Abstract
The phycobiliproteins of the blue-green algae Synechococcus sp. and Aphanocapsu sp. were characterized with respect to homogeneity, isoelectric point, and subunit composition. Each of the biliproteins consisted of two different noncovalently associated subunits, with molecular weights of about 20,000 and 16,000 for phycocyanin, 17,500 and 15,500 for allophycocyanin, and 22,000 and 20,000 for phycoerythrin. Covalently bound chromophore was associated with each subunit.
Keywords: Synechococcus, Aphanocapsa, molecular weights, gel electrophoresis, DEAE-Sephadex, antisera
Full text
PDF
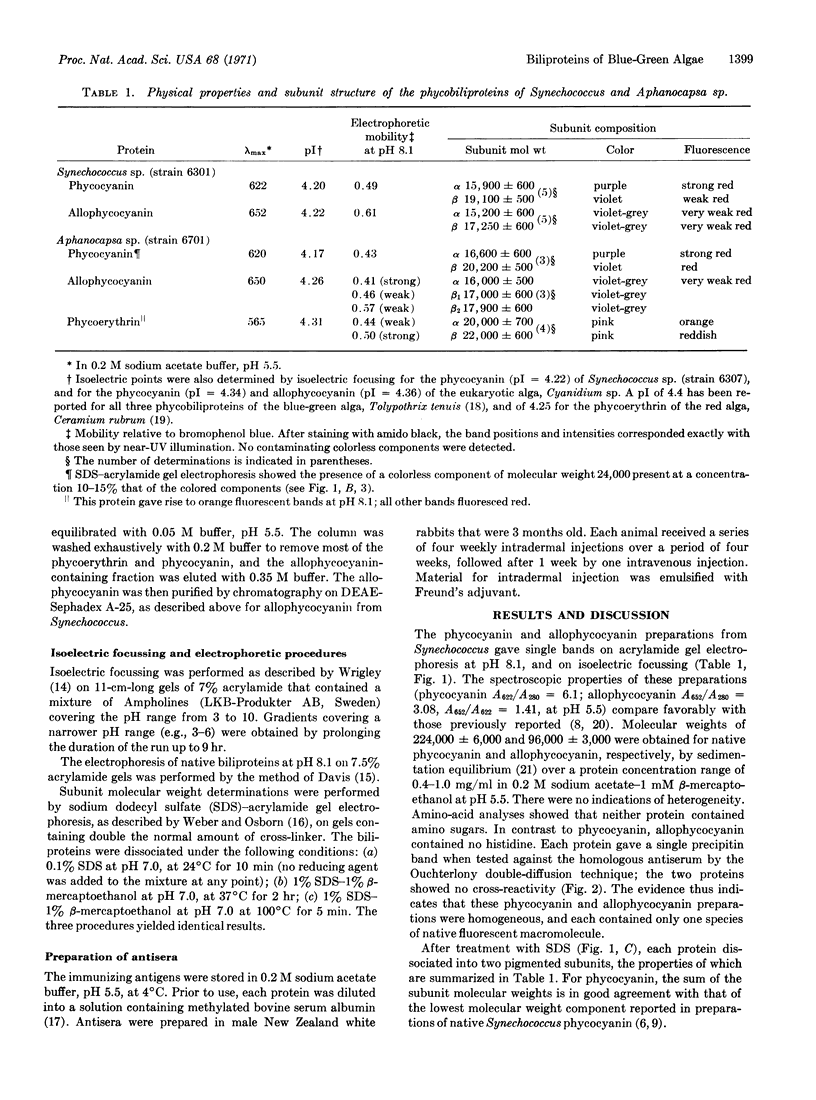
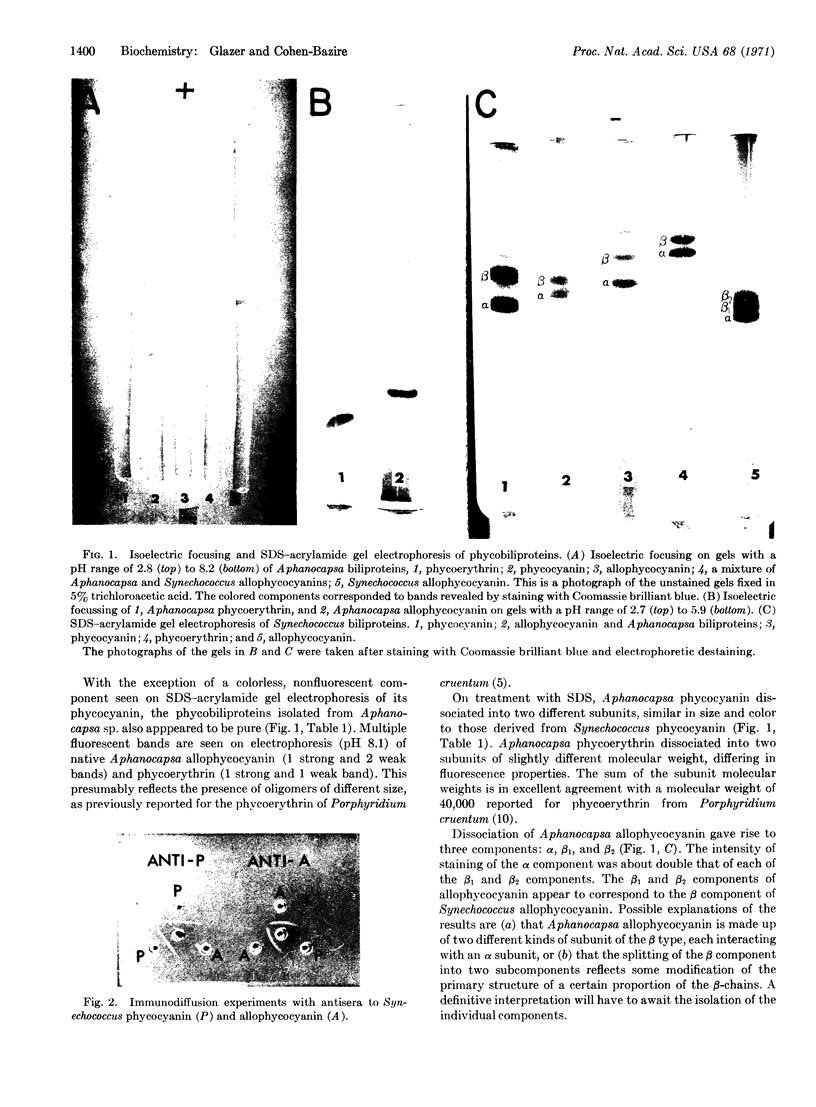

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig I. W., Carr N. G. C-phycocyanin and allophycocyanin in two species of blue-green algae. Biochem J. 1968 Jan;106(2):361–366. doi: 10.1042/bj1060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eriksson-Quensel I. B. The molecular weights of phycoerythrin and phycocyan. I. Biochem J. 1938 Mar;32(3):585–589. doi: 10.1042/bj0320585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E. Properties and Ultrastructure of Phycoerythrin From Porphyridium cruentum. Plant Physiol. 1969 Nov;44(11):1629–1638. doi: 10.1104/pp.44.11.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A., Crespi H. L., Katz J. J. Effect of side-chain deuteration on protein stability. Biochemistry. 1965 Jul;4(7):1213–1225. doi: 10.1021/bi00883a002. [DOI] [PubMed] [Google Scholar]
