Abstract
This study evaluated the efficacy of percutaneous radiofrequency ablation (RFA) for the treatment of metachronous liver metastases of gastric cancer. We enrolled a total of 44 patients who underwent percutaneous RFA for the treatment of metachronous liver metastases after resection of a primary gastric adenocarcinoma from January 2002 to November 2011. The primary endpoint of this study was overall survival (OS) and recurrence-free survival (RFS) after RFA. Systemic chemotherapy was combined with RFA in 40 patients; the OS and RFS of the patients with liver-only metastasis who underwent RFA and chemotherapy were 20.9 months (95 % CI 18.4–23.4) and 9.8 months (95 % CI 9.2–10.5), respectively. On multivariate analysis, the factors independently, negatively associated with OS were extrahepatic metastatic lesions (HR 12.6, 95 % CI 3.7–42.9; p = 0.001), no chemotherapy (HR 43.3, 95 % CI 7.4–251.3; p = 0.001), and tumor number ≥2 (HR 2.6, 95 % CI 1.2–5.9; p = 0.015). The factors independently, negatively associated with RFS were extrahepatic metastatic lesions (HR 3.6, 95 % CI 1.6–7.8; p = 0.003) and bilobar intrahepatic distribution (HR 3.9, 95 % CI 1.5–9.9; p = 0.001). The efficacy of percutaneous RFA for metachronous liver metastases of gastric cancer is limited to patients with a single, unilobar metastasis without extrahepatic metastatic lesions. Combined systemic chemotherapy is very important for the prolongation of OS.
Keywords: Radiofrequency ablation, Stomach neoplasms, Metastasis, Chemotherapy
Introduction
Liver metastasis from gastric cancer (LMGC) has a very poor prognosis and there are no effective treatment modalities [1]. Liver metastases can be found in 5–9 % of patients with gastric cancer, and cancer recurrence and metastatic patterns are associated with locoregional, peritoneal, and hematogenous metastases; all metastatic patterns are found simultaneously in most cases [2–4]. The vast majority of patients with LMGC may in fact have systemic disease. Only a very small subset of patients with LMGC are candidates for surgical resection as the disease is often associated with extrahepatic metastatic lesions: peritoneal dissemination, lymph node metastases, and direct cancer invasion of other organs [1, 4, 5].
The benefits of surgical resection for LMGC are unsatisfactory; systemic chemotherapy is a standard treatment approach for most patients with this disease [1, 6, 7]. Radiofrequency ablation (RFA) is a commonly-used alternative to surgery for tumor ablation [8]. The treatment efficacy of RFA for LMGC, however, remains poorly defined. In this study, we evaluated the efficacy of percutaneous RFA for treatment of metachronous liver metastases of gastric cancer after curative resection of the primary lesion.
Patients and methods
Patients
At Chonnam National University Hwasun Hospital in Jeonnam, Korea, a total of 1,342 patients with recurrent or metastatic gastric cancer received palliative chemotherapy between January 2002 and November 2011. The criteria for inclusion in our study of RFA for liver metastases were as follows: (1) histologically confirmed gastric adenocarcinoma; (2) presence of metachronous liver metastases, arising more than 3 months after surgery; (3) percutaneous complete ablation of the liver metastases was feasible; (4) no evidence of anastomotic site recurrence; (5) absence of extrahepatic metastatic lesions (preferable), or presence of minimal extrahepatic metastatic lesions with liver metastases considered suitable for RFA. Of the original 1,342 patients, a total of 229 (17 %) had liver metastases. Of these 229 patients, 185 were not considered eligible for RFA due to tumor growth in multiple liver segments, extensive extrahepatic metastatic lesions, and/or unfavorable clinical conditions such as poor performance status, old age, and cardiopulmonary dysfunction. We prospectively enrolled a total of 44 patients to undergo RFA for the treatment of metachronous liver metastasis after curative margin-negative (R0) resection of the primary gastric adenocarcinoma. All data were prospectively recorded and only the survival data was updated from the cancer registry at the time of analyses. Eastern Cooperative Oncology Group performance status (ECOG PS) was evaluated according to the established criteria. This study was approved by the Institutional Review Board of Chonnam National University Medical School Research Institution (2012-110). The recommendations of the Declaration of Helsinki for biomedical research involving human subjects were followed throughout.
Liver metastases were diagnosed during a pre-programmed follow-up schedule of physical examination, simple chest radiography, abdomal computed tomography (CT), endoscopy, and, if necessary, liver magnetic resonance imaging (MRI). Chest CT and bone scan or positron emission tomography were performed to evaluate for extrahepatic metastatic lesions if liver metastases were detected. A total of five patients received a needle aspiration biopsy just before RFA, as their abdominal CT and MRI scans did not show the typical enhancement pattern of liver metastases. All biopsy results demonstrated metastatic adenocarcinoma.
