Abstract
The dependence of prostate cancer on androgens provides a targeted means of treating advanced disease. Unfortunately, androgen deprivation therapies eventually become ineffective, leading to deadly castration-resistant prostate cancer (CRPC). One of many factors implicated in the transition to CRPC is the onset of de novo steroidogenesis. Although reactivation of steroid receptors likely plays a pivotal role in aggressive CRPC, little is understood regarding the mechanisms whereby prostate cancer cells initiate and maintain steroidogenesis. We hypothesize that steroidogenic factor 1 (SF1, NR5A1, AD4BP), a key regulator of steroidogenesis in normal endocrine tissues, is expressed in CRPC where it stimulates aberrant steroidogenesis and fuels aggressive growth. Notably, SF1 is not expressed in normal prostate tissue. Our results indicated that SF1 was absent in benign cells but present in aggressive prostate cancer cell lines. Introduction of ectopic SF1 expression in benign human prostate epithelial cells (BPH-1) stimulated increased steroidogenic enzyme expression, steroid synthesis, and cell proliferation. In contrast, data from an aggressive human prostate cancer cell line (BCaPT10) demonstrated that SF1 was required for steroid-mediated cell growth because BCaPT10 cell growth was diminished by abiraterone treatment and short hairpin RNA–mediated knockdown of SF1 (shSF1). SF1-depleted cells also exhibited defective centrosome homeostasis. Finally, whereas xenograft experiments in castrated hosts with BCaPT10 control transplants grew large, invasive tumors, BCaPT10-shSF1 knockdown transplants failed to grow. Therefore, we conclude that SF1 stimulates steroid accumulation and controls centrosome homeostasis to mediate aggressive prostate cancer cell growth within a castrate environment. These findings present a new molecular mechanism and therapeutic target for deadly CRPC.
The prostate is a hormone-dependent organ that relies on androgens synthesized by the testes for development, growth, and maintenance. Circulating testosterone also stimulates cell growth and proliferation of cancerous prostate epithelial cells. Thus, androgen deprivation therapy (ADT) by castration or by medical disruption of the hypothalamic-pituitary-gonadal (HPG) axis remains the cornerstone of treatment for metastatic prostate cancer based on the pioneering work of Huggins and Hodges (1). After systemic testosterone levels drop, the prostate cancer shrinks as a result of cellular apoptosis (2). Unfortunately, this success is typically short lived, and most patients become resistant to ADT within 3 years (3). Prostate cancer that progresses despite low circulating androgen levels is referred to as castration-resistant prostate cancer (CRPC), for which there is currently no cure.
Recent efforts for treatment of CRPC have centered on anti-androgen receptor (AR) therapy in combination with or sequential to steroid synthesis inhibition and other forms of chemotherapy but have only short-lived success. Resistance invariably develops due to several proposed mechanisms including expression of AR mutants that confer increased promiscuity, ligand independence, or increased coactivator binding in addition to AR inhibitors demonstrating agonist instead of antagonist activity (4–9). Recently, a series of studies have shown that hormone-deprived cancer cells can acquire the machinery to promote intratumoral hormone synthesis. Results from cell line models and patient tissue biopsies exposed an increase in the presence and activity of steroidogenic enzymes that resulted in de novo androgen synthesis within a chronically hormone-deprived environment (10–12). Despite the destructive consequences caused by local steroid production, the mechanisms by which cancer cells initiate and maintain expression of steroidogenic enzymes in prostate cancer cells is not known. Normally, de novo steroid production is confined to the gonads and adrenal cortex and is exquisitely regulated by hypothalamic and pituitary hormones. It is clear, however, that classic control via the HPG axis does not play a role in regulating steroidogenesis within CRPC because intratumoral steroid production occurs in the face of GnRH agonist or antagonist treatment, which are components of ADT.
Steroidogenic factor 1 (SF1, AD4BP, NR5A1, FTZ-F1) is best known for 2 critical roles in endocrine tissues: first, as a potent regulator of steroidogenesis within the adrenal glands and gonads throughout pre- and postnatal life, and, second, for cell survival and proliferation in development and maintenance of endocrine organs (13–16). As an essential regulator of steroidogenesis, SF1 acts as a transcription factor to drive the expression of genes involved in cholesterol metabolism and conversion to steroid hormones (17–21). In contrast to postnatal steroidogenesis within the adrenals and gonads, but similar to CRPC, the onset of steroid synthesis during development is independent of HPG/adrenal control and instead relies on paracrine signals that up-regulate SF1 expression (22–26). Mouse models with targeted disruption of Sf1 developed fewer cells within the steroidogenic lineage and expressed low levels of steroidogenic enzymes in the adrenals and gonads (27–29). Likewise, humans with SF1 mutations exhibit a wide range of phenotypes, but typically include reduced masculinization or sex reversal in males and adrenal insufficiency (30–32). Although the absence of SF1 disrupts steroid synthesis, its presence can push otherwise nonsteroidogenic cells toward a steroidogenic fate. Studies showed that ectopic SF1 expression in embryonic stem cells or bone marrow cells induced cell differentiation toward a steroidogenic fate and caused steroid production (33–35). Furthermore, abnormal SF1 expression has been implicated in promoting aberrant steroidogenesis in diseased states such as ovarian and adrenal cancers and endometriosis (36–38).
Independent of its steroidogenic actions, SF1 also promotes proliferation and cell survival. Mice with global deletion of Sf1 did not form the ventral medial hypothalamus and although the adrenogenital primordial ridge formed, within days it regressed by apoptosis, before the onset of steroidogenesis (14, 39–42). Conversely, transgenic mice that overexpressed SF1 within adrenal cortical cells experienced increased cell proliferation and the formation of adrenal tumors (36). Mechanisms by which SF1 stimulates proliferation are not known, but recent studies determined that SF1 was required to maintain proper centrosome homeostasis and genetic stability during cell division within steroidogenic cells (43, 44).
SF1 is not expressed in the normal prostate, but its presence in benign prostate hyperplasia or prostate cancer has not yet been examined. Based on its role in normal endocrine tissues, we hypothesized that aberrant SF1 expression is the mechanism by which prostate cancer cells acquire the capacity for de novo steroidogenesis that fuels aggressive growth in CRPC. Here we report that SF1 is expressed in aggressive forms of prostate cancer. In addition, gain- and loss-of-function experiments determined that SF1 functions as a critical mediator in stimulating steroidogenic enzyme expression and synthesis of steroids in prostate epithelial cells. Finally, SF1 maintained centrosome stability and promoted cell proliferation in cell culture and within xenograft tumor transplants. In summary, this report presents SF1 as a potential new target for therapy against aggressive CRPC.
Materials and Methods
For details, see Supplemental Materials and Methods published on The Endocrine Society's Journals Online web site at http://end.endojournals.org.
Plasmids
The pcDNA3-P2A-mCherry plasmid was made by inserting P2A-mCherry via restriction digest with KpnI and subsequent ligation into the pcDNA3 vector (Life Technologies). The pcDNA3-P2A-mCherrySF1 vector consists of full-length SF1 cDNA coupled via the self-cleaving viral peptide P2A to mCherry, resulting in bicistronic expression of these proteins (45). SF1 was ligated downstream of the P2A-mCherry via restriction digest with EcoRI of the previously described CMV5-SF1 plasmid (46).
Cell cultures
MA-10 (generously provided by Dr. Mario Ascoli, University of Iowa, College of Medicine, Iowa City, Iowa), BPH-1 (47), BCaPNT1, BCaPT1, and BCaPT10 cell lines (kind gifts from Dr. William Ricke, University of Wisconsin, Department of Urology, Madison, Wisconsin; for details regarding these cell lines, see Supplemental Materials and Methods, Supplemental Table 1) and H293, DU145, PC3, and LNCaP cell lines (American Type Culture Collection) were all cultured in RMPI-1640 (HyClone) supplemented with 5% fetal bovine serum, 2 mmol/L l-glutamine, and 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco) at 37°C under 5% CO2. For cell growth assays, 10 μM abiraterone acetate (Selleck Chemicals), 10 nM estradiol (Sigma-Aldrich), 10 nM synthetic progestin R5020 (PerkinElmer Life and Analytical Sciences Inc), and 100 nM dihydrotestosterone (DHT) (Sigma-Aldrich) were used.
