Abstract
The role that estrogens play in the aging lung is poorly understood. Remodeling of the aging lung with thickening of the alveolar walls and reduction in the number of peripheral airways is well recognized. The present study was designed to address whether estrogen deficiency would affect age-associated changes in the lungs of female C57BL/6J mice. Lungs isolated from old mice (24 months old, estrogen-deficient) demonstrated decreased lung volume and decreased alveolar surface area. There was no difference in alveolar number in the lungs of old and young mice (6 months old, estrogen-replete). Estrogen replacement restored lung volume, alveolar surface area, and alveolar wall thickness to that of a young mouse. Estrogen receptor-α (ERα) protein expression increased without a change in ERβ protein expression in the lung tissue isolated from old mice. In the lungs of old mice, the number of apoptotic cells was increased as well as the activation of matrix metalloproteinase-2 and ERK. Young mice had the highest serum 17β-estradiol levels that decreased with age. Our data suggest that in the aging female mouse lung, estrogen deficiency and an increase of ERα expression lead to the development of an emphysematous phenotype. Estrogen replacement partially prevents these age-associated changes in the lung architecture by restoration of interalveolar septa. Understanding the role of estrogens in the remodeling of the lung during aging may facilitate interventions and therapies for aging-related lung disease in women.
Physiological aging of the lung is associated with enlargement of alveolar airspaces, a decrease in exchange surface area, and the loss of supporting tissue for peripheral airways, resulting in decreased static elastic recoil of the lung and increased residual volume and functional residual capacity (1). Aged lungs also demonstrate a homogeneous dilatation of airspaces that is accompanied by thickening of the alveolar walls as well as a reduction in the number of peripheral airways (2, 3). Verbeken and co-workers proposed that absence of alveolar wall destruction characterizes elderly vs emphysematous lungs (3).
Huang et al (4) have shown that there is a reduction in elastic fiber content and an increase in collagen content in the lung parenchyma with aging in male C57BL/6J (B6) mice. Large decreases in airway resistance reflect these structural changes, suggesting that aging effects on the lung parenchyma did not parallel the aging effects in the airways of B6 male mice (4). Despite these and other studies on male mice, the role estrogens may play in maintaining the structure and function of the female lung with aging is poorly understood.
Estrogen action is mediated via the 2 estrogen receptor (ER) subtypes, ERα and ERβ. Human and experimental studies have reported the expression of both ER subtypes in the lungs of humans and rodents (5, 6). The ER subtypes have unique roles in estrogen-dependent gene regulation, and the transcriptional activities of ERα and ERβ differ depending on the cell and tissue context (7). In light of the fact that there are 40 or more different cell types in the lung (8) and most tissues express predominantly one ER subtype over another, expression of both ERα and ERβ in the same cell type would predict a more complex regulation of estrogen-mediated gene expression. Couse et al (9) found that mouse lung had the highest ratio of ERα to ERβ mRNA of all nonreproductive tissues examined. Any change in the expression of these ER subtypes could therefore play a role in age-related lung disease.
Studies using young (5 months of age) ERβ-knockout mice showed that ERβ was necessary for the maintenance of the extracellular matrix (ECM) composition in the lung, and loss of ERβ led to abnormal lung structure (10). Based on these and other studies, we hypothesized that estrogen deficiency associated with aging could induce age-associated injury of the lungs in female individuals and that estrogen replacement might prevent or protect against some of these effects. We found an aging phenotype in the lungs of these mice that was characterized by an increase in markers of ECM turnover and a change in the ratio of ERα to ERβ expression.
Materials and Methods
Animals
Female B6 mice were purchased from The Jackson Laboratory. For the initial studies on aging, we used intact female mice that were 6 months of age (young) and 24 months of age (old). For the second study, mice were ovariectomized (Ovx) at 16 months of age using the previously described procedure that has been approved by the Committee for Animal Safety at the University of Miami School of Medicine (11). The characteristics of the estrous cycle have been extensively studied in B6 mice (12). Briefly, regular estrous cycles in mice from puberty (around 1 month of age) up to 3 months, but not thereafter, require the presence of males. Many mice have irregular and lengthened cycles, which last 6 days or longer, after 10 months of age. Persistent vaginal cornification due to tonic estrogen secretion occurs between ages 11 and 16 months. Its age of onset is inversely correlated with its duration. Persistent anestrus, characterized by the absence of circulating estrogens, starts at about 18 months of age.
The mice received either placebo or 17β-estradiol (E2) (0.025 mg) 90-day release pellets 2 weeks after Ovx and were killed at 22 months of age (11). The 3-mm pellets were implanted sc into the back of the mice using a sterile trochar and forceps. These pellets were replaced every 90 days during the course of the experiment. When the mice were killed, serum concentrations of E2 were measured by a competitive immunoassay kit (estradiol EIA test; Diagnostic Systems Laboratories Inc).
