAs that used HA-binding proteins for detection and rooster comb HA as standards. We show that the binding efficiency of HA-binding protein in the ELISA is a function of HA polymer size. Using gel electrophoresis, we show that HA secreted from orbital cells is primarily comprised of polymers more than 500 000. We modified a commercially available ELISA by using 1 million molecular weight HA as standard to accurately measure HA of this size. We demonstrated that IL-1β-stimulated HA secretion is at least 2-fold greater than previously reported, and activation of the TSH receptor by an activating antibody M22 from a patient with Graves' disease led to more than 3-fold increase in HA production in both fibroblasts/preadipocytes and adipocytes. These effects were not consistently detected with the commercial ELISA using rooster comb HA as standard and suggest that fibroblasts/preadipocytes may play a more prominent role in HA remodeling in Graves' ophthalmopathy than previously appreciated.
Graves' ophthalmopathy (GO) is an autoimmune disorder in which the pathogenesis appears to involve binding of autoantibodies to TSH receptors (TSHRs) on cells in the retro-orbital space leading to tissue remodeling that may lead to optic nerve compression (1). According to histologic studies, retro-orbital tissue from GO patients is characterized by interstitial edema and hyaluronan (hyaluronic acid [HA]) accumulation (2), which is the likely source of tissue swelling and proptosis, and appears to be responsible for the major signs and symptoms in severe GO.
The mechanism through which Graves' autoantibodies (Graves' disease [GD]-IgG) initiate remodeling in such a specific, nonthyroid tissue is still a subject of debate. In the current model for GO pathogenesis, GD-IgGs are suggested to target fibroblasts in the retro-orbital tissue (1). Although several ideas exist for what happens after antibody binding, the most straightforward hypothesis is that autoantibody binding to TSHR on orbital fibroblasts induces differentiation into adipocytes (adipogenesis), which causes adipose tissue expansion, increased TSHR expression, and HA production. Orbital fibroblasts from patients with GO (GOFs) in culture are capable of undergoing differentiation into adipocytes when cultured in certain mediums and are therefore considered preadipocytes. TSHR stimulation with activating antibodies or TSH has been shown to up-regulate adipogenic markers in GOFs (3) and other cell types (4, 5). After differentiation, GOFs demonstrate greater stimulation of HA production as well as increased expression of HA synthase (HAS) genes upon TSHR activation (6–8). However, increased HA production could come from fibroblasts/preadipocytes or adipocytes, or both.
Difficulties in delineating GO pathogenesis stem from the fact that TSHR stimulation of undifferentiated GOFs in vitro does not produce a consistent HA response. Studies using commercial ELISAs to measure HA production showed only small changes in HA secretion from undifferentiated compared with adipocyte-differentiated GOFs (Adipo-GOFs) (9, 10). Pharmacologic stimulation of TSHR signaling pathways was shown to increase HA yield and HAS expression in undifferentiated GOFs but not with TSH treatment (10, 11). Transfection of constitutively active TSHR into GOFs led to significant increases in HA secretion and up-regulation of HAS isoforms, but in these experiments, signaling pathways were artificially robustly induced (7). Under certain conditions, the GD-IgG activating monoclonal antibody from a patient with GD (M22) and IL-1β both moderately increased HA in undifferentiated GOFs. However, changes were 2-fold at most (12, 13). In contrast, studies that measured HA synthesis using radiolabeled precursor incorporation detected higher-fold increases in HA production in response to IL-1β (14, 15). Although HA levels measured in ELISAs are the result of a steady state between synthesis and degradation, the difference in measurements between the 2 methods suggests that the commercially available ELISAs may underestimate HA production.
The physiochemical properties of HA greatly differ from most factors usually studied in ELISAs. HA is a linear, highly anionic, nonsulfated polysaccharide found in the extracellular matrix. HA exists in different lengths, depending on its tissue source and disease state. HA from amniotic membrane extracts has an average molecular weight of 3 million (16), whereas HA from rooster comb is reported to range from 800 to 1000 (17–19). In vitro, cultured synovial membranes produce polydisperse HA mixtures ranging in size from 5000 to more than 3 million (20, 21), and HA polymers secreted from myofibroblasts are usually more than 500 000 (22, 23). However, the most commonly applied, commercially available ELISAs (24) used to measure HA concentration do not take HA size into account nor consider whether differences in size profiles between samples have any effect on concentration measurements. In these assays, HA concentration is dependent on binding to an HA-binding protein (HABP). The efficiency of binding of HAs of various polymer sizes to HABP, however, has not been considered in the GO field. Previous experiments, in which radiolabeled precursor incorporation into HA was measured, differences in size are not taken into account when determining synthetic rate. Thus, determining the size distribution of HA secreted by orbital fibroblasts is not only important to interpreting HA assays but also may be an overlooked aspect in GO pathogenesis.
Using an HABP-independent method to measure HA from GOFs in vitro, we show that HA secreted from GOFs has a markedly different size distribution than the rooster comb HA used as standards in commercial ELISAs. Furthermore, HA polymer size affects its binding to HABP. Because of these differences, HA ELISAs underestimate changes in HA secretion from GOFs. Based on data from our HABP-independent studies, we used an HA ELISA with a 1 million molecular weight HA standard that accurately measures HA concentrations in our system and demonstrated a greater effect of IL-1β that was unique to GOF preadipocytes. Most importantly, we showed that TSHR stimulation by both TSH and monoclonal-activating antibody M22 increases HA secretion in undifferentiated GOFs. These changes were obscured using rooster comb HA standard provided in commercial ELISAs and suggest that the importance of TSHR signaling pathways in preadipocyte GOFs has been undervalued in the pathogenesis of GO.