Western blots
Cell lysates were collected using radioimmunoprecipitation assay buffer supplemented with protease inhibitor complex (Sigma-Aldrich). Protein content was measured using the DC protein assay (Bio-Rad Laboratories); 35 μg of protein was loaded and separated on a 12% SDS polyacrylamide gel, transferred to a nitrocellulose membrane, and blocked in Tris-buffered saline with 0.1% Tween 20 and 0.25% gelatin (TBSTG). The membrane was incubated overnight in TBSTG containing primary antibody SF1 (1:1000; kindly provided by Ken-Ichirou Morohashi, Kyushu University, Fukuoka, Japan) or GAPDH (AM4300, 1:500; Ambion) followed by incubation for 1 hour in goat anti-rabbit horseradish peroxidase or anti-mouse horseradish peroxidase conjugated (Pierce), respectively. Blots were developed using ECL reagent (Pierce) and autoradiography film.
Transfections
Nucleofection of BPH-1 and BCaPT10 cells was performed using the Amaxa Nucleofector Kit R (Lonza) as described in the manufacturer's recommended protocol. In brief, 2 × 106 cells were nucleofected with 4.5 μg of plasmid DNA on setting T-009 of a Nucleofector-II device (Lonza). Cells for transient transfection experiments were allowed to recover overnight and then plated for further experiments. Cells for stable transfection experiments were allowed to recover overnight and then were plated at 30% confluence in 350 μg/mL hygromycin (MP Biosciences). These cells were then grown until all control nonresistant cells were dead and colonies began to form in resistant cultures. These resistant colonies were pooled and maintained in 350 μg/mL hygromycin.
Gene expression
Total RNA was collected using an RNeasy mini kit (Qiagen), and 1 μg of RNA was used in an RT reaction using the SuperScript II cDNA First-Strand Synthesis System (Invitrogen). A 10-μL quantitative real time-PCR reaction volume was used with 1× SYBR Green PCR Master Mix (Applied Biosystems, 2 μL of 1:4 diluted cDNA, and 300 nM primer. Reactions were performed on an Applied Biosystems 7300 real-time PCR system (Applied Biosystems). Results were calculated using the comparative CT method and normalized to 36B4 RNA using RNA collected from at least 3 separate biological replicates (48). Primers are listed in Supplemental Table 2.
Liquid chromatography–tandem mass spectrometry (LC/MS-MS) hormone measurements
Cells were plated at 1.5 × 105 cells/well in a 6-well plate and allowed to grow overnight after which the medium was changed to a phenol red–free medium supplemented with 5% charcoal strip serum (hormone-free medium) (Sigma-Aldrich) and allowed to grow 24 hours. Cells were then incubated with 2.5 μM pregnenolone spiked-hormone free medium for 18 hours. The cell medium was then collected for steroid extraction and measurement by LC/MS-MS (for details, see Supplemental Materials and Methods, Supplemental Tables 3 and 4).
Cell growth assay
Cells were plated at 5 × 103 in each well of a 96-well plate and then treated with the indicated compound and grown for the reported time. 3-(4,5-Dimethyl-thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT) assays were performed using a CellTiter 96-well aqueous nonradiometric cell growth assay (Promega), according to the manufacturer's guidelines. Absorbance was read at 490 and 650 nm. Results are reported as the mean values of 4 replicates ± SE for each treatment and time from at least 3 independent experiments.
5-Bromo-2′-deoxyuridine (BrdU) proliferation assay
Cells were plated at 1.5 × 105 cells per well in a 6-well plate and grown for 48 hours. They were then incubated in medium containing 10 μM BrdU (Sigma Aldrich) for 2 hours. Processing for immunocytochemical analysis was performed as described below with the addition of a 20-minute 2 N HCl incubation directly after fixation. A primary antibody against BrdU (555627; BD Pharmingen), a secondary donkey anti-mouse Cy3-conjugated antibody (Jackson ImmunoResearch), and 4′,6-diamidino-2-phenylindole (DAPI) treatment to stain nuclei were used. At least 150 cells were assessed for BrdU positivity per condition per slide from at least 3 independent experiments.
Immunocytochemistry
Cells were grown on coverslips and fixed for 10 minutes with ice-cold methanol. After blocking in blocking serum (PBS–0.5% Triton X-100 with 5% donkey sera), primary antibody incubation was performed overnight at 4°C with γ-tubulin (1:1000, T5326; Sigma-Aldrich) and SF1 (1:500; kindly provided by Ken-Ichirou Morohashi, Kyushu University, Fukuoka, Japan) followed by 1-hour room temperature incubation with the secondary antibody and counterstaining with DAPI. The coverslips were then mounted in antifade medium (PBS containing 80% glycerol and 0.2% n-propyl gallate) on glass slides.
Nude mouse kidney capsule cell xenografts
BCaPT10 cells (100 000) were embedded into 30 μL of neutralized rat tail collagen (BD Biosciences) and allowed to set overnight in maintenance medium. Grafts were then transplanted under the kidney capsule of a gonadectomized nude mouse of at least 8 weeks of age (Charles River Laboratories). Gonadectomy was performed 10 to 14 days before transplantation surgery. Kidney capsule transplantation was performed as described previously (49, 50) http://mammary.nih.gov/tools/mousework/Cunha001/index.html). Grafts and adjacent kidney tissue were collected after 2 months of growth under the kidney capsule. Tumor volume was estimated by the formula width × length × depth × π/6, which underestimates invasive tumor volume (51). Mice were housed at 25°C in conventional polycarbonate cages with 3.2-mm corncob bedding (Harlan) on a 12-hour light/dark cycle. All the procedures described were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
Cell grafts and adjacent kidney tissue were fixed overnight in 4% paraformaldehyde at 4°C. Tissues were then dehydrated in an ethanol gradient, cleared in xylenes, and embedded in paraffin blocks. Sections were stained with hematoxylin and eosin or processed for immunohistochemical analysis. For immunohistochemical analysis, sections were dewaxed and rehydrated, and the Vector Mouse on Mouse (M.O.M.) (Vector Laboratories) or rabbit IgG kits were used as indicated by the manufacturers with anti-SV40 T Ag (Santa Cruz Biotechnology), Ki67 (Abcam), SF1 (clone N1665; Invitrogen), 3-β-hydroxysteroid dehydrogenase (3-βHSD) (kindly provided by Ken-Ichirou Morohashi, Kyushu University, Fukuoka, Japan), and a 3,3′-diaminobenzidine (DAB) peroxidase substrate kit (Vector Laboratories).
Statistical analysis
Statistics were performed using Prism version 4.0 software (GraphPad Software, Inc).