Lung tissue analysis/immunohistochemistry
For studies of morphometry and histology, mouse lungs were inflated with 10% neutral buffered formalin under 25 cm water pressure. Lungs were postfixed by immersion into 10% formaldehyde for 48 hours and then transferred to PBS. The volume of the fixed whole lungs was determined by immersion. Lung volume was normalized to body weight. Left lung lobes were processed and prepared into 4-μm sections for hematoxylin-eosin and trichrome staining (13).
Alveoli count and surface area estimation by design-based stereology
Before sampling, the absolute volume of each lung was determined by fluid displacement (14). To randomize tissue orientation, sampling of the tissue blocks and sections was performed according to the method described by Hyde et al (15). Alveoli number was counted using design-based stereology as described previously (16). In short, 2 serial sections of 3 μm were on the same slide. One section was used as the reference section and the other as the look-up section to count alveolar bridges. An alveolar bridge was counted whenever there was a discontinuous septum on the reference section and continuous in the look-up section. To measure the alveolar surface area, the multicascade method was used as described previously (16, 17).
Western analysis
Tissue samples were cut in 2-mm pieces and homogenized in lysis buffer. Protein was extracted with lysis buffer, and the concentration was assessed using the Pierce BCA protein assay kit. For ERα, ERK, phosphorylated ERK, cleaved caspase 3, and β-actin, 10 to 50 μg of protein lysate was fractionated on 10% polyacrylamide gels and transferred to nitrocellulose membranes as previously described (18). For immunoprecipitation experiments, 100 μg of protein extract were incubated with ERβ antibody or normal goat IgG for 1 hour at 4°C, followed by the addition of Protein G-PLUS Agarose overnight (Santa Cruz Biotechnology, Inc). The resulting Protein G-antibody conjugate was centrifuged at 4°C and washed 4 times with PBS (pH 7.4). The final pellet was resuspended in PBS, sample buffer was added, and the mixture was boiled for 3 minutes before analysis as described above. Immunoreactive bands were determined by exposing the nitrocellulose blots to a chemiluminescence solution (Santa Cruz Biotechnology) followed by exposure to X-Omat AR film (Eastman Kodak Co). The specificity of the signal was demonstrated by incubating blots with an excess of the corresponding specific immunizing peptide. Densitometry was performed using ImageJ version 1.45 software (National Institutes of Health) to determine relative amounts of protein. Experiments were performed in duplicate.
Hydroxyproline assay
Hydroxyproline content was determined according to the method of Reddy and Enwemeka (19). Briefly, individual samples of lung tissue were weighed and homogenized in hypotonic buffer (20). An equal volume of 10N HCl was added to the samples before drying at 110°F for 24 hours, the pH was adjusted, and the samples were vortexed and further incubated with perchloric acid and p-dimethylbenzaldehyde for 20 minutes at 60°C. Hydroxyproline standard was prepared according to a standard solution (0.5–12 μg). Hydroxyproline content was read at 557 nm, using the SoftMax Pro Software (Molecular Devices Corp), after cooling.
Matrix metalloproteinase-2 activity
Tissue supernatants were collected to assess matrix metalloproteinase-2 (MMP-2) activity as described (21). Standards (Chemicon) were electrophoresed in parallel. Zymography gels (Novex) were incubated for 24 hours for MMP-2 activity in 50mM Tris buffer, allowing determination of total proteolytic MMP activities with no interference from their associated tissue inhibitors. Densitometry, using ImageJ software from the National Institutes of Health, was used to determine relative MMP-2 activity.
Apoptosis detection
Apoptosis was evaluated using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay following the manufacturer's protocol (ApopTag; Oncor Inc). Slides were moved in a grid manner so that 5 individual fields were counted. Each experimental slide had 3 sections from the same mouse so that triplicates were performed. Twenty-five fields for each section of lung were assessed. Lung tissue was scored as apoptotic only when nuclear labeling by 4′,6-diamidino-2-phenylindole (blue) and nuclear TUNEL labeling (green) colocalized, resulting in turquoise nuclei (22).
Statistical analysis
All values are expressed as mean ± SEM. Significance of differences between experimental groups was determined by ANOVA in combination with Newman-Keuls multiple-comparison test. A P value of <.05 was considered significant.
Results
Comparison of intact young and old mice
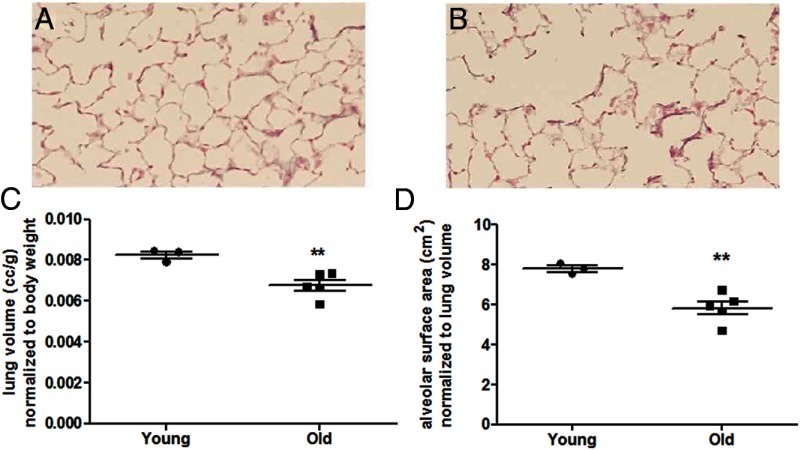
Body weight did not differ between young and old mice (Table 1). There was an expected decrease in uterine weight of old female B6 mice due to a decrease in circulating E2 (Table 1, P < .05). Histology (Figure 1, A and B) demonstrated the development of enlarged air spaces in the old B6 mouse. The lung volume of the old mice decreased to 0.0068 ± 0.0003 mL/g compared with young mice (0.0083 ± 0.0002 mL/g, Figure 1C, P < .005). Alveolar surface area decreased in old mice (Figure 1D, P < .05). No change in alveolar number (0.197 ± 0.03 million in young mice vs 0.155 ± 0.01 million in old mice) was detected in the old mice compared with young mice.