Materials and Methods
Primary orbital fibroblast cell culture
Primary orbital fibroblasts from Graves' and non-Graves' patients were generously provided by Rebecca Bahn (Mayo Clinic, Rochester, Minnesota) and harvested as previously described (8). Fibroblast strains were obtained from 4 Graves' patients undergoing orbital decompression surgery (GOFs) and 2 patients with no history of Graves' disease (normal orbital fibroblasts, NOFs). Use of these samples was approved by the Mayo Clinic Institutional Review Board, and studies were carried out according to the Institutional Review Board guidelines. Thawed cells were propagated in growth medium (DMEM, 4.0mM L-glutamine and sodium pyruvate, 10% fetal bovine serum (FBS) and 1% penicillin/gentamicin solution), in a humidified 10% CO2 incubator at 37°C. Differentiation was induced in confluent fibroblast cultures with adipogenic medium (DMEM, 4.0mM L-glutamine and sodium pyruvate, 10% FBS, 1% penicillin/gentamicin solution, 0.1mM indomethacin, 0.1μM dexamethasone, 0.5mM 3-isobutyl-1-methylxanthine, and 10-μg/mL insulin). Adipogenesis was confirmed as the level of adiponectin increased more than 106-fold (25). For experiments, cell passage was no greater than passage 10.
HA induction
GOFs and NOFs were grown to confluence in 12-well plates in growth medium until proliferation was arrested through contact inhibition, verified visually by phase-contrast microscopy when less than 1% of cells were seen dividing. This ensured an equal number of cells per well during the course of the experiment. Cells were treated with growth medium without or with IL-1β (10 ng/mL; Sigma), activating TSHR antibody M22 (100 ng/mL; Kronos), or bovine TSH (100 mU/mL; Sigma). Adipocytes were pretreated 3 days and then switched to adipogenic medium without or with IL-1β, M22, or TSH and differentiated 7 days. Preadipocytes were treated for a total of 10 days to match the treatment time of adipocytes. Medium was replaced every 3–4 days, and conditioned media were collected, combined, and stored at 4°C overnight or −20°C for long-term storage.
For TSHR inhibition experiments, TSHR antagonist National Institutes of Health Chemical Genomics Center number 00229600 (TSHR-ant) was synthesized by the National Center for Advancing Translational Science, National Institutes of Health as previously reported (26). GOF preadipocytes were pretreated with TSHR-ant (10μM) for 24 hours. Cells were treated with bovine TSH (100 mU/mL; Sigma) or M22 (100 ng/mL) in growth medium without or with TSHR-ant (10μM). Medium was refreshed after 2 days, and total treatment time was 4 days.
For HA time-course experiments, GOFs were grown to confluence in growth medium, then starved for 1 day in low-serum medium containing 1% FBS. Cells were treated with hyaluronidase from Streptomyces hyalurolyticus (1 U/mL; Sigma) in Hanks' balanced salt solution for 1 hour at 37°C to remove existing HA. After several washes with Hanks' balanced salt solution, cells were switched to low-serum medium with IL-1β (10 ng/mL). Conditioned media were collected and stored at −20°C.
HA polyacrylamide gel electrophoresis (PAGE)
Conditioned media were filter concentrated 5 times (Corning Spin-X UF, MWCO 5,000). To enrich for HA, conditioned media were digested with proteinase K (50 μg/mL; Roche) for 4 hours at 55°C followed by boiling for 10 minutes for enzyme inactivation. Negative controls were produced by treating sample aliquots with hyaluronidase for 16 hours at 37°C. A total of 20 μL of sample was mixed with 5-μL loading buffer (Tris/Borate/EDTA [TBE], 0.02% bromophenol blue and 2M sucrose). Select-HA LoLadder and HiLadder (Sigma) were used as molecular weight standards. HA was separated on 3%–12% Bis-Tris NativePAGE gels (10 well, 1 mm thick; Life Technologies) in and X-cell SureLock Mini cell apparatus. Outer and inner buffer chambers were filled with precooled TBE. Gels were run at 300 V for 1 hour, rinsed in water, then transferred to staining solution (0.005% Stains-All [Sigma], 50% ethanol, 50% water) in the dark overnight. Gels were destained in water and imaged on a flatbed scanner. Densitometry analysis was done using ImageJ.
HA/HABP blotting
HA samples were separated by PAGE as described above. After electrophoresis, gels were soaked in freshly prepared HA depolymerization buffer (0.2mM cupric sulfate and 2mM ascorbic acid in TBE) for 30 minutes at room temperature with shaking, then washed 3 times for 10 minutes in TBE. HA was transferred to nylon membranes in 0.5× TBE at constant 100 mA overnight at 4°C in a Bio-Rad Mini Trans-Blot apparatus. Immediately after transfer, membranes were blocked with Odyssey blocking buffer (LiCor) containing 1% sodium dodecyl sulfate for 1 hour at room temperature. To detect HA, membranes were incubated with 5-mg/mL biotin-HABP (Calbiochem) in Odyssey blocking buffer plus sodium dodecyl sulfate overnight. Blots were washed 2 times with 1× phosphate-buffered saline/Tween 20 (0.1% Tween 20), rinsed with PBS, and then incubated in the dark with diluted IRDye 800CW Streptavidin (1:10 000; LiCor) for 30 minutes. Blots were imaged on a LiCor Odyssey CLX.