Results
SF1 is expressed in aggressive prostate cancer cell lines
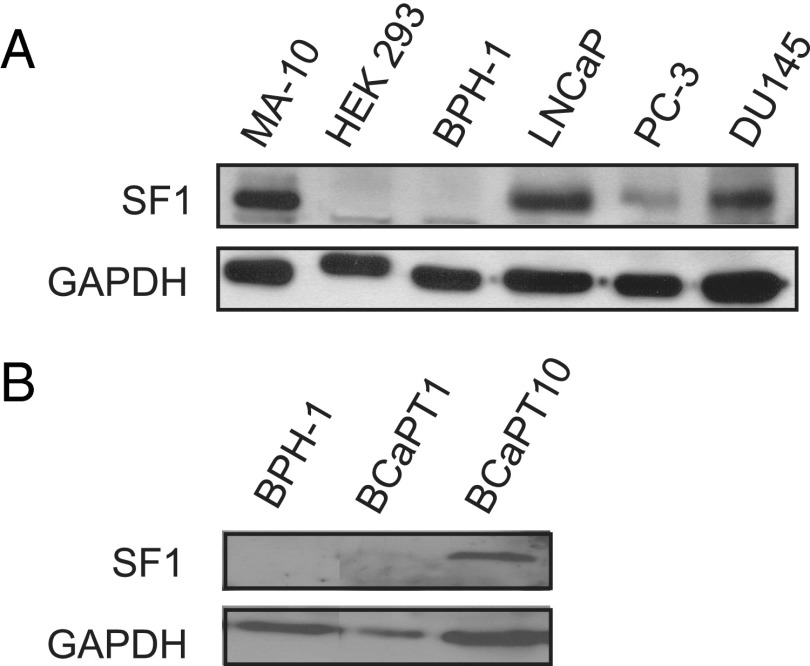
SF1 stimulates steroidogenesis and proliferation in normal endocrine organs and disease states associated with aberrant steroidogenesis (22, 24). We investigated a panel of prostate cancer cell lines for the presence of SF1 protein to explore the potential for its activity in aggressive prostate cancer. SF1 was not expressed in HEK-293 (negative control) or benign prostate epithelial (BPH-1) cell lines. In contrast, compared with MA-10 Leydig cells (positive control), SF1 was detected in all aggressive prostate cancer cell lines tested including LNCaP, PC-3, and DU145 (Figure 1A).
Figure 1.
SF1 is present in prostate cancer cell lines. A, Western blot for SF1 protein on lysates from MA-10 rat Leydig (positive control), HEK-293 human embryonic kidney (negative control), BPH-1 human prostate epithelial–derived, and LNCaP, PC-3, and DU145 human prostate cancer–derived cell lines. GAPDH was used as a loading control. B, Western blot from lysates from parent BPH-1 cells alongside BCaPT1 and BCaPT10 cell lines. Each panel shows representative images from at least 3 independent experiments.
LNCaP, PC-3, and DU145 cell lines are all derived from metastatic prostate cancer (52–54), suggesting that SF1 is expressed minimally in late- and end-stage cancer. To investigate the stage at which SF1 expression might first be detected in prostate disease, we evaluated human prostate epithelial cell lines derived from xenografts that represent distinct stages of prostate development and cancer progression. This xenograft model was described previously and is outlined in the Supplemental Materials and Methods (50). When these cell lines were regrafted as xenografts into nude mouse hosts, each graft produced a signature morphology that included benign prostate duct epithelium (no tumor, BCaPNT1 cell line), discrete, localized tumors (BCaPT1 cell line), large, invasive tumors (BCaPT10 cell line), and CRPC (BCaPM-T10 cell line) (50) (Ricke, W. A., unpublished data, Supplemental Table 1). Results show that SF1 was not detected in parent BPH-1 cells or in BCaPNT1 or BCaPT1 cell lines that were previously reported to produce nontumorigenic tissue or localized tumors; however, SF1 expression was present in the BCaPT10 and BCaPM-T10 cell lines, previously documented to form aggressive and castration-resistant tumors (Figure 1B and Supplemental Figure 1). Taken together, these data suggest that SF1 expression marks aggressive prostate cancer cells.
SF1 is necessary and sufficient for steroidogenesis in prostate epithelial cell lines
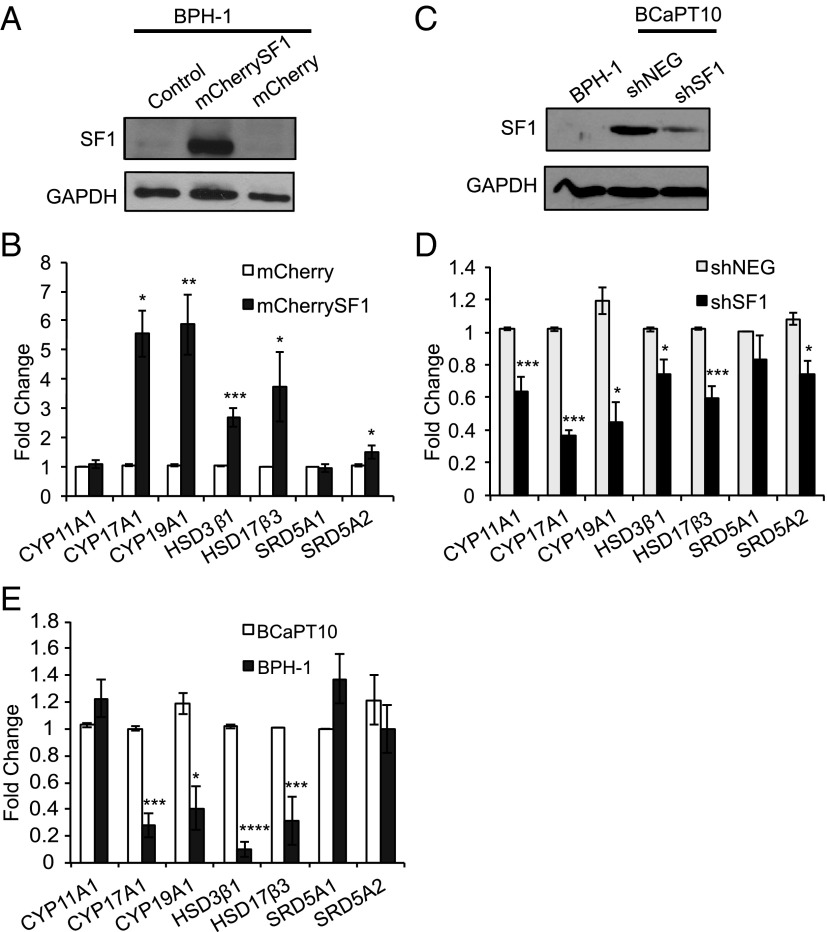
Once we established that SF1 was present in aggressive prostate cancer cells, gain- and loss-of-function experimental paradigms were used to test whether the presence of SF1 stimulated expression of steroidogenic enzymes and increased steroid synthesis. To induce SF1 activity in benign prostate epithelial cells, an expression vector containing coding sequences for SF1 and mCherry fluorescent protein (mCherrySF1) was transiently transfected into BPH-1 cells, which lack endogenous SF1 expression (Figures 1A and 2A). Ectopic expression of SF1 significantly increased transcript levels of steroidogenic enzymes CYP17A1 (5.6-fold, P < .05), HSD3B1 (2.7-fold, P < .005), HSD17B3 (3.7-fold, P < .05), and CYP19A1 (5.9-fold, P < .01) compared with BPH-1 cells transfected with the mCherry control plasmid (Figure 2B). Neither steroidogenic enzyme SRD5A1 or SRD5A2 is a direct target of SF1, and there was no significant difference in transcript levels of SRD5A1. However, SRD5A2 was slightly increased (1.5-fold, P < .05), which may be attributed to its response to increased nuclear receptor activity caused by local production of steroids or indirect actions (55).
Figure 2.
SF1 regulates steroidogenic enzyme expression in prostate cells. A, Western blot for SF1 protein in BPH-1 cells nucleofected with control (mCherry) or SF1-mCherry expression constructs (mCherrySF1) compared with parent BPH-1 cells (mock, negative control). B, Steroidogenic enzyme mRNA levels of BPH-1–Cherry-SF1 cells (dark gray bars) measured by quantitative real-time PCR and reported as fold change from -mCherry controls (white bars). C, SF1 protein expression by Western blot in BCaPT10 cells stably transfected with an SF1 targeting shRNA construct (shSF1) vs nontargeting control (shNEG) compared with parent BPH-1 cells (negative control). D, Steroidogenic enzyme transcript levels for -shSF1 cells (black bars) reported as fold change relative to -shNEG cells (light gray bars). E, Steroidogenic enzyme transcript levels comparing parent BPH-1 cells (dark gray bars) vs BCaPT10 cancer cells (white bars) reported as fold change relative to BCaPT10 cells. B, D, and E are shown as the average fold change ± SEM from at least 3 independent experiments. *, P < .05; **, P < .01; ***, P < .005; ****, P < .001; Student t test, two-tailed, equal variance.