Table 1.
Body Weight, Uterine Weight, and E2 Levels of Young and Old B6 Mice
| Mice | Body Weight, g | Uterine Weight, g Wet Weight | E2 Levels, pg/mL |
|---|---|---|---|
| Young (6 mo, n = 7) | 25.6 ± 3.4 | 0.08 ± 0.006a | 18.4 ± 1.7a |
| Old (24 mo, n = 9) | 29.3 ± 5.3 | 0.06 ± 0.001 | 13.3 ± 0.6 |
P < .05 compared with old.
Figure 1.
Lung volume and alveolar surface area is reduced in aging female mice. A and B, Representative photomicrographs of the lung of a 6-month-old female B6 mouse (A), and a 24-month-old female B6 mouse (B) (hematoxylin-eosin staining at ×40 magnification). C, Lung volume normalized to body weight. D, Alveolar surface area normalized to lung volume. Data are expressed as scatter plots of at least 3 mice per group. **, P < .005.
ER expression
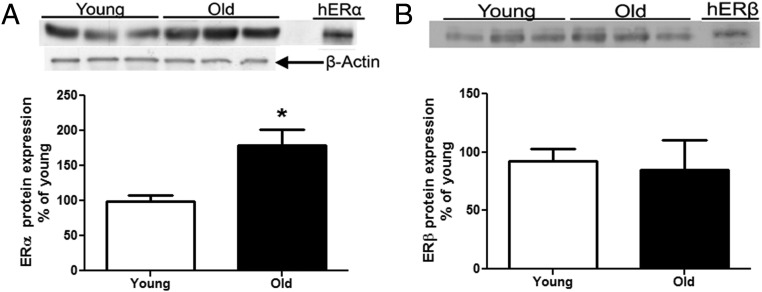
We found that there was an age-associated increase in the protein expression of ERα but not ERβ in the lungs of mice isolated from old mice compared with those isolated from young mice (Figure 2, A and B, P < .05).
Figure 2.
ERα protein expression increases in the lungs of old, estrogen-deficient female B6 mice (24 months). A and B, Lung tissue lysates from young (6 months) and old (24 months) mice were collected and Western analysis performed for ERα and β-actin loading control and ERβ (B). Shown are representative Western blots of ER protein expression. Data are graphed as the mean ± SEM of 2 experiments, 5 mice per group. The arrow indicates the β-actin loading control. *, P < .05. Abbreviation: hER, human recombinant ER.
MMP-2 activity
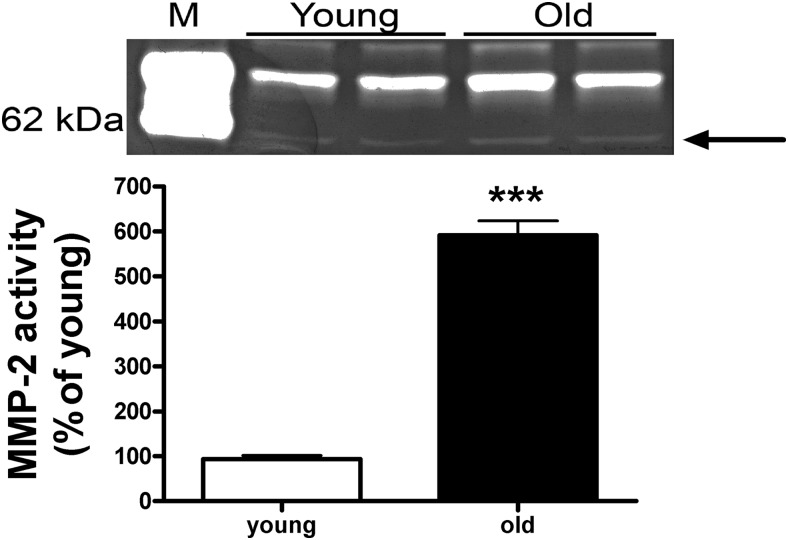
A change in ECM turnover may result from a dysregulation of MMPs and/or collagen production. MMP-2 activity was increased in lungs isolated from old mice compared with those of young mice (Figure 3, P < .005).
Figure 3.