Enzyme-linked immunosorbent assay
A commercial ELISA was obtained from Corgenix and run according to the manufacturer's instructions with 1 exception. In addition to the supplied standards that were diluted 1:10, the 500- and 800-ng/mL rooster comb standards were diluted 1:5 to produce 1000- and 1600-ng/mL standards, respectively. Binding of different sized HA (Lifecore) to HABP was measured using the ELISA platform but substituting various HAs of the following HA preparations: 20 000 (actual 28 600), 200 000 (actual 215 000), and 1 million (actual 1.01 million). We analyzed rooster comb HA (lot 095K3786; Sigma) and found the size range to be from 50 000 to 500 000. Modified ELISAs for measurement of HA secretion were based on the Corgenix kit, with the rooster comb standard replaced by freshly prepared 1 million molecular weight HA.
Statistical analyses
Significance was determined using Student's t test.
Results
Cultured orbital fibroblasts secrete primarily high molecular weight (HMW) HA
A modified PAGE technique was used to assess the size distribution of HA from GOFs and NOFs and from these cells after differentiation to adipocytes (Adipo-GOF and Adipo-NOF, respectively).
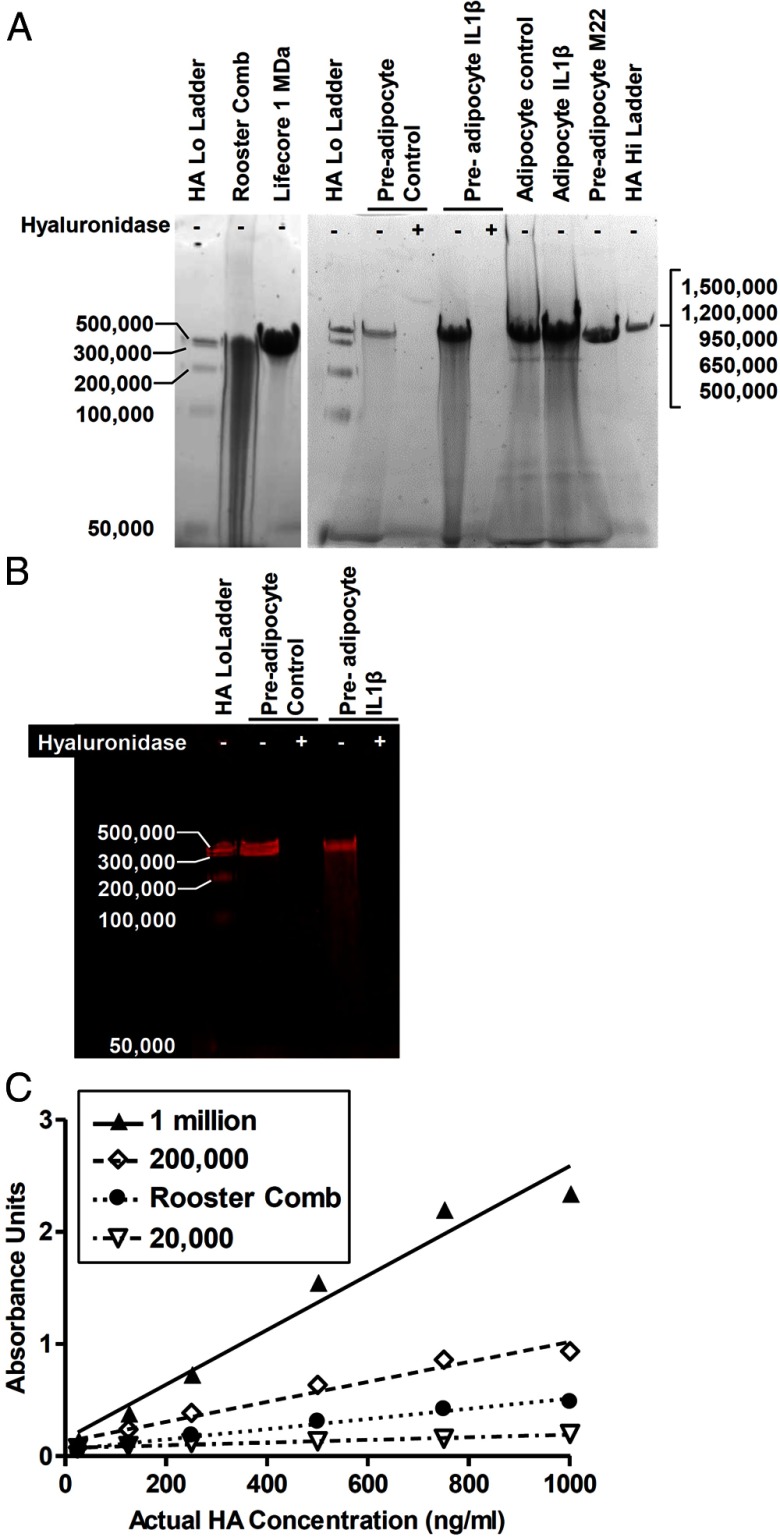
Previous HA electrophoresis experiments used polyacrylamide gels to resolve low molecular weight (LMW) HA, ranging from 5000 to 500 000, and agarose gels to separate HMW HA, 500 000 and above. Here, 3%–12% NativePAGE polyacrylamide gels were used to visualize both LMW and HMW HA on the same gel. Individual bands, ranging from 50 000 to 500 000 in the Select-HA LoLadder, were resolved, whereas HA from the Select-HA HiLadder migrated as a single band with a mobility similar to that of 500 000 HA (Figure 1A). When visualized with Stains-All, the amount of HA polymers greater than 500 000 can be analyzed by the intensity in the single band, because the density of the band was found to be proportional to the amount of HA from 125 to 4000 ng (slope = 3.3 ± 0.2 arbitrary units/ng; R2 = 0.98).
Figure 1.