To examine the effect of SF1 depletion in aggressive prostate cancer cells, we used BCaPT10 cells, which represented the earliest stage of SF1 expression in the prostate epithelial cancer progression model and were not derived from metastatic cancer cells (Figure 1B and Supplemental Figure 1). These cells express AR and both estrogen receptors α and β, which makes BCaPT10 an attractive cell line to study the hormonal effects of SF1 expression (Supplemental Figure 2 and Supplemental Table 5). The BCaPT10 cell line was stably transfected with SF1-targeting short hairpin (sh) RNA (shSF1) and compared with cells expressing nontargeting control shRNA (shNEG). shRNA targeting SF1 caused approximately 75% knockdown as measured by Western blot, which was correlated with a significant biological effect (Figure 2, C and D). Loss of SF1 expression in BCaPT10 prostate cancer cells caused a significant decrease in transcript levels of steroidogenic enzymes CYP11A1 (0.63-fold, P < .005), CYP17A1 (0.37-fold, P < .005), HSD3B1 (0.75-fold, P < .05), HSD17B3 (0.59-fold, P < .005), and CYP19A1 (0.44-fold, P < .05) (Figure 2D). Similar to the knock-in studies with BPH-1 cells, no change was detected in SRD5A1, but SRD5A2 was affected (0.74-fold, P < .05), presumably because of altered local steroid concentrations.
We next evaluated the steroidogenic potential in SF1-positive, aggressive prostate cancer cells by comparing transcript levels in BCaPT10 with those in BPH-1 cell lines. Compared with BCaPT10 cells, steroidogenic enzyme transcript levels were significantly decreased in BPH-1 cells for CYP17A1 (0.28 fold, P < .005), HSD3B1 (0.10 fold, P < .001), HSD17B3 (0.32 fold, P < .005), and CYP19A1 (0.41 fold, P < .05). There were no differences in CYP11A1, SRD5A1, or SRD5A2 transcripts (Figure 2E).
To optimize our capacity to assess steroidogenic activity within these cells in the absence of stromal interactions and stimulatory signals of an in vivo environment, we provided an excess of an upstream steroid precursor, pregnenolone. Although supplementation with pregnenolone prevented our assessment of de novo steroid synthesis from cholesterol (bypasses STAR and CYP11A1), it allowed us to measure the presence and activity of subsequent steroid enzymes, leading to intracellular steroid metabolism, even at low levels (Figure 3A). Our results indicated that ectopic expression of SF1 in BPH-1 cells (BPH-1–mCherrySF1) was sufficient to stimulate more conversion of pregnenolone to progesterone than BPH-1–mCherry control cells (1.7-fold, P < .05) (Figure 3B). The requirement of SF1 for steroidogenic activity was supported by a significant decrease in progesterone accumulation in BCaPT10-shSF1 vs BCaPT10-shNEG cells (0.4-fold, P < .05) (Figure 3B). In agreement with these data, dehydroepiandrosterone (DHEA) and DHT measurements followed a similar but less robust trend (DHEA: +SF1mCherry, 1.4-fold, P = .19; shSF1, 0.65-fold, P = .15; DHT: +SF1mCherry, 1.2-fold, P = .27; shSF1, 0.39-fold, P = .16) (Figure 3B). No appreciable difference was detected between treatments for androstanediol (back door or alternative pathway) or testosterone. In summary, these data suggest that the presence of SF1 affects the expression of steroidogenic enzymes, most notably, CYP17A1, HSD3B1, HSD17B3, and CYP19A1, each known targets of SF1 regulation (13, 18–20, 56) (Figure 3A), and that SF1 activity is sufficient to alter steroid metabolism in these cells.
Figure 3.
SF1 stimulates steroidogenic enzyme activity in prostate epithelial cells. A, Classic (Δ5 [left] and Δ4 [middle]) and alternative pathways for sex steroid production are shown. Each steroidogenic enzyme except SRD5A1, SRD5A2, and RDH5 is regulated by SF1 (★, known SF1 target genes; ★?, genes that have SF1 binding sites within their proximal promoter but have not been directly tested for SF1 regulation). To optimize steroid detection, cells were incubated in medium spiked with an upstream steroid precursor, pregnenolone (shaded box). Unshaded boxes denote steroids that were subsequently measured by LC/MS-MS from cell culture media (nanograms per milliliter of medium). B, Presence of SF1 exerted a significant effect on progesterone production (*, P ≤ .05, Student t test, one-tailed, equal variance); low levels of DHEA and DHT were detected (note scale difference), which followed the same trend as progesterone in the absence or presence of SF1. White bars, BPH-1–mCherry; dark gray bars, BPH-1–mCherry-SF1; light gray bars, BCaPT10-shNEG; black bars, BCaPT10-shSF1. Androstanediol concentrations were below the limits of detection.
SF1 promotes prostate cancer cell growth and proliferation
Previous studies have shown that SF1 overexpression stimulated proliferation of adrenal carcinoma cells, whereas treatment with SF1 shRNA or inverse agonists blocked this effect (36, 57). To determine whether SF1 can promote proliferation in prostate epithelial cells, we assessed cell growth in our SF1-modified cell lines after 24, 48, and 96 hours in culture. BPH-1 cells expressing ectopic SF1 exhibited a significant increase in cell growth at 48 and 96 hours compared with that in controls (P < .05 at 48 hours; P < .001 at 96 hours) (Figure 4A). No difference in activated caspase-3 was detected in BPH-1 cells transfected with mCherry control vs mCherry-SF1 plasmids (results not shown), suggesting that the observed cell growth was caused by increased cellular proliferation rather than alterations in apoptosis. Proliferation studies showed that BPH-1–mCherry-SF1 cells incorporated more BrdU than the negative control cell line (9% increase, P < .05) (Figure 4B).
Figure 4.
SF1 increases prostate cell growth by stimulating cell proliferation. A and B, Ectopic expression of SF1 within BPH-1 cells promotes cell growth. MTT assays comparing cell growth at 24, 48, and 96 hours of culture (A) and quantification of immunocytochemical staining for BrdU incorporation in mCherry (white bars) and mCherrySF1-expressing (dark gray bars) BPH-1 cells (B). C and D, Loss of SF1 expression in BCaPT10 cells slows cell growth. MTT assays (C) and BrdU incorporation quantification (D) performed in shNEG (light gray bars) and shSF1-expressing (black bars) BCaPT10 cells. E, BCaPT10 and BPH-1 cells were treated with vehicle (NTC, white bars), 10 μM abiraterone (ABI, gray bars), or abiraterone plus steroid hormones (black bars, 100 nM DHT, 10 nM estradiol, and 10 nM progestin). B and D results are reported as percentages of positive BrdU-labeled cells per total number of DAPI-stained nuclei. A minimum of 200 cells per condition was used from each of at least 3 independent experiments. Each result is shown as the average ± SEM from at least 3 independent experiments. A–D, Student t test, two-tailed, equal variance. E, One-way ANOVA. *, P < .05; **, P < .01; ***, P < .005; ****, P < .001.