- Kao O., Berns D. S. The monomer molecular weight of C-phycocyanin. Biochem Biophys Res Commun. 1968 Nov 8;33(3):457–462. doi: 10.1016/0006-291x(68)90595-0. [DOI] [PubMed] [Google Scholar]
- Mieras G. A., Wall R. A. Sub-units of the algal biliprotein phycoerythrin. Biochem J. 1968 Mar;107(1):127–128. doi: 10.1042/bj1070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G. J., Riggs A. F. Aggregation properties of C-Phycocyanin from Anacystis nidulans. Biochim Biophys Acta. 1969 May;181(1):234–243. doi: 10.1016/0005-2795(69)90246-3. [DOI] [PubMed] [Google Scholar]
- PLESCIA O. J., BRAUN W., PALCZUK N. C. PRODUCTION OF ANTIBODIES TO DENATURED DEOXYRIBONUCLEIC ACID (DNA). Proc Natl Acad Sci U S A. 1964 Aug;52:279–285. doi: 10.1073/pnas.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E., Berns D. S. Protein-protein interaction. The phycocyanin system. Biochemistry. 1965 Dec;4(12):2597–2606. doi: 10.1021/bi00888a008. [DOI] [PubMed] [Google Scholar]
- Teale F. W., Dale R. E. Isolation and spectral characterization of phycobiliproteins. Biochem J. 1970 Jan;116(2):161–169. doi: 10.1042/bj1160161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]