PAGE of HAs and HA-HABP interaction. A, Representative PAGE. HA samples were run on a 3%–12% NativePAGE gel and visualized with Stains-All. The Select-HA LoLadder in lanes 1 and 4 contains equal masses of 5 HA polymers ranging from 50 000 to 500 000 in size, which were separated as 4–5 distinct bands. Rooster comb HA (lane 2) was a polydisperse mixture with a significant LMW component. A HA standard of 1 million in size (Lifecore 1 MDa) is shown in lane 3. HA secreted from orbital fibroblasts migrated as a single band with mobility similar to 1 million MW HA; size distribution showed little change with treatment of IL-1β, M22, or differentiation. Hyaluronidase controls indicate bands were primarily HA; hyaluronidase treatment (+) of the lysates before electrophoresis degraded the band compared with untreated lysates (−). The Select-HA HiLadder in lane 12, comprised of equal masses of HA polymers sized 500 000 to 1.5 million, migrated as a single band. B, HA from undifferentiated GOFs (preadipocytes) was depolymerized and transferred to a nylon membrane. Degradation by hyaluronidase and reactivity with biotin-HABP confirmed that PAGE bands were virtually entirely HA. HABP signal was brightest for HMW bands even though equal masses of HAs of different sizes were applied. C, Purified 20 000, 200 000, and 1 million MW HA polymers and rooster comb HA were processed in a commercial HA ELISA. The HA-HABP interaction was determined by the colorimetric signal and demonstrates that, for a given mass of HA, HABP signal is greater for larger polymers. A mixture of lower size HAs, such as rooster comb, results in a signal much lower than the HMW HA seen in fibroblast samples.
HA was purified from conditioned media collected from orbital fibroblasts, and the size distribution of cell-secreted HA was compared with HA from rooster comb extracts and purified HA of various sizes. Rooster comb HA was shown to be a polydisperse mixture with a significant proportion of LMW polymers and apparent sizes ranging from 50 000 to 500 000 (Figure 1A). In contrast, HA from orbital fibroblasts migrated as single HMW bands (Figure 1A) with a similar mobility to purified 1 million molecular weight (MW) HA (Figure 1A, lane 3). Hyaluronidase treatment confirmed that HA was the dominant species in those bands (Figure 1A), and an HA/HABP blot demonstrated binding of HABP to these bands that was abolished by hyaluronidase treatment (Figure 1B).
HABP preferentially binds HMW HA
We found that HAs of different sizes bind differently to a fixed amount of HABP as is present in commercial kits (Figure 1C); 20 000 MW HA bound very poorly. Rooster comb HA bound less than the purified 200 000 HA, which bound moderately well. Most importantly, 1 million MW HA, which was most similar in size to cell-secreted HA, bound most efficiently to the HABP. Thus, it is clear that in order to accurately measure HA, a standard for the ELISA must be of a similar size to the predominant HA in the sample, and using rooster comb HA, which is comprised of a broad range of HA sizes that are less than 500 000 MW, would not accurately measure HA secreted by orbital fibroblasts and adipocytes. We, therefore, used a modified ELISA, in which the standard was 1 million MW HA for all our measurements.
IL-1β regulates HA secretion differently in GOFs and NOFs
Because IL-1β has been shown to regulate HA secretion in a number of reports (14, 15), we decided to confirm the accuracy and sensitivity of the modified ELISA using undifferentiated GOFs, which secreted less HA than Adipo-GOFs (see below). Because optimal adipogenesis requires cells to be cell cycle-arrested at confluence, to compare cells under similar conditions, we compared HA secretion from undifferentiated and differentiated cells at confluence. HA electrophoresis on 3%–12% NativePAGE gels was used as an HABP-independent assay to determine the effects of IL-1β treatment on HA secretion. According to densitometry results from multiple GOF strains, total HA in the conditioned media was on average 6.7-fold greater than controls after 10 days of continuous IL-1β treatment, which was similar to that measured by the modified ELISA (see below) but was several fold higher than that detected using the commercial ELISA (1.3-fold). PAGE also showed that the size distribution of HA secreted by GOFs under several culture conditions (Figure 1A), including IL-1β, did not change and was predominantly HMW.