Evaluation of MTT assays showed similar growth rates for BCaPT10-shSF1 and BCaPT10-shNEG cells after 24 hours, but cell growth was significantly slowed at 48 and 96 hours in BCaPT10-shSF1 compared with BCaPT10-shNEG cells (P < .005 at 48 hours; P < .001 at 96 hours) (Figure 4C). Decreased cell growth in these cells was attributed to less cell proliferation as measured by a decrease in BrdU incorporation (13% less, P < .05) (Figure 4D). No change in cell apoptosis was detected in BCaPT10-shSF1 or BCaPT10-shNEG cells over time (data not shown).
To test whether SF1-mediated changes in cell growth were associated with the presence of steroids, we incubated BCaPT10 cells with abiraterone, an inhibitor of CYP17A1 activity that essentially shuts down steroid metabolism (58–60). BCaPT10 cell growth was significantly decreased upon treatment with abiraterone (P < .01); BPH-1 cell growth was not affected by abiraterone treatment (nonsteroidogenic cell line control) (Figure 4E). Steroids were added back, individually or in combination, to rescue the abiraterone-mediated decrease in BCaPT10 cell growth; results show that a mixture of progesterone, estradiol, and DHT was successful (Figure 4E). These results explained why we failed to observe inhibition of BCaPT10 cell growth when the antagonists flutamide (AR inhibitor) or fulvestrant (estrogen receptor inhibitor) were added to the cell culture medium (data not shown). Taken together, these data suggest that multiple steroid metabolites work together to promote cell growth in BCaPT10 cells.
SF1 localizes to the centrosome to stabilize centrosome homeostasis in prostate cancer cells
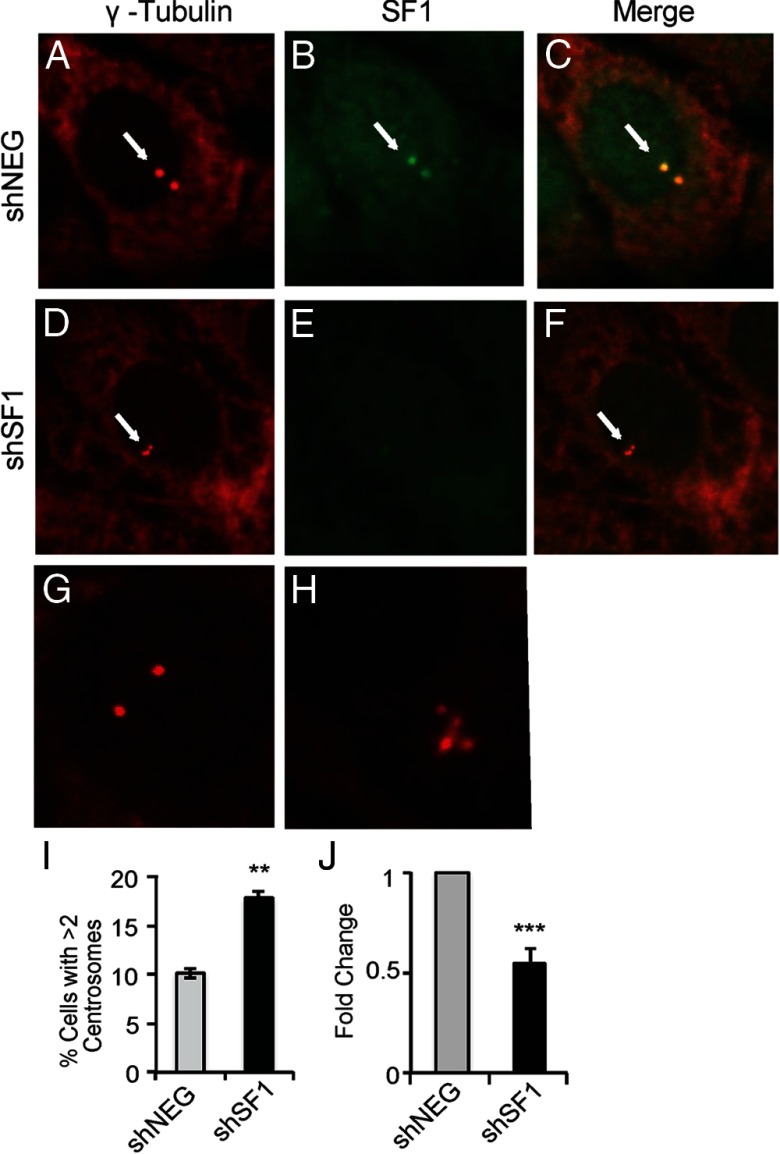
In addition to its role as a transcriptional regulator in the nucleus, recent studies established that SF1 also colocalized to centrosomes in MA-10 Leydig and Y1 adrenocortical cells to prevent centrosome overduplication and stabilize genetic material during cell division (43, 44). Our results show that this activity was conserved in BCaPT10 prostate epithelial cancer cells. Similar to other steroidogenic cell lines, SF1 colocalized with γ-tubulin in BCaPT10 cells, which was lost on expression of shRNA targeting SF1 (Figure 5, A–F). Moreover, SF1 depletion in BCaPT10 cells caused aberrant centrosome duplication, resulting in an increase in the number of cells with greater than 2 centrosomes indicative of genetic instability (Figure 5, G, H, and I). These cells also expressed significantly less cyclin D1 (CCND1) (Figure 5J). Taken together with the cell growth and proliferation studies reported above, these data suggest that SF1 contributes to centrosome stability and control of cell growth.
Figure 5.
SF1 is located at the centrosome to prevent centrosome overduplication in prostate cancer cells. Representative images for immunofluorescence with antibodies targeting γ -tubulin (A, D, G, and H) and SF1 (B and E) and an image of both antibody signals merged (C and F) in BCaPT10 cells expressing shNEG (A, B, and C) or shSF1 (D, E, and F). Arrows highlight centrosomes. Higher magnification views of γ -tubulin–stained shNEG (G) and shSF1 (H) cells show representative images of centrosome numbers. I, Quantification of cells with greater than 2 centrosomes, as a percentage of total DAPI-stained nuclei. At least 150 cells per slide per condition were counted. J, Cyclin D1 transcript levels measured by quantitative real-time PCR and reported as fold change from shNEG-expressing BCaPT10 cells. I and J results are shown as the average ± SEM from at least 3 independent experiments. **, P < .01; ***, P < .005; Student t test, two-tailed, equal variance.
SF1 mediates growth of BCaPT10 prostate cell xenografts within a steroid-depleted environment
To test the importance of SF1 in maintaining prostate cancer cell growth in vivo, xenografts containing BCaPT10-shNEG or BCaPT10-shSF1 cells were placed under the kidney capsule of castrated nude mice and grown for 2 months. Gross evaluation of the tumors indicated that grafts from BCaPT10-shSF1 cells were stunted in growth compared with shNEG grafts (Figure 6, A and B), which was confirmed upon measuring tumor volume (shNEG tumor volume, 8.5 ± 2.2 mm3, n = 7; shSF1 tumor volume, 0.84 ± 0.26 mm3, n = 4; P < .05) (Figure 6C). Hematoxylin and eosin staining of grafts illustrated that BCaPT10-shNEG samples contained an epithelial population with polygonal cells in indistinct nests and cords (Figure 6D). Cells exhibited signs of anisocytosis and anisokaryosis, and there are numerous mitotic figures scattered throughout (Figure 6F). Renal tissue juxtaposed to the xenograft contains multiple neoplastic cells within nodules and as individual cells in the interstitium (Supplemental Figure 3). In contrast, BCaPT10-shSF1 samples harbor small and rare nodules of collagen-rich, cell-poor tissue with few spindle cells (Figure 6, E and G). Substantial BCaPT10 tumor cell proliferation was evident in shNEG xenografts compared with that in shSF1 xenografts as detected by the presence of mitotically active cells (compare Figure 6F with Figure 6G) and an increased number of Ki67-stained cells (compare Figure 6H with Figure 6I) that were also verified as human cancer cells by the presence of SV40 large T Ag (Figure 6, F–I, insets). Finally, xenograft serial sections were stained for SF1 and 3βHSD for evidence of steroidogenic activity. Most cells in BCaPT10-shNEG samples exhibited positive nuclear staining for SF1 along with positive cytoplasmic 3βHSD staining (Figure 6J, 3βHSD, inset). As expected, rare cells in BCaPT10-shSF1 xenograft sections were positive for both SF1 and 3βHSD antigens (Figure 6I, 3βHSD inset), supporting evidence for SF1 knockdown in the transplanted cells (Figure 2C). In summary, these complementary results from xenograft and in vitro experiments provide evidence that the presence of SF1 in BCaPT10 xenografts is required to form large, invasive tumors in the absence of exogenous steroids.