MMP-2 activity is increased in lungs of old, estrogen-deficient female B6 mice (24 months). Zymography was performed on lung tissue protein extracts from young (6 months) and old (24 months) mice to measure MMP-2 activity. A representative zymogram is shown of 2 mice per group. Arrow denotes the active MMP-2 band. Data are graphed as the mean ± SEM of 5 mice per group. ***, P < .0005. M represents the standard for zymography.
ERK signaling
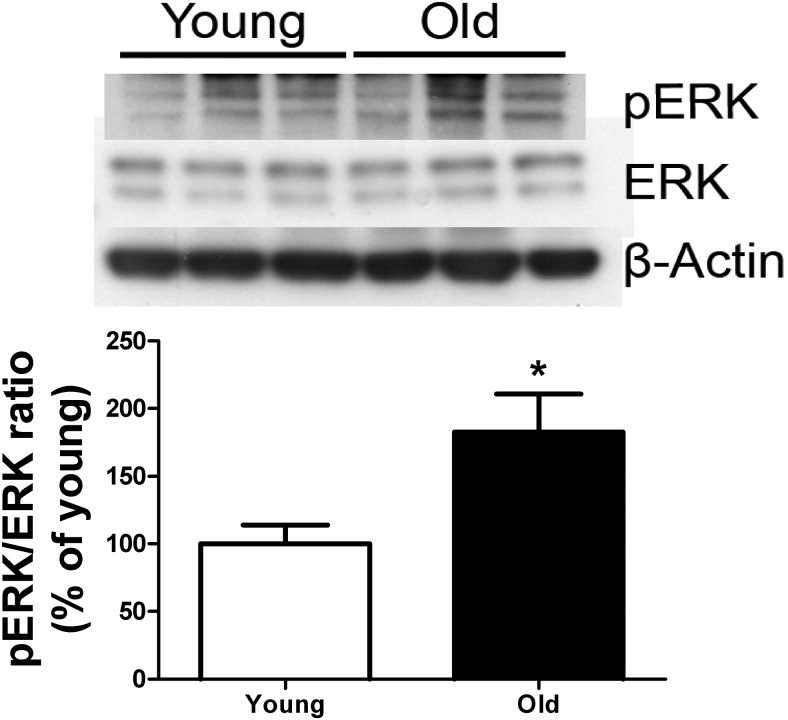
Recent literature has shown that changes in ERK activation affects apoptosis and changes in ER expression (23). In this study, we investigated the ERK signaling pathway in lung tissue isolated from young and old female mice and found an increase in ERK activation in old lung tissue compared with lung tissue from young mice (Figure 4, P < .05).
Figure 4.
ERK activation increases in lung tissue from old, estrogen-deficient female B6 mice (24 months). Western analysis was performed on tissue isolated from young (6 months) and old (24 months) mice. Shown is a representative Western blot and β-actin loading control of 3 mice per group. Data are graphed as the ratio of phospho-ERK(pERK)/ERK. *, P < .05.
Estrogen replacement studies
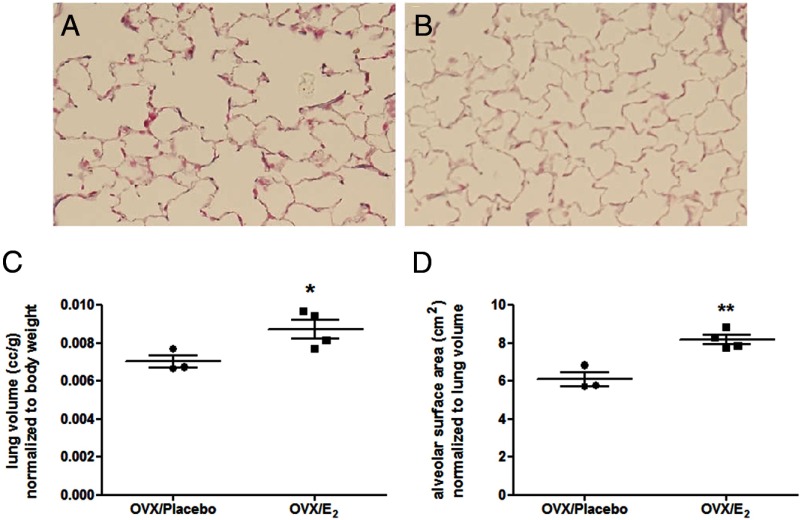
We investigated whether E2 replacement could affect the phenotype of aging lung. Mice underwent Ovx at 16 months of age and received either placebo or E2 pellets. Effectiveness of the Ovx procedure was confirmed by a reduction in uterine weight per body weight. Although Ovx reduced uterine weight (0.02 ± 0.003 g) compared with E2 replacement (0.10 ± 0.07 g), body weight did not differ between the groups (Ovx, 28.3 ± 0.01 g; E2, 29.8 ± 0.01 g). E2 replacement prevented airspace enlargement and restored lung volume and alveolar surface area to that of a young mouse (Figure 5, P < .05 and P < .005, respectively). In addition, we found that estrogen treatment restored alveolar wall thickness close to that of a young mouse (young, 3.2 ± 0.32 μm; E2 treated, 3.08 ± 0.069 μm).