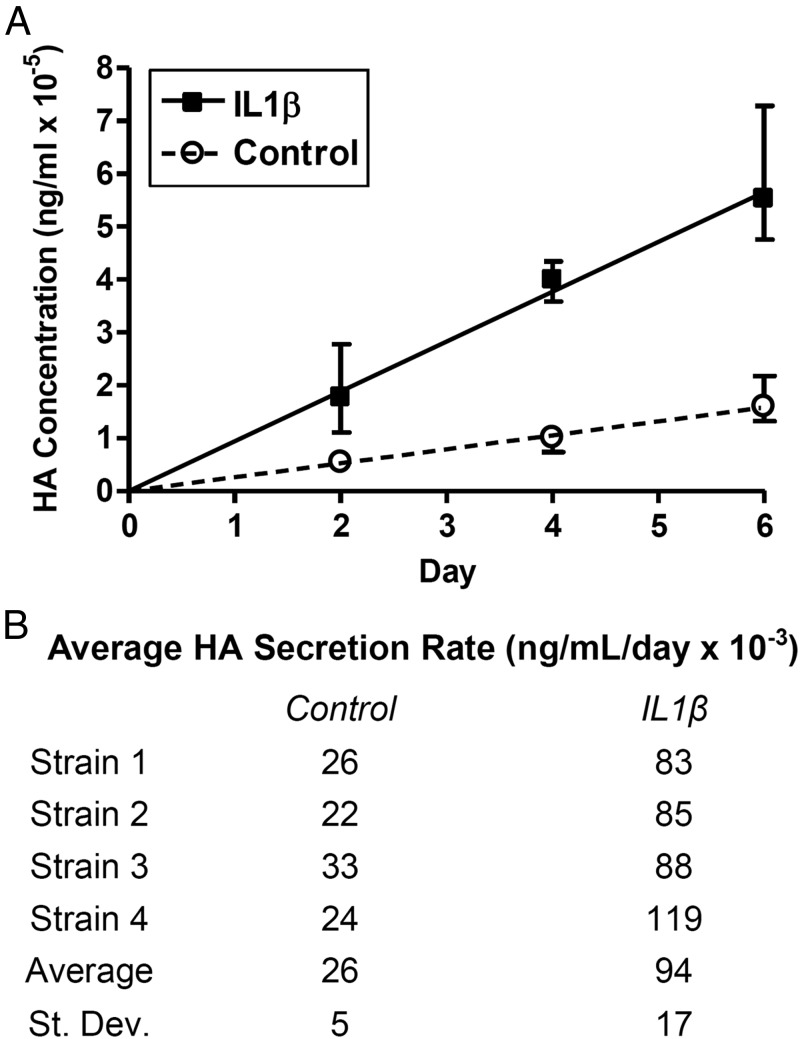
In order to make more accurate and sensitive measurements of multiple samples, all further HA measurements were performed using the modified ELISA. Because the HA secreted by orbital fibroblasts under all the culture conditions explored in this study migrated with an apparent size between 500 000 and 1.5 million, we used a 1 million MW HA as standard in the modified ELISA. In contrast to the commercial ELISA, the modified ELISA measured significant increases in HA secretion with IL-1β treatment of GOF preadipocytes after as early as 2 days (Figure 2A). Total HA levels increased at constant rates under both control and IL-1β-stimulated culture conditions, but cells treated with IL-1β secreted HA at a faster rate. Over 6 days, the average rate of HA secretion for the 4 strains was 3.6-fold greater in GOFs treated with IL-1β than control media (Figure 2B) and was even higher when additional GOFs were studied (7.8-fold) (Figure 3).
Figure 2.
Time course of HA secretion by undifferentiated GOFs. Serum-starved, hyaluronidase-treated undifferentiated GOFs from 4 strains were cultured with or without IL-1β for 2, 4, and 6 days. Total HA from conditioned media was measured using the modified ELISA. A, HA increased linearly over time in both conditions, and the slope, defined as average HA secretion rate, was greater after IL-1β treatment. The lines were forced through the origin, and error bars indicate SD of data from 4 samples. B, Average secretion rate for individual strains show evidence of strain-to-strain variation.
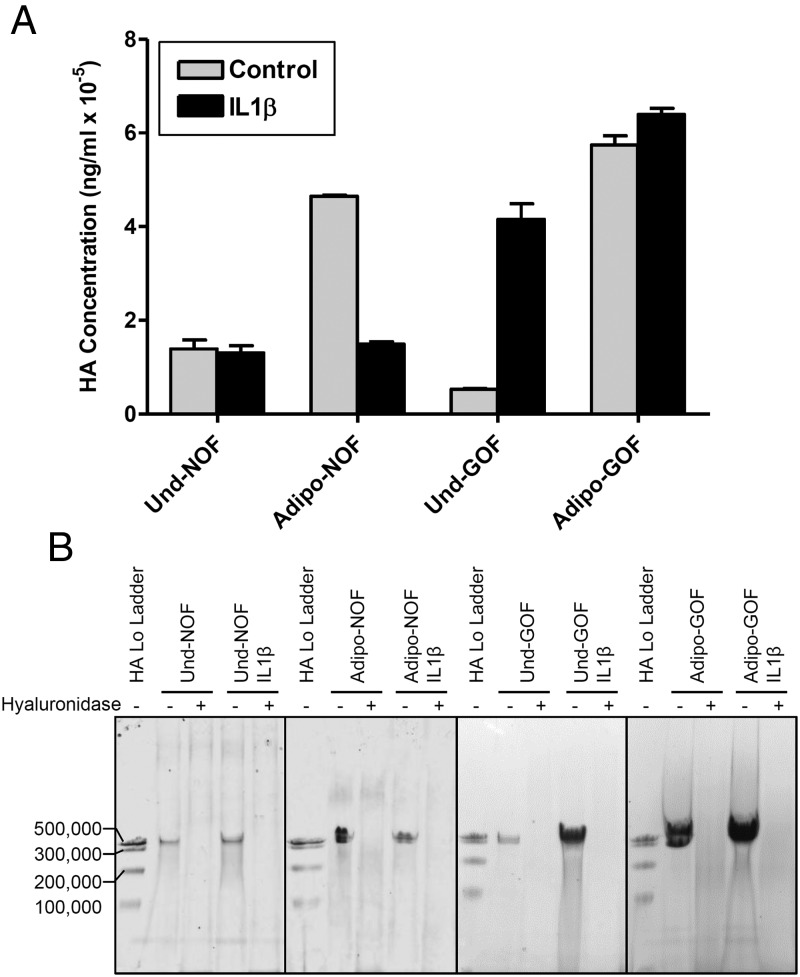
Figure 3.
IL-1β regulation of HA secretion by NOFs and GOFs. A, Undifferentiated NOFs (Und-NOF), Adipo-NOFs, undifferentiated GOFs (Und-GOF), or Adipo-GOFs were treated with IL-1β for 10 days, and secreted HA was measured with the modified ELISA. GOF samples consisted of combined conditioned media from all 4 GOF strains. NOF samples consist of combined conditioned media from 2 NOF strains. The bars represent the mean ± range of duplicate samples run in the same assay. P values for each sample were as follows: Und-NOF, more than 0.05; Adipo-NOF, less than 0.05; Und-GOF, less than 0.05; and Adipo-GOF, more than 0.05. B, Representative PAGE gels show conditioned media from NOFs and GOFs next to their hyaluronidase controls. Both cell types secreted HA polymers more than or equal to 500 000 MW before and after adipogenesis. Treatment with IL-1β did not result in a significant amount of LMW HA, indicating that significant HA degradation did not take place.