Figure 6.

SF1 mediates growth of BCaPT10 prostate cell xenografts within a steroid-depleted environment. Tissue images of shNEG (A) and shSF1 (B) cells grafted under the kidney capsule of castrated nude mice after 2 months of growth. White arrows denote tissue derived from grafts surrounded by the kidney. C, Tumor volumes from each individual sample (n = 7 shNEG, n = 4 shSF1) are represented with the mean and SD marked by error bars. *, P < .05; Student t test, two-tailed, equal variance. Hematoxylin and eosin–stained sections from shNEG-derived (D) and shSF1-derived (E) tumors (K, kidney; G, graft, dotted line separates graft from kidney). F and G, High-magnification hematoxylin and eosin images of grafts from shNEG (F) and shSF1 (G) cells showing cell architecture corresponding to neighboring serial sections used for immunohistochemical analysis with anti-SV40 T Ag (brown, insets), which was used to validate their origin from the parent BCaPT10 cell line. F, White arrows mark examples of mitotic figures in dividing cells within the shNEG graft that were also positive for SV40 T Ag (white arrowheads within inset). H and I, Ki67-stained cells (brown) comparing cell proliferation between shNEG (H) and shSF1 (I) grafts; insets indicate serial sections stained with SV40 T Ag with white arrows highlighting examples of cells stained for both Ki67 and SV40 T Ag. J and K, Immunohistochemical analysis was performed to identify SF1 positive cells (brown) in shNEG (J) and shSF1 (K) grafts; insets show serial sections stained for 3βHSD. White arrows indicate examples of cells positive for both SF1 and 3βHSD. Scale bars correspond to 0.5 mm (D and E), 0.05 mm (F, G and F and G insets), 0.1 mm (H, I, J, and K), and .01 mm (H, I, J, and K insets). 3,3′-DAB staining was counterstained with hematoxylin (blue, F and G insets and H, I, J, and K).
Discussion
Based on the recent discovery that CRPC cells can acquire the capacity for de novo steroid synthesis, we hypothesized that SF1, an established regulator of steroidogenesis, cell survival, and proliferation in normal endocrine organs, is aberrantly expressed in prostate tumors. We report that SF1 is expressed in prostate cancer cells where it stimulates local, autonomous steroid production necessary to support proliferation and survival. Our results suggest that this occurs within prostate cancer cells that progress independently from exogenous steroids. The normal prostate gland does not express SF1 or exhibit steroidogenic enzyme activity. Thus, establishing the presence of SF1 and its pro-steroidogenic and growth promoting activities within cancerous prostate cells uncovers a critical molecular mechanism to explain local steroid production that drives aggressive prostate cancer.
It has long been established that SF1 is a potent regulator of steroidogenesis (15, 17, 18, 61). Our data suggest that this role is conserved within prostate epithelial cell lines. Our gain- and loss-of-function studies implicated SF1 as a key mediator in regulating steroidogenic enzyme transcript levels and steroid metabolism activity in BPH-1 and BCaPT10 cells. SF1-expressing BCaPT10 cells (BCaPT10-shNEG) produced significantly more steroids than BPH-1 cells (mCherry and mCherrySF1), suggesting that other unknown mediators were induced in these cells during their transition from parent BPH-1 to BCaPT10 cells, which together with SF1, promoted an aggressive phenotype. Progesterone was the primary metabolite measured in each cell line; downstream steroids including DHEA and DHT were also detected. Our data suggested that ectopic expression of SF1 within prostate epithelial cells was sufficient to stimulate steroidogenic enzyme gene expression. A notable exception was CYP11A1. Further, knockdown of SF1 in BCaPT10 cells caused a more profound decrease in expression of CYP17A1, CYP19A1, and HSD17B3 compared to that of CYP11A1 or HSD3B. Precedence for differential regulation by SF1 was previously illustrated by reports that found that CYP11A1 was relatively insensitive, whereas CYP17A1, especially its 17–20 lyase isoform, was extremely sensitive to SF1 depletion in the H295R adrenocortical carcinoma cell line (62). In addition, SF1 regulatory activity is sensitive to a cadre of stimulatory signals, such as cAMP, MAPK, protein kinase C, and others, which contribute to differential regulation of downstream targets (63–68).
Differential regulation of steroidogenic enzymes by SF1 would probably be important when considering it as a target to block local steroid synthesis vs potential systemic complications. One of the most recent clinical advances in the fight against intratumoral hormone synthesis in CRPC is the use of specific steroidogenic enzyme inhibitors such as the CYP17A1 inhibitor, abiraterone acetate (69–71). Although therapy delays disease progression, concomitant administration of external adrenal steroids is required, and resistance occurs within a matter of months (72). This resistance has been attributed to up-regulation of CYP17A1 and a rebound in steroid production within tumor cells (72, 73). It is intriguing to consider whether the profound effects of SF1 on CYP17A1 in prostate epithelial cells could be specific to the 17–20 lyase isoform as reported in H295R adrenal cells (62). This property, along with its more comprehensive effects on other enzymes within the sex steroid pathway, presents SF1 as an attractive target for CRPC therapy, because it could potentially act as an adrenal-sparing blocker of steroidogenesis in addition to preventing or delaying the development of resistance to abiraterone treatment.
SF1 expression has been shown to promote aberrant cell growth in adrenal and ovarian cancer as well as endometriosis (24, 36–38). Likewise, we found that SF1 modulated cell growth and proliferation in prostate epithelial cells, which are normally nonsteroidogenic. BCaPT10 prostate cancer cell growth was slowed to the same rate as that of SF1-negative BPH-1 cells when treated with abiraterone; BPH-1 cells were not affected. These results support previous studies that implicate the importance of steroids in the growth of aggressive prostate cancer cells (72, 73). Further, these data indicate that the presence of SF1 is required to stimulate the production of steroids that promotes rapid cell growth. It was noteworthy, but not without precedence, that a combination of progesterone, DHT, and estradiol was required to rescue cell growth in abiraterone-treated BCaPT10 cells. This finding is consistent with epidemiologic reports of the human disease; whereas it has long been recognized that androgens have permissive roles in prostate cancer, they are insufficient to induce it on their own (74). Furthermore, promiscuity of the AR has been implicated in reactivation of the receptor (75, 76). Meanwhile, the role of local estrogens has been established in promoting abnormal cell proliferation in the prostate, especially when present in combination with androgens (77). Similar to these reports, our in vivo model did not progress when exposed to either estrogen or testosterone alone (50). It is also recognized that the presence of estrogen is required to up-regulate the progesterone receptor and its activity (78). In addition and similar to our findings, LnCaP xenografts synthesized greater concentrations of progesterone relative to androgens (12). Taken together, our data suggest that androgens are insufficient to promote BCaPT10 cancer cell growth on their own, consistent with the human disease. This point is interesting to consider, especially in light of in vivo situations in which tumor-stromal interactions and multiple steroid metabolites probably play an important role in tumor growth.
