Abstract
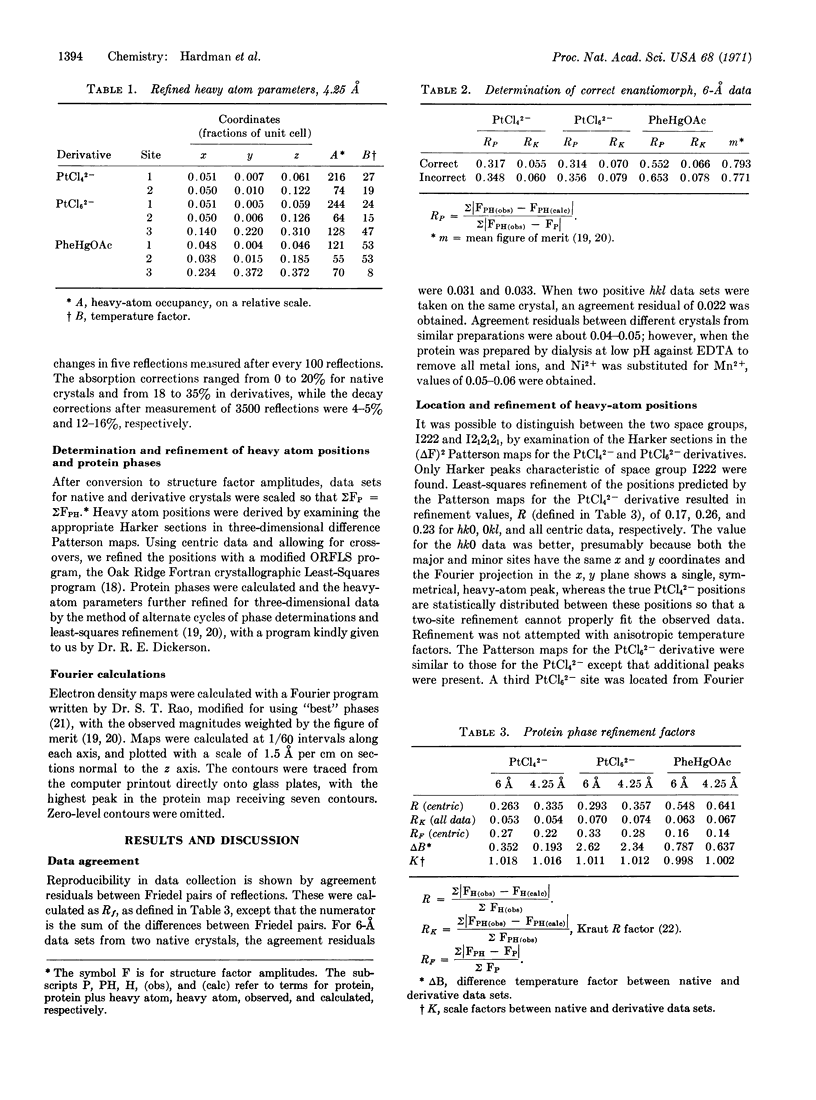
An electron density map produced by x-ray diffraction analysis of concanavalin A has been calculated to 4.25 Å from data of three isomorphous heavy atom derivatives. The crystals are orthorhombic, with unit-cell dimensions of 63.1, 87.0, and 89.2 Å for a, b, and c, respectively. The space group is I222, with eight asymmetric units per unit cell. The crystal asymmetric unit contains 27,000 daltons of protein and reflects the chemically unique component (protomer) within the oligomer. Separate chemical studies indicate that the protomer consists of two different polypeptide chains. Four protomers cluster around the intersection of three mutually perpendicular two-fold rotation axes to form a molecule of 108,000 daltons. The molecule can also be subdivided into two-protomer units of 54,000 daltons. Within the two-protomer unit, there are significantly more contacts joining the protomers than there are between adjacent two-protomer units that form the total molecule. These results provide a possible explanation for disagreement in molecular weights obtained in previous ultracentrifugal studies.