We next compared the effects of IL-1β on HA secretion by undifferentiated GOFs, Adipo-GOFs, undifferentiated NOFs, and Adipo-NOFs (Figure 3). NOFs secreted HA at a level higher than GOFs but were not affected by IL-1β treatment in contrast to stimulation found with GOFs. Differentiation to adipocytes increased HA secretion by NOFs (3.3-fold) and GOFs (12.8-fold), but IL-1β decreased HA secretion by differentiated NOFs, whereas it had little effect in differentiated GOFs.
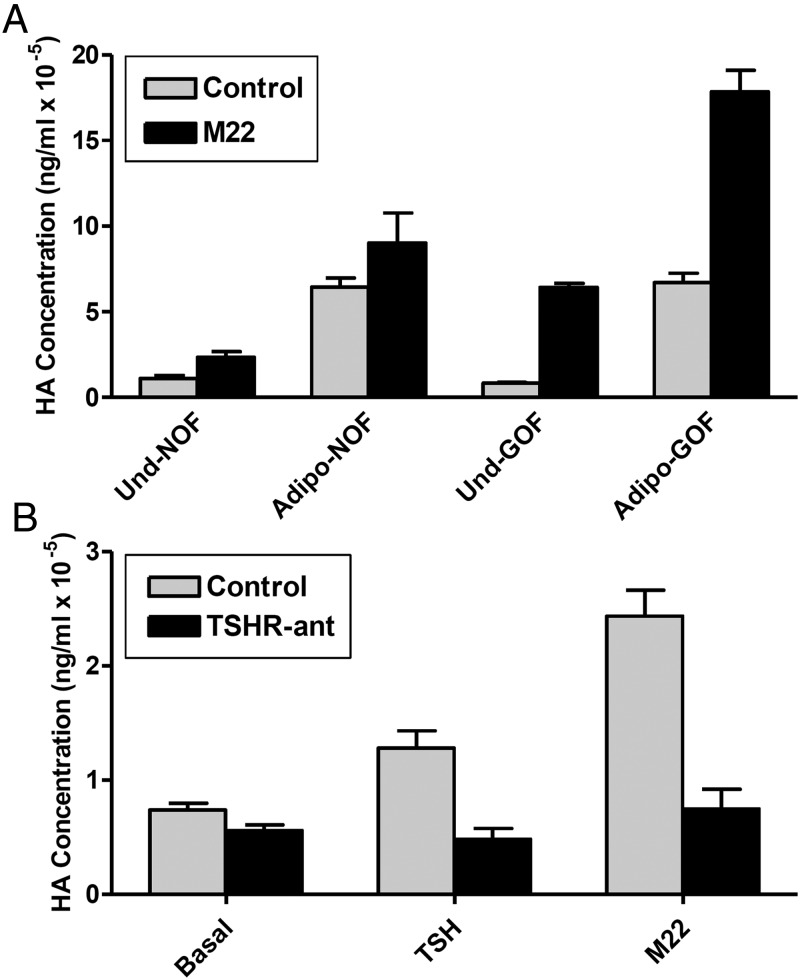
TSHR activation is sufficient for HA induction in GOFs
TSHR activation by the monoclonal antibody M22 stimulated HA secretion to a much greater extent in GOFs than NOFs (Figure 4A). Undifferentiated NOFs (1.8-fold) and Adipo-NOFs (1.5-fold) exhibited small increases in HA secretion in response to exposure to TSHR-activating antibody M22. In contrast, M22 stimulated HA secretion by undifferentiated GOFs and Adipo-GOFs by 7.6- and 2.4-fold, respectively. Of note, the fold increase in Adipo-GOFs was from an elevated control level.
Figure 4.
TSHR regulation of HA secretion in GOFs and NOFs. A, Undifferentiated NOFs (strain 1) (Und-NOF), Adipo-NOFs (strain 1), undifferentiated GOFs (strain 4) (Und-GOF), or Adipo-GOFs (strain 4) (Adipo-GOF) were treated with M22 for 10 days. Only GOFs showed significantly increased HA with M22, and fold induction was greater in GOF preadipocytes than adipocytes (7.5 and 2.7, respectively). The bars represent the mean ± SD of 3 biological replicates of each representative OF strain. P values for each sample were as follows: Und-NOF, 0.05; Adipo-NOF, more than 0.05; Und-GOF, less than 0.05; and Adipo-GOF, less than 0.05. B, Und-GOFs were treated with TSH or M22 without or with 10μM TSHR antagonist for 4 days. TSH induction was 50% of M22 induction, and TSHR antagonist inhibited both TSH and M22 HA induction to basal levels. The bars represent the mean ± range of 2 biological replicates of GOF strain 4. Basal, P > .05; TSH, P < .05; and M2, P < .05.
We used an antagonist of TSHR activation (25, 26) to determine whether the stimulation of HA secretion caused by M22 was mediated by TSHR, because it has been suggested that M22 may have other receptor targets (10–12, 27). TSH alone stimulated HA secretion in undifferentiated GOFs as soon as 4 days. However, HA levels only reached 50% of what was found with M22 (Figure 4B). The effects of TSH and M22 were completely inhibited by the TSHR antagonist.