Previous studies using Sf1-null mice unveiled nonsteroidogenic roles for SF1 when it was discovered that fetal adrenal and gonad development first arrested and then regressed by apoptosis before steroidogenic onset (14, 41). In many cases, however, it is difficult to separate the steroidogenic vs nonsteroidogenic roles for SF1. The mechanisms by which SF1 promotes cell survival and growth may, at least in part, be explained by its activity at the centrosome. Previous studies showed that SF1 localized to the centrosome and stabilized division of genetic material during cytokinesis, but only in steroidogenic cells (43, 44). SF1 stabilizes centrosome homeostasis by regulating activity of DNA-dependent protein kinase/Akt activity. Notably, this occurs exclusively at the centrosome and illustrates a role for SF1 that is independent of its nuclear function for activating transcription (79). Our results indicated that this mechanism is conserved in BCaPT10 prostate cancer cells that have acquired SF1 expression and a steroidogenic phenotype. Based on these findings, together with the cell growth data, we conclude that SF1 plays 2 distinct roles to promote cell growth in BCaPT10 prostate cancer cells: stimulation of steroid synthesis and stabilization of centrosome homeostasis.
Finally, we transplanted xenografts of BCaPT10 cells into the kidney capsule of castrated nude mice to evaluate the role of SF1 in prostate cancer cells that were in an in vivo environment devoid of circulating testosterone. Whereas xenografts from control cells (BCaPT10-shNEG) developed prominent prostate tumors after 2 months, cells within BCaPT1-shSF1 grafts failed to grow. These data suggest that, similar to our in vitro abiraterone cell growth assays, SF1 controlled tumor growth in a hormone-depleted environment in vivo, which is especially clinically relevant to CRPC. The development of SF1 inverse agonists and small molecule inhibitors has opened the door for targeting SF1 in disease (80–82). In particular, isoquinolinone compounds effectively reduced SF1 target genes, steroidogenesis, and proliferation of adrenocortical carcinoma cells (82). Further studies will evaluate the effectiveness of these compounds within prostate cancer cells, both in vitro and in vivo.
Current treatments for CRPC are insufficient to stop progression to the deadly form of the disease. Although androgen-dependent growth can be partially targeted with ADT and steroidogenic enzyme inhibitors, the mechanisms by which intratumoral steroid synthesis is initiated and maintained must be uncovered for progress to be made in development of new treatments. Our data suggest that SF1 reprises its endocrine-centric roles within rogue prostate cancer cells where it stimulates local, autonomous steroid production and supports cell proliferation and survival to promote CRPC. The discovery of SF1 expression and action in aggressive prostate cancer cells presents a molecular insight into the development of intra-tumoral steroidogenesis and an appealing new target for treating CRPC.
Acknowledgments
We thank the following contributors: Drs. Ian Bird and David Abbott for input on evaluation of steroid synthesis, and Drs Ruth Sullivan and Marie Pinkerton for histopathology consultation.
This work was supported by The University of Wisconsin-Madison Graduate School (J.S.J.), the National Institutes of Health (NIH) Endocrinology and Reproductive Physiology Program (Training Grant NIH 5T32-HD041921-07 to S.R.L.), Experimental Pathology Laboratory, UWCCC Cancer Center Support (NIH Grant P30 CA014520), the National Primate Research Center (NIH Grant RR000167 to C.J.H. and T.Z.), the Clinical and Translational Science Award program through the National Institutes of Health National Center for Advancing Translational Sciences (NIH Grant UL1TR000427 to C.J.H. and T.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADT
- androgen deprivation therapy
- AR
- androgen receptor
- BrdU
- 5-bromo-2′-deoxyuridine
- CRPC
- castration-resistant prostate cancer
- DAPI
- 4′,6-diamidino-2-phenylindole
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- HPG
- hypothalamic-pituitary-gonadal
- 3βHSD
- 3-β-hydroxysteroid dehydrogenase
- LC/MS-MS
- liquid chromatography–tandem mass spectrometry
- MTT
- 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- SF1
- steroidogenic factor 1
- sh
- short hairpin.
References
- 1. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297 [DOI] [PubMed] [Google Scholar]
- 2. Tammela T. Endocrine treatment of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:287–295 [DOI] [PubMed] [Google Scholar]
- 3. Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–462 [DOI] [PubMed] [Google Scholar]
- 4. Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406 [DOI] [PubMed] [Google Scholar]
- 5. Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087–38094 [DOI] [PubMed] [Google Scholar]
- 6. Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490 [DOI] [PubMed] [Google Scholar]
- 7. Zhang X, Morrissey C, Sun S, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One. 2011;6:e27970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinkamp MP, O'Mahony OA, Brogley M, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69:4434–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vis AN, Schröder FH. Key targets of hormonal treatment of prostate cancer. Part 1: the androgen receptor and steroidogenic pathways. BJU Int. 2009;104:438–448 [DOI] [PubMed] [Google Scholar]
- 10. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825 [DOI] [PubMed] [Google Scholar]
- 12. Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415 [DOI] [PubMed] [Google Scholar]
- 13. Hoivik EA, Lewis AE, Aumo L, Bakke M. Molecular aspects of steroidogenic factor 1 (SF-1). Mol Cell Endocrinol. 2010;315:27–39 [DOI] [PubMed] [Google Scholar]
- 14. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490 [DOI] [PubMed] [Google Scholar]
- 15. Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993;7:852–860 [DOI] [PubMed] [Google Scholar]
- 16. Morohashi K, Iida H, Nomura M, et al. Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol Endocrinol. 1994;8:643–653 [DOI] [PubMed] [Google Scholar]
- 17. Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6:1249–1258 [DOI] [PubMed] [Google Scholar]
- 18. Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267:17913–17919 [PubMed] [Google Scholar]
- 19. Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem 1993;268:7494–7502 [PubMed] [Google Scholar]
- 20. Bakke M, Lund J. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine 3′,5′-monophosphate-responsive sequence in the bovine CYP17 gene. Mol Endocrinol. 1995;9:327–339 [DOI] [PubMed] [Google Scholar]
- 21. Mascaró C, Nadal A, Hegardt FG, Marrero PF, Haro D. Contribution of steroidogenic factor 1 to the regulation of cholesterol synthesis. Biochem. J. 2000;350(Pt 3):785–790 [PMC free article] [PubMed] [Google Scholar]
- 22. Schimmer BP, White PC. Minireview: Steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Shaughnessy PJ, Baker P, Sohnius U, Haavisto AM, Charlton HM, Huhtaniemi I. Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology. 1998;139:1141–1146 [DOI] [PubMed] [Google Scholar]
- 24. Ferraz-de-Souza B, Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, NR5A1) and human disease. Mol Cell Endocrinol. 2011;336:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol Cell Endocrinol. 2012;351:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang CCJ, Yao HH. Diverse functions of Hedgehog signaling in formation and physiology of steroidogenic organs. Mol Reprod Dev. 2010;77:489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeyasuria P, Ikeda Y, Jamin SP, et al. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610–1619 [DOI] [PubMed] [Google Scholar]
- 29. Zhao L, Bakke M, Hanley NA, et al. Tissue-specific knockouts of steroidogenic factor 1. Mol Cell Endocrinol. 2004;215:89–94 [DOI] [PubMed] [Google Scholar]
- 30. Mello MP, França ES, Fabbri HC, Maciel-Guerra AT, Guerra-Júnior G. Multifunctional role of steroidogenic factor 1 and disorders of sex development. Arq Bras Endocrinol Metabol. 2011;55:607–612 [DOI] [PubMed] [Google Scholar]