Figure 5.
Age-related lung changes are prevented with E2 treatment. A and B, Representative trichrome stain of lungs isolated from old Ovx female B6 mice receiving either placebo (A) or E2 replacement (B) as described in Materials and Methods. Magnification, ×40. C, Lung volume normalized to body weight. D, Alveolar surface area normalized to lung volume. Data are expressed as scatter plots of at least 3 mice per group. *, < .05; **, P < .005.
ER status
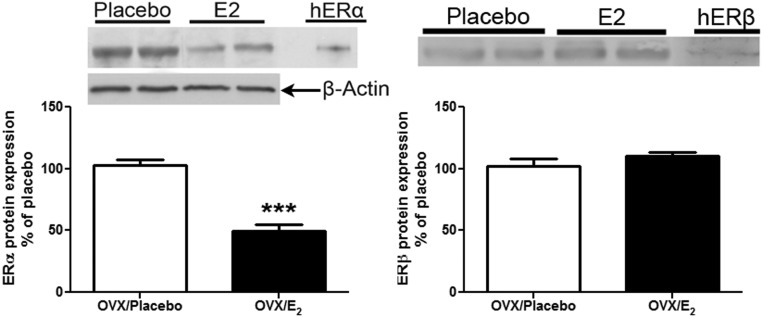
We found that E2 replacement prevented the increase of ERα protein expression in the aging lung by 50% over Ovx lung tissue (Figure 6A, P < .0005). ERβ protein expression did not change (Figure 6B). There was no difference in ER subtype expression between old non-Ovx mice compared with old Ovx mice (data not shown).
Figure 6.
E2 replacement prevents the age-related increase of ERα protein expression in lung tissue from estrogen-deficient female B6 mice. Lung tissue lysates from old Ovx placebo- and E2-treated mice were collected, and Western analysis was performed for ERα and β-actin loading control (A) or ERβ (B). Representative Western blots are shown. Data are graphed as the mean ± SEM of 2 experiments, 5 mice per group. The arrow indicates the β-actin loading control. *, P < .05; **, P < .005. Abbreviation: hER, human recombinant ER.
MMP-2 activity and hydroxproline content
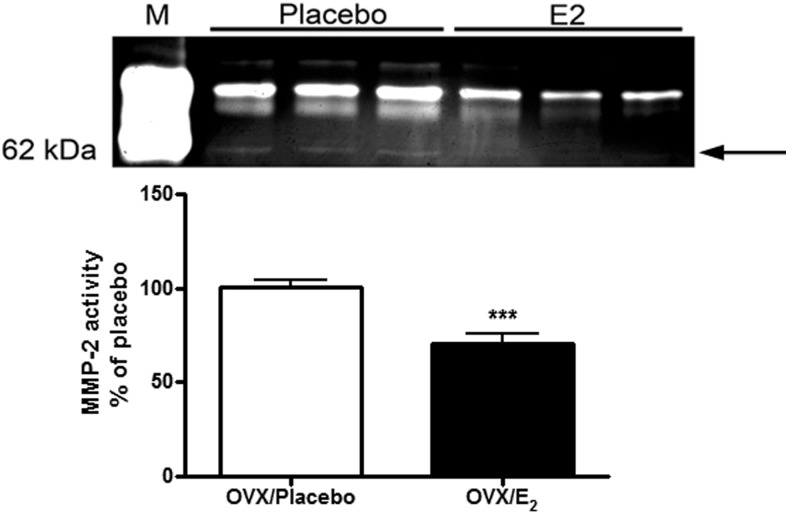
E2 replacement restored the age-associated alveolar wall alteration and was associated with decreased MMP-2 activity (Figure 7, P < .0005). Hydroxyproline content did not increase (0.018 ± 0.0048 for placebo vs 0.0169 ± 0.0008 for E2-treated).
Figure 7.
E2 replacement prevents the age-related increase of MMP-2 activity in lung tissue from estrogen-deficient female B6 mice. Lung tissue lysates from old Ovx placebo- and E2-treated mice were collected, and zymography was performed as described in Materials and Methods. A representative zymogram is shown. Data are graphed as the mean ± SEM of 2 experiments, 3 mice per group. The arrow indicates the active MMP-2 band. ***, P < .0005. M represents the standard for zymography.
Apoptosis
Although increased rates of apoptosis have been found primarily in humans with severe emphysema, TUNEL staining in mice has demonstrated variable results (24). To characterize apoptosis in this aging model, an ApopTag apoptosis detection kit was used to detect focal in situ staining inside early apoptotic nuclei and apoptotic bodies. There were 9.3 ± 1.2 apoptotic cells in the lungs of Ovx old mice (placebo, Figure 8A, P < .05) compared with 3.4 ± 0.5 in young mice. E2 treatment of old mice prevented the increased number of apoptotic cells (Figure 8A, 6 ± 0.6 cells) compared with mice that were placebo-treated (Figure 8A, P < .05). There was no difference in the number of apoptotic cells between E2-treated old mice and young mice. Additionally, Western blot analysis was used to measure caspase-3 activation, a marker of apoptosis, to validate the TUNEL staining. Although there was an increase in cleaved caspase-3 after Ovx (compared with old mice), E2 treatment of old Ovx mice prevented increased protein expression of cleaved caspase-3 (Figure 8B, P < .05).