RFA technique
Percutaneous hepatic lesion RFA was performed, under local and intravenous anesthesia, by two experienced interventional radiologists with 14 and 6 years of experience, respectively. RFA was performed using an internally-cooled monopolar electrode with a 3 cm active tip (Valleylab, Boulder, CO, USA) and a 150 W radiofrequency generator (Cool-tip RF System, Radionics, Burlington, MA, USA). A standard grounding pad (Valleylab, Boulder, CO, USA) was placed on the patient’s back. All RFA was performed under real-time sonographic guidance with a 1–5 MHz curved probe (LOGIQ E9, GE Healthcare, Milwaukee, WI, USA). Radiofrequency current was applied to each lesion for 12 min at 150 W to create a radius of ablation at least 10 mm larger than the largest tumor diameter. If the tumors were larger than 2.5 cm in greatest diameter, we performed multiple overlapping ablations to cover the entire tumor, with a 5 mm ablative margin. After RFA, the intrahepatic electrode track was cauterized to minimize bleeding and prevent tumoral seeding.
One hour after the initial treatment, contrast-enhanced dynamic CT was performed. When the thickness of the ablative margin was at least 0.5 cm for the index tumor, the treatment was considered finished. When a residual enhanced lesion was seen on dynamic CT, an additional RFA was immediately performed.
Follow-up
Local therapeutic and technical efficacy was evaluated by contrast-enhanced dynamic CT scanning 1 month after RFA. Clinical tumor recurrence and response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.0) [9], by subsequent CT scanning after every two or three courses of chemotherapy. When patients did not receive chemotherapy, CT scanning was performed every 3 months after RFA, or in the case of clinical suspicion for recurrence.
Chemotherapy
Peri-procedural systemic chemotherapy was administered to patients just before (n = 23) or after (n = 17) RFA (within 3 weeks before or after RFA). In patients with no evidence of recurrence, chemotherapy was maintained for 6 months. When follow-up CT showed intrahepatic or extrahepatic recurrence, second-line chemotherapy was initiated or best supportive care was performed, according to the clinical situation. Chemotherapy regimens included a variety of agents such as taxanes, oxaliplatin, irinotecan, cisplatin, 5-fluorouracil (5-FU), and TS-1. Doublet chemotherapy regimens were most commonly used.
Statistics
Kaplan–Meier analysis was applied to assess clinical factors affecting overall survival (OS) and recurrence-free survival (RFS), and the significance of differences in survival curves was determined by the log-rank test. OS was defined as the period from the date of RFA to the date of death from any cause. RFS was defined as the period from the date of RFA to the date of disease progression or death, whichever occurred first. If neither event had occurred at the time of the last record, the patient was censored at that time. The factors included in univariate survival analysis were age, gender, ECOG PS, tumor size, tumor number, presence of liver-only metastases, presence of extrahepatic metastatic lesions, and intrahepatic tumor distribution (unilobar or bilobar). Multivariate regression analysis using the Cox proportional hazards regression model (stepwise forward procedure), was performed to achieve an adjusted hazard ratio (HR) to determine the prognostic factors for OS and RFS. A 2-tailed p < 0.05 was considered significant for all analysis. The SPSS software package, version 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Patient characteristics
The baseline characteristics of the 44 patients are listed in Table 1. All patients had gastric adenocarcinoma and there were no distant metastases identified at the time of initial operation. All patients received curative resection for their gastric cancer, combined with D2 lymph node dissection. The median follow-up time (from surgery to death or last follow-up date) was 31.7 months, with a range of 10.5–57.1 months. The median patient age was 64 years, with a range of 38–83 years. A total of 26 patients (59.1 %) were male, and 18 patients (40.9 %) were female. The median time to development of liver metastases after surgery was 12.9 months (95 % CI 3.9–37.7 months). A total of 13 patients (29.5 %) had simultaneous extrahepatic metastatic lesions: 11 patients (25 %) had metastases to intraabdominal lymph nodes; 1 patient had peritoneal seeding nodules; one patient had a metastatic pleural nodule. A total of 30 patients (68.2 %) had a unilobar intrahepatic distribution, and 14 patients (31.8 %) had bilobar intrahepatic metastatic tumors. A total of 23 patients had a single liver metastasis, 20 patients had 2 liver metastatic tumors, and only 1 patient had 3 liver metastases. The median size of the largest metastatic liver tumor was 2 cm, with a range of 1–2.7 cm. A total of 40 patients (90.9 %) received systemic chemotherapy, and 18 patients (41 %) received second-line chemotherapy. Only four patients did not receive systemic chemotherapy due to patient’s refusal; these patients had liver-only metastases. All patients with extrahepatic metastatic lesions received chemotherapy. The most commonly used chemotherapy regimen was a oxaliplatin and 5-FU doublet (n = 16, 40 %). The specific chemotherapy regimens are shown in Table 2.