Discussion
Preferential binding of HABP to HMW HA
Unlike other chemical reactions involving large macromolecules, an HA polymer is equally reactive along its entire surface, and the kinetic assumptions used to design traditional ELISAs would not apply. In a mixture of HA polymers, overall, HABP signal would be the result of some sort of averaged binding, dominated by the signal from larger polymers. The polymer size and polydispersity of rooster comb HA will vary lot to lot depending on the age, breed, and hormonal state of the rooster and method of extraction. Obtaining these details about the HA supplied in commercial HA ELISA kits is difficult. We found that commercially acquired rooster comb HA contained a significant LMW component despite supplier claims. This rooster comb HA sample likely had a similar polydispersity to the HA ELISA standard, because its concentration measurements were virtually 1:1 with actual concentration. The LMW HA extracted from rooster comb bound less HABP than the same mass unit of HMW HA. Therefore, ELISAs on HA from orbital fibroblasts would follow a pattern akin to 1 million MW HA, where concentration would be overestimated at low concentrations and underestimated at high ones. Analyzing the size distribution of samples and then using the appropriately sized HA as standard revealed major errors in the measurements made using currently available ELISAs with rooster comb HA as standard. These findings led us to reexamine some basic hypotheses in GO pathogenesis.
NOFs differ from GOFs in their regulation of HA secretion by IL-1β
Both NOFs and GOFs exhibited marked increases in HA secretion when differentiated into adipocytes. One of the unexpected outcomes was the absence of a stimulatory response to IL-1β in undifferentiated NOFs compared with the robust response in undifferentiated GOFs. In addition, IL-1β inhibited HA secretion in differentiated NOFs, whereas it had little effect in differentiated GOFs. The reason(s) for these differences is not apparent at the present time. The greater effect of IL-1β in GOF preadipocytes compared with adipocytes confirm the findings in a previous report (28) that undifferentiated GOFs have a robust response to IL-1β. Under all conditions we tested, IL-1β did not change HA size distribution. In our system, HA degradation apparently did not occur, because no LMW HA was observed. Our finding of no HA degradation in GOF cultures supports the conclusion of a previous study (29).
TSHR's role in matrix remodeling may begin before GOF differentiation
We found that the ability of TSHR activation to stimulate HA secretion in undifferentiated GOFs is much greater than previously thought. We cannot distinguish whether HA induction is the result of increased synthase expression/activity or decreased hyaluronidase expression/activity. Both undifferentiated GOFs and NOFs responded to M22, although GOFs responded to a greater degree. Undifferentiated GOFs have been reported to exhibit higher levels of TSHR expression compared with NOFs (8, 30–33) that may account for the amplified effect of M22 in GOFs. Both cell types are expected to up-regulate TSHR expression with adipogenesis (34–36), and this may account for the greater effects of M22 in orbital adipocytes compared with undifferentiated fibroblasts. In some previous studies, TSHR activation in undifferentiated GOFs by TSH was found not to increase HA secretion (10, 12). The authors concluded that TSHR activation was not a primary pathway for HA secretion and that signaling by M22 must occur through a different receptor. Our data do not exclude effects by M22 being mediated by other receptors, but, as we have shown previously (37), they do demonstrate that TSHR activation alone (by TSH) is capable of stimulating HA secretion. Moreover, the findings that the specific TSHR antagonist fully inhibited HA secretion stimulated by TSH and M22 suggest that TSHR is the major target of M22 with regard to HA secretion.
In conclusion, we have shown that measurement of HA levels by assays that use HABP to bind HA are dependent on the size(s) of HA in the sample and in the standards. We suggest that before using an HABP-dependent ELISA, it is necessary to determine the size of the predominant HA polymer in the samples and then to use a similarly sized purified HA as standard. Using this approach, we have been able to accurately and sensitively measure HA levels in the conditioned mediums from orbital fibroblasts and adipocytes in culture. Our confirmation of previous data by Kumar et al (12) that TSHR activation can lead to increased HA secretion from undifferentiated GOFs suggests that fibroblasts and adipocytes in the orbital tissue of patients with Graves' disease may be responsible for the matrix remodeling found in GO. Moreover, these data are consistent with the idea that matrix remodeling may occur early in the pathogenesis of GO before any increase in adipocyte number.
Acknowledgments
We thank Dr Rebecca Bahn for providing the Graves' and non-Graves' orbital fibroblasts used in this study.
This work was supported by the National Institutes of Health Intramural Research Program 1Z01 K011006.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Adipo-GOF
- adipocyte-differentiated GOF
- Adipo-NOF
- adipocyte-differentiated NOF
- FBS
- fetal bovine serum
- GD
- Graves' disease
- GO
- Graves' ophthalmopathy
- GOF
- orbital fibroblast from patients with GO
- HA
- hyaluronic acid
- HABP
- HA-binding protein
- HAS
- HA synthase
- HMW
- high molecular weight
- LMW
- low molecular weight
- M22
- activating monoclonal antibody from a patient with GD
- MW
- molecular weight
- NOF
- normal orbital fibroblast
- PAGE
- polyacrylamide gel electrophoresis
- TBE
- Tris/Borate/EDTA
- TSHR
- TSH receptor
- TSHR-ant
- TSHR antagonist National Institutes of Health Chemical Genomics Center number 00229600.