Keywords: x-ray diffraction, electron density map, isomorphous replacement, subunits, molecular weight
Full text
PDF
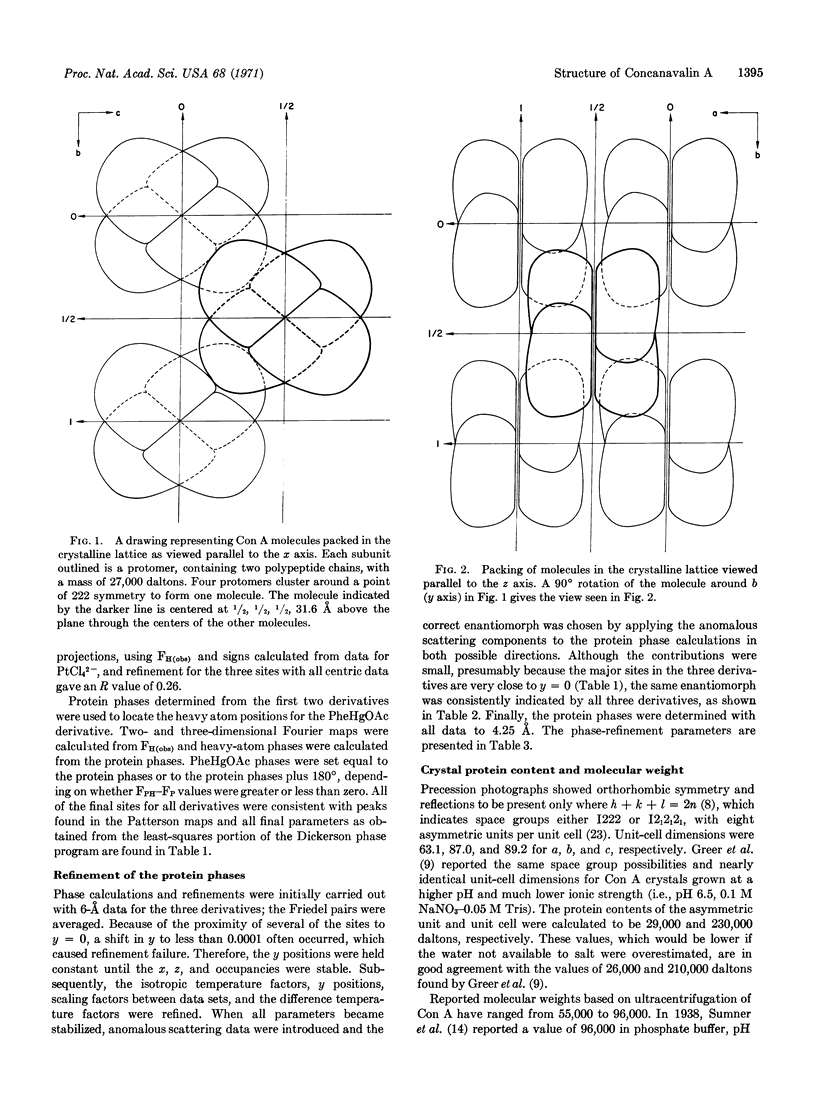

Images in this article
Selected References
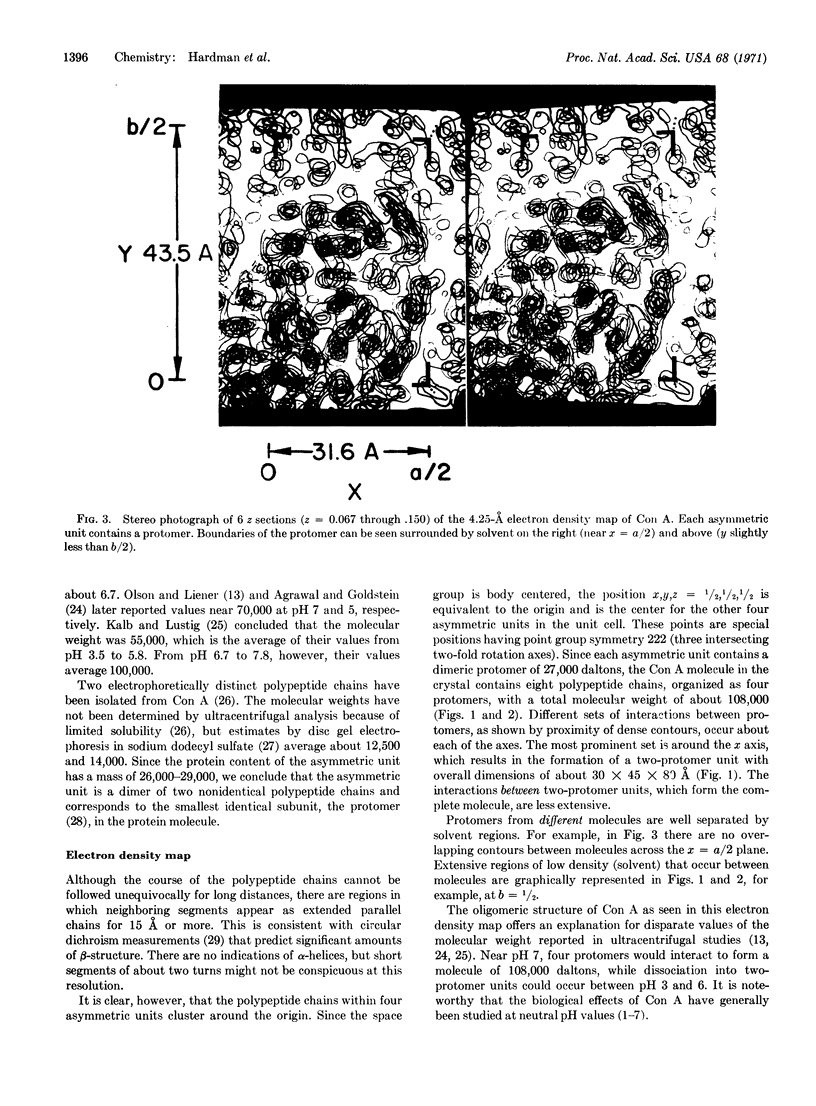
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Physical and chemical characterization of concanavalin A, the hemagglutinin from jack bean (Canavalia ensiformis). Biochim Biophys Acta. 1967 Feb 21;133(2):376–379. doi: 10.1016/0005-2795(67)90081-5. [DOI] [PubMed] [Google Scholar]
- Anggård E., Matschinsky F. M., Samuelsson B. Prostaglandins: enzymatic analysis. Science. 1969 Jan 31;163(3866):479–480. doi: 10.1126/science.163.3866.479. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Greer J., Kaufman H. W., Kalb A. J. An x-ray crystallographic study of concanavalin A. J Mol Biol. 1970 Mar 14;48(2):365–366. doi: 10.1016/0022-2836(70)90169-5. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUT J., SIEKER L. C., HIGH D. F., FREER S. T. Chymotrypsinogen: a three-dimensional fourier synthesis at 5 angstrom resolution. Proc Natl Acad Sci U S A. 1962 Aug;48:1417–1424. doi: 10.1073/pnas.48.8.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Lustig A. The molecular weight of concanavalin A. Biochim Biophys Acta. 1968 Oct 21;168(2):366–367. doi: 10.1016/0005-2795(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Kay C. M. The presence of beta-structure in concanavalin A. FEBS Lett. 1970 Jul 29;9(2):78–80. doi: 10.1016/0014-5793(70)80317-9. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Beychok S. Immunochemical studies on blood groups. 43. The interaction of blood group substances from various sources with a plant lectin, concanavalin A. J Immunol. 1969 Jun;102(6):1354–1362. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Liener I. E. Some physical and chemical properties of concanavalin A, the phytohemagglutinin of the jack bean. Biochemistry. 1967 Jan;6(1):105–111. doi: 10.1021/bi00853a018. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Liener I. E. The association and dissociation of concanavalin A, the phytohemagglutinin of the jack bean. Biochemistry. 1967 Dec;6(12):3801–3808. doi: 10.1021/bi00864a025. [DOI] [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. 13. The interaction of concanavalin A with alpha-mannans from a variety of microorganisms. J Biol Chem. 1968 Apr 25;243(8):2003–2007. [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wyckoff H. W., Doscher M., Tsernoglou D., Inagami T., Johnson L. N., Hardman K. D., Allewell N. M., Kelly D. M., Richards F. M. Design of a diffractometer and flow cell system for X-ray analysis of crystalline proteins with applications to the crystal chemistry of ribonuclease-S. J Mol Biol. 1967 Aug 14;27(3):563–578. doi: 10.1016/0022-2836(67)90059-9. [DOI] [PubMed] [Google Scholar]