Figure 8.
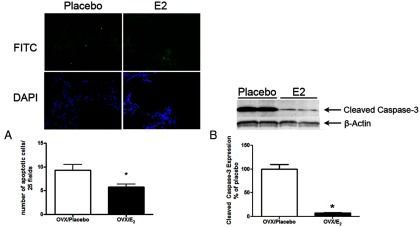
Apoptosis after Ovx in aging B6 female mice is decreased by E2 treatment. A, An ApopTag assay was performed on lung tissue sections from Ovx old female B6 mice treated with placebo or E2. The upper panels are stained with fluorescein isothiocyanate (FITC) (nuclear TUNEL labeling) as indicated in Materials and Methods; the lower panels are stained with 4′,6-diamidino-2-phenylindole (DAPI) (nuclear staining). *, P < .05. Five fields per section were evaluated, and data are shown as the mean ± SEM of 5 mice per group. B, Cleaved caspase-3 protein expression was visualized by Western analysis. *, P < .05, n = 3 mice per group. Arrows indicate cleaved caspase-3 and β-actin loading controls.
Discussion
In this study, we demonstrate for the first time that estrogen-deficient aging B6 female mice developed an emphysematous phenotype. This raises the question of the role of estrogen deficiency in the pathogenesis of chronic obstructive pulmonary disease (COPD) in women. With estrogen replacement, changes in lung architecture were ameliorated, and the architecture resembled that of a young mouse. These findings support the observation that approximately 75% of older postmenopausal women (age 56.6 years and older) who develop COPD are never-smokers (25–27); this does not reflect the almost 2 times higher standardized mortality rate for women compared with men with COPD (26). To our knowledge, data on the effects of hormone replacement therapy on the development or clinical course of COPD in women are not available.
There are few studies on the expression of ER subtypes in human lungs and the mechanisms of estrogen action and ER expression in normal lung. In mouse and human lung, ERβ is the predominant ER subtype (28). We have previously shown that both ERα and ERβ are present in human lung tissue and smooth muscle cells isolated from adult female lungs (18, 29). In the present study, we not only detected both ER subtypes by Western analysis but also found that lungs isolated from old mice had increased ERα protein expression with no change in ERβ expression compared with young B6 mice. Although these findings contrast with the studies by Patrone et al (30), they agree with other studies in adult human lung that detect expression of both receptors in adult lung tissue obtained from patients with lung cancer (31).
Estrogens are also fundamental in lung development. In the absence of estrogens, the lung loses normal alveolar septation. When estradiol is replaced, regeneration of the alveoli occurs and is dependent on the ER (32). Estrogen deprivation, however, may induce changes in the ER subtype ratio. Because ER levels in target tissues determine estrogen responsiveness, changes in the ER subtype ratio may lead to a decrease in E2-mediated action (33, 34). The effect of estrogen deficiency is well recognized in other organs, including the kidney. In the glomerulus, E2 replacement to estrogen-deficient mice increases ERβ levels, whereas in other tissue and cell types, E2 decreases ERα levels through direct transcriptional repression of ERα gene expression (35, 36). Ongoing experiments will determine whether the timing of estrogen replacement has an effect on lung ER subtype expression in our model.
In this report, the aging lung phenotype was characterized by increased MMP-2 activity that was prevented by E2 replacement. Our group has previously shown that activation of the ER contributes to the progression of pulmonary lymphangioleiomyomatosis via MMP-2–induced invasion (18). Estrogens, in particular, have potent regulatory influences on collagen and MMP expression, molecules important for ECM homeostasis in the kidney, and the retina as well as the lung (21, 37–39). In fact, the loss of ERβ in ERβ-knockout mouse lungs and the increased effects of ERα results in increased expression of membrane type-1 metalloproteinase, MMP-2 (active form), and tissue inhibitors of metalloproteinases, suggesting that lung ECM is regulated by ERβ (10). Decreased alveolar septa would decrease the exchange surface area and also enlarge alveolar sacs with the consequence of losing the supporting tissue for peripheral airways. This results in decreased static elastic recoil of the lung and increased residual volume and functional residual capacity.
Age-related changes in the ECM of the lung also affect altered lung mechanics with respect to the airways and lung parenchyma (40). Collagen (types I and III) and elastin are the major components of the framework of the alveolar structure and are critical in the mechanical function of the lung (41). Elastic fibers influence lung compliance at low lung volumes, whereas collagen fibrils are more important at high lung volumes (4). Early aging changes in male mice (age <20 months) include increased lung volume and static compliance and a loss of elastic fiber content in the lung parenchyma (4). In older male mice (age greater than 20 months), there is a change in airway resistance and an increase in the size of alveoli. To our knowledge, it is unknown whether age-dependent changes occur differently over time during aging in female mice. We would speculate that the contribution of age-related estrogen deficiency and/or decreased estrogen action could lead to changes similar to those reported in our model.