- 31. Ozisik G, Achermann JC, Jameson JL. The role of SF1 in adrenal and reproductive function: insight from naturally occurring mutations in humans. Mol Genet Metab. 2002;76:85–91 [DOI] [PubMed] [Google Scholar]
- 32. Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22:125–126 [DOI] [PubMed] [Google Scholar]
- 33. Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997;17:3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yazawa T, Mizutani T, Yamada K, et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111 [DOI] [PubMed] [Google Scholar]
- 35. Gondo S, Okabe T, Tanaka T, et al. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717–4725 [DOI] [PubMed] [Google Scholar]
- 36. Doghman M, Karpova T, Rodrigues GA, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987 [DOI] [PubMed] [Google Scholar]
- 37. Ramayya MS, Sheng M, Moroz K, Hill SM, Rowan BG. Human steroidogenic factor-1 (hSF-1) regulates progesterone biosynthesis and growth of ovarian surface epithelial cancer cells. J Steroid Biochem Mol Biol. 2010;119:14–25 [DOI] [PubMed] [Google Scholar]
- 38. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol. 1999;13:239–253 [DOI] [PubMed] [Google Scholar]
- 39. Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662 [DOI] [PubMed] [Google Scholar]
- 40. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486 [DOI] [PubMed] [Google Scholar]
- 41. Sadovsky Y, Crawford PA, Woodson KG, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA. 1995;92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ingraham HA, Lala DS, Ikeda Y, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312 [DOI] [PubMed] [Google Scholar]
- 43. Wang CY, Chen WY, Lai PY, Chung BC. Distinct functions of steroidogenic factor-1 (NR5A1) in the nucleus and the centrosome. Mol Cell Endocrinol. 2013;371:148–153 [DOI] [PubMed] [Google Scholar]
- 44. Lai PY, Wang CY, Chen WY, et al. Steroidogenic factor 1 (NR5A1) resides in centrosomes and maintains genomic stability by controlling centrosome homeostasis. Cell Death Differ. 2011;18:1836–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim JH, Lee SR, Li LH, et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6:e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jorgensen JS, Nilson JH. AR suppresses transcription of the LHβ subunit by interacting with steroidogenic factor-1. Mol Endocrinol. 2001;15:1505–1516 [DOI] [PubMed] [Google Scholar]
- 47. Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24 [DOI] [PubMed] [Google Scholar]
- 48. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Sudilovsky D, Zhang B, et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61:6064–6072 [PubMed] [Google Scholar]
- 50. Ricke WA, Ishii K, Ricke EA, et al. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118:2123–2131 [DOI] [PubMed] [Google Scholar]
- 51. Franco OE, Jiang M, Strand DW, et al. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978;21:274–281 [DOI] [PubMed] [Google Scholar]
- 53. Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979;17:16–23 [PubMed] [Google Scholar]
- 54. Horoszewicz JS, Leong SS, Chu TM, et al. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132 [PubMed] [Google Scholar]
- 55. Torres JM, Ortega E. Differential regulation of steroid 5α-reductase isozymes expression by androgens in the adult rat brain. FASEB J. 2003;17:1428–1433 [DOI] [PubMed] [Google Scholar]
- 56. Young M, McPhaul MJ. A steroidogenic factor-1-binding site and cyclic adenosine 3′,5′-monophosphate response element-like elements are required for the activity of the rat aromatase promoter in rat Leydig tumor cell lines. Endocrinology. 1998;139:5082–5093 [DOI] [PubMed] [Google Scholar]
- 57. Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J Clin Endocrinol Metab. 2009;94:2178–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P45017α (17α-hydroxylase/C17–20 lyase). J Steroid Biochem Mol Biol. 1994;50:267–273 [DOI] [PubMed] [Google Scholar]
- 59. Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017α (17α-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471 [DOI] [PubMed] [Google Scholar]
- 60. Rowlands MG, Barrie SE, Chan F, et al. Esters of 3-pyridylacetic acid that combine potent inhibition of 17α-hydroxylase/C17,20-lyase (cytochrome P45017α) with resistance to esterase hydrolysis. J Med Chem. 1995;38:4191–4197 [DOI] [PubMed] [Google Scholar]
- 61. Busygina TV, Vasiliev GV, Klimova NV, Ignatieva EV, Osadchuk AV. Binding sites for transcription factor SF-1 in promoter regions of genes encoding mouse steroidogenesis enzymes 3βHSDI and P450c17. Biochemistry Mosc. 2005;70:1152–1156 [DOI] [PubMed] [Google Scholar]
- 62. Li LA, Chang YC, Wang CJ, Tsai FY, Jong SB, Chung BC. Steroidogenic factor 1 differentially regulates basal and inducible steroidogenic gene expression and steroid synthesis in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. 2004;91:11–20 [DOI] [PubMed] [Google Scholar]
- 63. Ito M., Richard NY, Jameson JL. Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol Endocrinol. 1998;12:290–301 [DOI] [PubMed] [Google Scholar]
- 64. Ito M, Park Y, Weck J, Mayo KE, Jameson JL. Synergistic activation of the inhibin α-promoter by steroidogenic factor-1 and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol. 2000;14:66–81 [DOI] [PubMed] [Google Scholar]
- 65. Hammer GD, Krylova I, Zhang Y, et al. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526 [DOI] [PubMed] [Google Scholar]
- 66. Jacob AL, Lund J, Martinez P, Hedin L. Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J Biol Chem. 2001;276:37659–37664 [DOI] [PubMed] [Google Scholar]
- 67. Chen WY, Juan LJ, Chung BC. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol Cell Biol. 2005;25:10442–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sakai N, Terami H, Suzuki S, et al. Identification of NR5A1 (SF-1/AD4BP) gene expression modulators by large-scale gain and loss of function studies. J Endocrinol. 2008;198:489–497 [DOI] [PubMed] [Google Scholar]
- 69. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–1246 [DOI] [PubMed] [Google Scholar]
- 72. Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wilding G. 1995 Endocrine control of prostate cancer. Cancer Surv. 1995;23:43–62 [PubMed] [Google Scholar]
- 75. Monge A, Jagla M, Lapouge G, et al. Unfaithfulness and promiscuity of a mutant androgen receptor in a hormone-refractory prostate cancer. Cell Mol Life Sci. 2006;63:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Southwell J, Chowdhury SF, Gottlieb B, et al. An investigation into CAG repeat length variation and N/C terminal interactions in the T877A mutant androgen receptor found in prostate cancer. J Steroid Biochem Mol Biol. 2008;111:138–146 [DOI] [PubMed] [Google Scholar]
- 77. Ellem SJ, Risbridger GP. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer. 2007;7:621–627 [DOI] [PubMed] [Google Scholar]
- 78. Vu Hai MT, Warembourg M, Milgrom E. Hormonal control of progesterone receptors. Ann NY Acad Sci. 1977;286:199–209 [DOI] [PubMed] [Google Scholar]
- 79. Wang CY, Kao YH, Lai PY, Chen WY, Chung BC. Steroidogenic factor 1 (NR5A1) maintains centrosome homeostasis in steroidogenic cells by restricting centrosomal DNA-dependent protein kinase activation. Mol Cell Biol. 2013;33:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Del Tredici AL, Andersen CB, Currier EA, et al. Identification of the first synthetic steroidogenic factor 1 inverse agonists: pharmacological modulation of steroidogenic enzymes. Mol Pharmacol. 2008;73:900–908 [DOI] [PubMed] [Google Scholar]
- 81. Doghman M, Madoux F, Hodder P, Lalli E. Identification and characterization of steroidogenic factor-1 inverse agonists. Methods Enzymol. 2010;485:3–23 [DOI] [PubMed] [Google Scholar]
- 82. Madoux F, Li X, Chase P, et al. Potent, selective and cell penetrant inhibitors of SF-1 by functional ultra-high-throughput screening. Mol Pharmacol. 2008;73:1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expression Patterns. 2005;5:756–762 [DOI] [PubMed] [Google Scholar]
- 85. Bhatt KV, Hu R, Spofford LS, Aplin AE. Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene. 2007;26:1056–1066 [DOI] [PubMed] [Google Scholar]
- 86. Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-α and ER-β in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–3182 [PubMed] [Google Scholar]