References
- 1. Iyer S, Bahn R. Immunopathogenesis of Graves' ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012;26:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hufnagel TJ, Hickey WF, Cobbs WH, Jakobiec FA, Iwamoto T, Eagle RC. Immunohistochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves' disease. Ophthalmology. 1984;91:1411–1419 [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves' ophthalmopathy. J Mol Endocrinol. 2011;46:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu S, Guan Q, Liu Y, et al. Role of extrathyroidal TSHR expression in adipocyte differentiation and its association with obesity. Lipids Health Dis. 2012;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu M, Lin RY. TSH stimulates adipogenesis in mouse embryonic stem cells. J Endocrinol. 2008;196:159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang L, Grennan-Jones F, Lane C, Rees DA, Dayan CM, Ludgate M. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves' orbitopathy. J Clin Endocrinol Metab. 2012;97:653–662 [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci. 2006;47:5197–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562 [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Bowen T, Grennan-Jones F, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem. 2009;284:26447–26455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Zeijl CJ, Fliers E, van Koppen CJ, et al. Effects of thyrotropin and thyrotropin-receptor-stimulating Graves' disease immunoglobulin G on cyclic adenosine monophosphate and hyaluronan production in nondifferentiated orbital fibroblasts of Graves' ophthalmopathy patients. Thyroid. 2010;20:535–544 [DOI] [PubMed] [Google Scholar]
- 11. van Zeijl CJ, Fliers E, van Koppen CJ, et al. Thyrotropin receptor-stimulating Graves' disease immunoglobulins induce hyaluronan synthesis by differentiated orbital fibroblasts from patients with Graves' ophthalmopathy not only via cyclic adenosine monophosphate signaling pathways. Thyroid. 2011;21:169–176 [DOI] [PubMed] [Google Scholar]
- 12. Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in graves' orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97:1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Steensel L, Hooijkaas H, Paridaens D, et al. PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves' ophthalmopathy patients. J Clin Endocrinol Metab. 2012;97:E944–E953 [DOI] [PubMed] [Google Scholar]
- 14. Imai Y, Ibaraki K, Odajima R, Shishiba Y. Analysis of proteoglycan synthesis by retro-ocular tissue fibroblasts under the influence of interleukin 1 β and transforming growth factor-β. Eur J Endocrinol. 1994;131:630–638 [DOI] [PubMed] [Google Scholar]
- 15. Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1β in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84:4079–4084 [DOI] [PubMed] [Google Scholar]
- 16. He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-α-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009;284:20136–20146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kikuchi T, Yamada H, Fujikawa K. Effects of high molecular weight hyaluronan on the distribution and movement of proteoglycan around chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2001;9:351–356 [DOI] [PubMed] [Google Scholar]
- 18. Shiedlin A, Bigelow R, Christopher W, et al. Evaluation of hyaluronan from different sources: Streptococcus zooepidemicus, rooster comb, bovine vitreous, and human umbilical cord. Biomacromolecules. 2004;5:2122–2127 [DOI] [PubMed] [Google Scholar]
- 19. Swann DA. Studies on hyaluronic acid. I. The preparation and properties of rooster comb hyaluronic acid. Biochim Biophys Acta. 1968;156:17–30 [PubMed] [Google Scholar]
- 20. Kawakami M, Suzuki K, Matsuki Y, et al. Hyaluronan production in human rheumatoid fibroblastic synovial lining cells is increased by interleukin 1 β but inhibited by transforming growth factor β 1. Ann Rheum Dis. 1998;57:602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campo GM, Avenoso A, D'Ascola A, et al. The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem. 2012;113:1852–1867 [DOI] [PubMed] [Google Scholar]
- 22. Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279:41453–41460 [DOI] [PubMed] [Google Scholar]
- 23. Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFβ1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haserodt S, Aytekin M, Dweik RA. A comparison of the sensitivity, specificity, and molecular weight accuracy of three different commercially available Hyaluronan ELISA-like assays. Glycobiology. 2011;21:175–183 [DOI] [PubMed] [Google Scholar]
- 25. Neumann S, Pope A, Geras-Raaka E, Raaka BM, Bahn RS, Gershengorn MC. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in Graves' orbital fibroblasts. Thyroid. 2012;22:839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumann S, Eliseeva E, McCoy JG, et al. A new small-molecule antagonist inhibits Graves' disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011;96:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181:4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50:2262–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith TJ, Bahn RS, Gorman CA. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: evidence for differences between retroocular and dermal fibroblasts. J Clin Endocrinol Metab. 1989;69:1019–1023 [DOI] [PubMed] [Google Scholar]
- 30. Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves' ophthalmopathy patients. Clin Endocrinol (Oxf). 2003;58:280–287 [DOI] [PubMed] [Google Scholar]
- 31. Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002 [DOI] [PubMed] [Google Scholar]
- 32. Stadlmayr W, Spitzweg C, Bichlmair AM, Heufelder AE. TSH receptor transcripts and TSH receptor-like immunoreactivity in orbital and pretibial fibroblasts of patients with Graves' ophthalmopathy and pretibial myxedema. Thyroid. 1997;7:3–12 [DOI] [PubMed] [Google Scholar]
- 33. Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves' ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300 [DOI] [PubMed] [Google Scholar]
- 34. Agretti P, De Marco G, De Servi M, et al. Evidence for protein and mRNA TSHr expression in fibroblasts from patients with thyroid-associated ophthalmopathy (TAO) after adipocytic differentiation. Eur J Endocrinol. 2005;152:777–784 [DOI] [PubMed] [Google Scholar]
- 35. Kumar S, Coenen MJ, Scherer PE, Bahn RS. Evidence for enhanced adipogenesis in the orbits of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab. 2004;89:930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotrophin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30:369–380 [DOI] [PubMed] [Google Scholar]
- 37. Turcu AF, Kumar S, Neumann S, et al. A small molecule antagonist inhibits thyrotropin receptor antibody-induced orbital fibroblast functions involved in the pathogenesis of Graves ophthalmopathy. J Clin Endocrinol Metab. 2013;98:2153–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]