Abstract
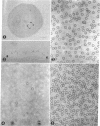
A method is described for determining the two-dimensional distribution of specific antigens on cell surfaces, and is applied to the D antigen of the Rh antigenic system. Rh-positive human erythrocytes are allowed to react with purified 125I-labeled human anti-Rho(D) γ-globulin antibodies, and the sensitized cells are then lysed at an air-water interface. The residual cell membranes are spread flat by surface forces, and are picked up on a carbon-strengthened collodion-coated electron microscope grid. The membranes are then stained with ferritin-conjugated goat antibodies directed against human γ-globulins. Only Rh-positive cells sensitized with anti-Rho(D) antibodies bind the ferritin-conjugated antihuman γ-globulins. The ferritin particles are found in small clusters on the membrane surface, and the number of such clusters per unit area agrees with the number of 125I-labeled anti-Rho(D) antibodies bound per unit area. The Rho(D) antigenic sites appear to be molecularly dispersed on the membrane surface, but in a random two dimensional array.
Keywords: ferritin-conjugated γ-globulins, surface antigens
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Hämmerling U., De Harven E., Boyse E. A., Old L. J. Antigenic structure of cell surfaces. An immunoferritin study of the occurrence and topography of H-2' theta, and TL alloantigens on mouse cells. J Exp Med. 1969 Nov 1;130(5):979–1001. doi: 10.1084/jem.130.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. C., Douglas S. D., Petz L. D., Fudenberg H. H. Ferritin-antibody localization of erythrocyte antigenic sites in immunohemolytic anemias. J Immunol. 1968 Oct;101(4):621–627. [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Glaser M., Simpkins H., Singer S. J., Sheetz M., Chan S. I. On the interactions of lipids and proteins in the red blood cell membrane. Proc Natl Acad Sci U S A. 1970 Mar;65(3):721–728. doi: 10.1073/pnas.65.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green F. A. Erythrocyte membrane sulfhydryl groups and Rh antigen activity. Immunochemistry. 1967 Jul;4(4):247–257. doi: 10.1016/0019-2791(67)90186-3. [DOI] [PubMed] [Google Scholar]
- Green F. A. Phospholipid requirement for Rh antigenic activity. J Biol Chem. 1968 Oct 25;243(20):5519–5521. [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- Hughes-Jones N. C. Reactivity of anti-D immunoglobulin G subunits. Nature. 1970 Jul 11;227(5254):174–175. doi: 10.1038/227174b0. [DOI] [PubMed] [Google Scholar]
- Hämmerling U., Aoki T., de Harven E., Boyse E. A., Old L. J. Use of hybrid antibody with anti-gamma-G and anti-ferritin specificities in locating cell surface antigens by electron microscopy. J Exp Med. 1968 Dec 1;128(6):1461–1473. doi: 10.1084/jem.128.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN B., MASOUREDIS S. P. EFFECT OF FORMALIN AND PERIODIC ACID ON RED CELL RHO (D) AND ENZYME-EXPOSED RHO (D) ANTIGEN. J Immunol. 1963 Aug;91:233–242. [PubMed] [Google Scholar]
- LEE R. E., FELDMAN J. D. VISUALIZATION OF ANTIGENIC SITES OF HUMAN ERYTHROCYTES WITH FERRITIN-ANTIBODY CONJUGATES. J Cell Biol. 1964 Nov;23:396–401. doi: 10.1083/jcb.23.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE P., CELANO M. J., VOS G. H., MORRISON J. The first human blood, ---/---, which lacks the 'D-like' antigen. Nature. 1962 Apr 21;194:304–305. doi: 10.1038/194304a0. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- MASOUREDIS S. P. Reaction of I-131 trace labeled human anti-Rho(D) with red cells. J Clin Invest. 1959 Feb;38(2):279–290. doi: 10.1172/JCI103800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASOUREDIS S. P. Relationship between RhO(D) genotype and quantity of 1131 anti-RhO(D) bound to red cells. J Clin Invest. 1960 Sep;39:1450–1462. doi: 10.1172/JCI104164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masouredis S. P., Dupuy M. E., Elliot M. Relationship between Rh-o(D) zygosity and red cell Rh-o(D) antigen content in family members. J Clin Invest. 1967 May;46(5):681–694. doi: 10.1172/JCI105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. D., Singer S. J. A technique for the specific staining of macromolecules and viruses with ferritin-antibody conjugates. J Mol Biol. 1971 Mar 28;56(3):633–635. doi: 10.1016/0022-2836(71)90407-4. [DOI] [PubMed] [Google Scholar]
- SINGER S. J. Preparation of an electron-dense antibody conjugate. Nature. 1959 May 30;183(4674):1523–1524. doi: 10.1038/1831523a0. [DOI] [PubMed] [Google Scholar]
- Schmidt P. J., Vos G. H. Multiple phenotypic abnormalities associated with Rh-null (---/---). Vox Sang. 1967 Jul;13(1):18–20. doi: 10.1111/j.1423-0410.1967.tb03383.x. [DOI] [PubMed] [Google Scholar]
- VOS G. H., VOS D., KIRK R. L., SANGER R. A sample of blood with no detectable Rh antigens. Lancet. 1961 Jan 7;1(7167):14–15. doi: 10.1016/s0140-6736(61)92183-3. [DOI] [PubMed] [Google Scholar]
- WESTERMAN M. P., PIERCE L. E., JENSEN W. N. A direct method for the quantitative measurement of red cell dimensions. J Lab Clin Med. 1961 May;57:819–824. [PubMed] [Google Scholar]
- Wolff I., Springer G. F. Observations on Rh active substances. Bibl Haematol. 1965;23:510–514. doi: 10.1159/000384300. [DOI] [PubMed] [Google Scholar]