We also observed age-related changes in apoptosis that could be prevented by E2 replacement. Inflammation and oxidant stress are common triggers of apoptosis. Multiple studies have shown that aging is associated with an increase in oxidant stress (42, 43). To our knowledge, there has been no evaluation of the time course of apoptosis in human lungs. It appears, however, that in COPD, which is proposed as premature aging of the lung (44), the normal balance between apoptosis and cell proliferation has shifted, resulting in the loss of lung structure. Male Klotho mice, a mouse model of premature aging, also exhibit increased apoptosis and airspace enlargement (45). In lungs isolated from aging estrogen-deficient female mice, there are changes in the expression of MMPs as well as increased apoptosis.
Finally, it is known that oxidant stress/aging is an important mediator of proliferation and results in activation of various signaling molecules and pathways, among which are the MAPKs (46). In states of oxidant injury such as hyperoxia, activated ERK signaling increases apoptosis (47). Despite the modest increase in ERK activation in our study, it is possible that estrogen deficiency increases oxidant stress and triggers a signaling cascade leading to an aging phenotype.
In summary, the change in the ratio of ER subtype expression and subsequent loss of E2 function may be responsible for the changes in lung structure and activation of signaling pathways that may be detrimental in estrogen-deficient states. We found that an increase in markers of ECM turnover and a change in the ratio of ERα to ERβ expression are hallmarks of an aging phenotype in the lungs of female B6 mice. These findings may also extend to male lungs in light of the recent report in which estrogen therapy, mediated through ERβ, prevented progression of pulmonary hypertension in male rats treated with monocrotaline (48). In our study, estrogen rescue prevented the change in the ratio of ER subtypes. These findings underscore the importance of understanding estrogens in the aging lung (49).
Acknowledgments
This work was partially supported by the James S. Fauver Pulmonary Fibrosis Research Fund (to M.K.G.) and the National Institutes of Health National Institute of Aging (to S.J.E.).
Portions of this manuscript were presented at the annual meeting of The Endocrine Society in Washington, DC, in 2009.
Disclosure Summary: M.K.G., R.C., V.Z., S.S., P.R., R.V., M.A., X.X., and S.J.E. have nothing to declare.
Footnotes
- B6
- C57BL/6J
- COPD
- chronic obstructive pulmonary disease
- E2
- 17β-estradiol
- ECM
- extracellular matrix
- ER
- estrogen receptor
- MMP-2
- matrix metalloproteinase-2
- Ovx
- ovariectomized
- TUNEL
- terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
References
- 1. Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205 [DOI] [PubMed] [Google Scholar]
- 2. Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest. 1992;101:800–809 [DOI] [PubMed] [Google Scholar]
- 3. Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992;101:793–799 [DOI] [PubMed] [Google Scholar]
- 4. Huang K, Rabold R, Schofield B, Mitzner W, Tankersley CG. Age-dependent changes of airway and lung parenchyma in C57BL/6J mice. J Appl Physiol. 2007;102:200–206 [DOI] [PubMed] [Google Scholar]
- 5. Massaro D, Clerch LB, Massaro GD. Estrogen receptor-α regulates pulmonary alveolar loss and regeneration in female mice: morphometric and gene expression studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L222–L228 [DOI] [PubMed] [Google Scholar]
- 6. Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med. 2008;264:128–142 [DOI] [PubMed] [Google Scholar]
- 7. Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578 [DOI] [PubMed] [Google Scholar]
- 8. Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis. 1977;116:705–777 [DOI] [PubMed] [Google Scholar]
- 9. Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138:4613–4621 [DOI] [PubMed] [Google Scholar]
- 10. Morani A, Barros RP, Imamov O, et al. Lung dysfunction causes systemic hypoxia in estrogen receptor β knockout (ERβ−/−) mice. Proc Natl Acad Sci U S A. 2006;103:7165–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliot SJ, Karl M, Berho M, et al. Smoking induces glomerulosclerosis in aging estrogen-deficient mice through cross-talk between TGF-β1 and IGF-I signaling pathways. J Am Soc Nephrol. 2006;17:3315–3324 [DOI] [PubMed] [Google Scholar]
- 12. vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. 2nd ed New York: Raven Press; 1994:1213–1314 [Google Scholar]
- 13. Shahzeidi S, Sarnstrand B, Jeffery PK, McAnulty RJ, Laurent GJ. Oral N-acetylcysteine reduces bleomycin-induced collagen deposition in the lungs of mice. Eur Respir J. 1991;4:845–852 [PubMed] [Google Scholar]
- 14. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60 [PubMed] [Google Scholar]
- 15. Hyde DM, Tyler NK, Putney LF, Singh P, Gundersen HJ. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:216–226 [DOI] [PubMed] [Google Scholar]
- 16. Fehrenbach H, Voswinckel R, Michl V, et al. Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J. 2008;31:515–522 [DOI] [PubMed] [Google Scholar]
- 17. Fehrenbach H. Animal models of pulmonary emphysema: a stereologist's perspective. Eur Respir Rev. 2006;15:136–147 [Google Scholar]
- 18. Glassberg MK, Elliot SJ, Fritz J, et al. Activation of the estrogen receptor contributes to the progression of pulmonary lymphangioleiomyomatosis via matrix metalloproteinase-induced cell invasiveness. J Clin Endocrinol Metab. 2008;93:1625–1633 [DOI] [PubMed] [Google Scholar]
- 19. Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229 [DOI] [PubMed] [Google Scholar]
- 20. Kassira N, Glassberg MK, Jones C, et al. Estrogen deficiency and tobacco smoke exposure promote matrix metalloproteinase-13 activation in skin of aging B6 mice. Ann Plast Surg. 2009;63:318–322 [DOI] [PubMed] [Google Scholar]
- 21. Potier M, Elliot SJ, Tack I, et al. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol. 2001;12:241–251 [DOI] [PubMed] [Google Scholar]
- 22. Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest. 2001;108:807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719 [DOI] [PubMed] [Google Scholar]
- 24. Wright JL, Cosio MG, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller RD, Hepper NG, Kueppers F, Gordon H, Offord KP. Host factors in chronic obstructive pulmonary disease in an upper Midwest rural community. Design, case selection, and clinical characteristics in a matched-pair study. Mayo Clin Proc. 1976;51:709–715 [PubMed] [Google Scholar]
- 26. Ringbaek T, Seersholm N, Viskum K. Standardised mortality rates in females and males with COPD and asthma. Eur Respir J. 2005;25:891–895 [DOI] [PubMed] [Google Scholar]
- 27. Song Y, Klevak A, Manson JE, Buring JE, Liu S. Asthma, chronic obstructive pulmonary disease, and type 2 diabetes in the Women's Health Study. Diabetes Res Clin Pract. 2010;90:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verma MK, Miki Y, Sasano H. 2011 Sex steroid receptors in human lung diseases. J Steroid Biochem Mol Biol. 2011;127:216–222 [DOI] [PubMed] [Google Scholar]
- 29. Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD. The role of female hormones on lung function in chronic lung diseases. BMC Womens Health. 2011;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patrone C, Cassel TN, Pettersson K, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor β. Mol Cell Biol. 2003;23:8542–8552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stabile LP, Siegfried JM. Estrogen receptor pathways in lung cancer. Curr Oncol Rep. 2004;6:259–267 [DOI] [PubMed] [Google Scholar]
- 32. Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1154–L1159 [DOI] [PubMed] [Google Scholar]
- 33. Vanderbilt JN, Miesfeld R, Maler BA, Yamamoto KR. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987;1:68–74 [DOI] [PubMed] [Google Scholar]
- 34. Webb P, Lopez GN, Greene GL, Baxter JD, Kushner PJ. The limits of the cellular capacity to mediate an estrogen response. Mol Endocrinol. 1992;6:157–167 [DOI] [PubMed] [Google Scholar]
- 35. Catanuto P, Doublier S, Fornoni A, et al. 17β-Estradiol and tamoxifen upregulate estrogen receptor β expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75:1194–1201 [DOI] [PubMed] [Google Scholar]
- 36. Saceda M, Lippman ME, Chambon P, et al. Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol Endocrinol. 1988;2:1157–1162 [DOI] [PubMed] [Google Scholar]
- 37. Elliot S, Catanuto P, Fernandez P, et al. Subtype specific estrogen receptor action protects against changes in MMP-2 activation in mouse retinal pigmented epithelial cells. Exp Eye Res. 2008;86:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lei J, Silbiger S, Ziyadeh FN, Neugarten J. Serum-stimulated α1 type IV collagen gene transcription is mediated by TGF-β and inhibited by estradiol. Am J Physiol. 1998;274:F252–F258 [DOI] [PubMed] [Google Scholar]
- 39. Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621 [DOI] [PubMed] [Google Scholar]
- 40. Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180 [DOI] [PubMed] [Google Scholar]
- 41. Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 2005;98:1892–1899 [DOI] [PubMed] [Google Scholar]
- 42. Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood). 2007;232:592–606 [PubMed] [Google Scholar]
- 43. Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247 [DOI] [PubMed] [Google Scholar]
- 44. Vogelmeier C, Bals R. Chronic obstructive pulmonary disease and premature aging. Am J Respir Crit Care Med. 2007;175:1217–1218 [DOI] [PubMed] [Google Scholar]
- 45. Ishii M, Yamaguchi Y, Yamamoto H, Hanaoka Y, Ouchi Y. Airspace enlargement with airway cell apoptosis in Klotho mice: a model of aging lung. J Gerontol A Biol Sci Med Sci. 2008;63:1289–1298 [DOI] [PubMed] [Google Scholar]
- 46. Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17:287–296 [DOI] [PubMed] [Google Scholar]
- 47. Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care. 2007;11:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Umar S, Iorga A, Matori H, et al. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med. 2011;184:715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maciewicz RA, Warburton D, Rennard SI. Can increased understanding of the role of lung development and aging drive new advances in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2009;6:614–617 [DOI] [PubMed] [Google Scholar]