Table 1.
Patient characteristics (n = 44)
| Characteristics | Number of patients (n = 44) |
|---|---|
| Age (years) | |
| Median (range) | 64 (38–83) |
| Gender | |
| Male/Female | 26/18 |
| ECOG PS | |
| 0/1 | 20/24 |
| Primary tumor pathologic stage | |
| II | 21 |
| III | 23 |
| Histologic grade | |
| Well differentiated | 37 |
| Poorly differentiated | 7 |
| Chemotherapy | |
| Yes/No | 40/4 |
| Tumor size (cm) | |
| Median (range) | 2 (1–2.7) |
| Tumor number | |
| 1 | 23 |
| ≥2 | 21 |
| Extrahepatic metastatic lesion | |
| No extrahepatic disease | 31 |
| Intraabdominal lymph node | 11 |
| Peritoneum | 1 |
| Pleura | 1 |
| Intrahepatic distribution | |
| Unilobar | 30 |
| Bilobar | 14 |
ECOG PS Eastern Cooperative Oncology Group performance status
Table 2.
Chemotherapy regimens (n = 40)
| Regimen | Number of patients | % |
|---|---|---|
| Oxaliplatin/5-FU | 16 | 40 |
| Irinotecan/5-FU | 8 | 20 |
| Taxane/Cisplatin | 12 | 30 |
| Weekly paclitaxel | 2 | 5 |
| TS-1 | 2 | 5 |
| Total | 40 | 100 |
Results of RFA
Radiofrequency ablation was technically successful for all tumors. A total of 42 patients showed complete disappearance of tumor enhancement in the contrast-enhanced CT acquired 1 h after treatment. Two patients with small residual tumors underwent immediate additional RFA. There were no significant adverse events. Almost all patients demonstrated mild to moderate abdominal pain and liver enzyme elevations. Both the abdominal pain and liver enzyme elevations resolved with supportive care. A total of three patients suffered from transient Gram-negative bacteremia. These patients were treated with antibiotics: two patients were treated with a third generation cephalosporin, one with a carbapenem. All three recovered without sequelae.
Survival and prognostic factors
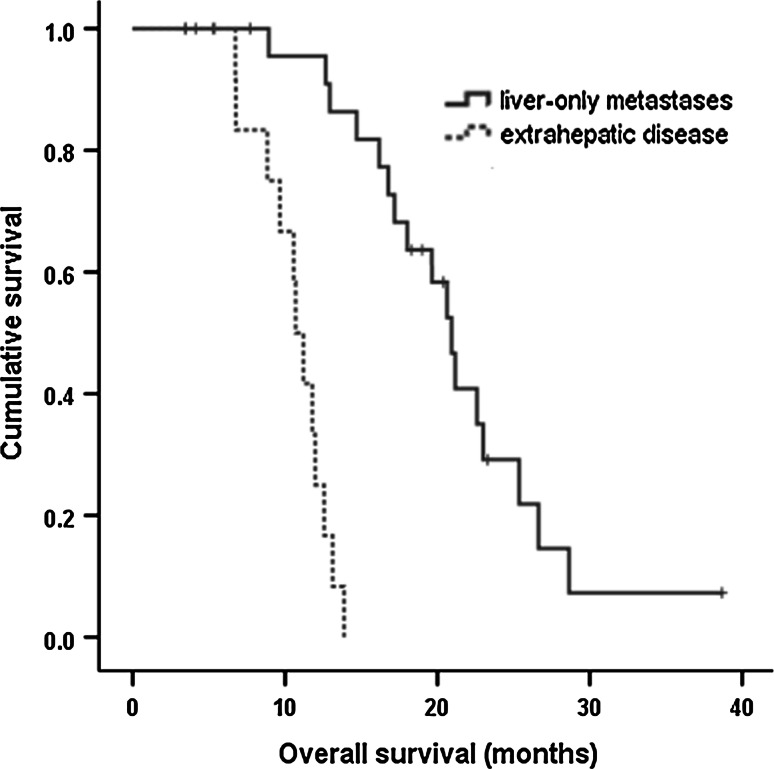
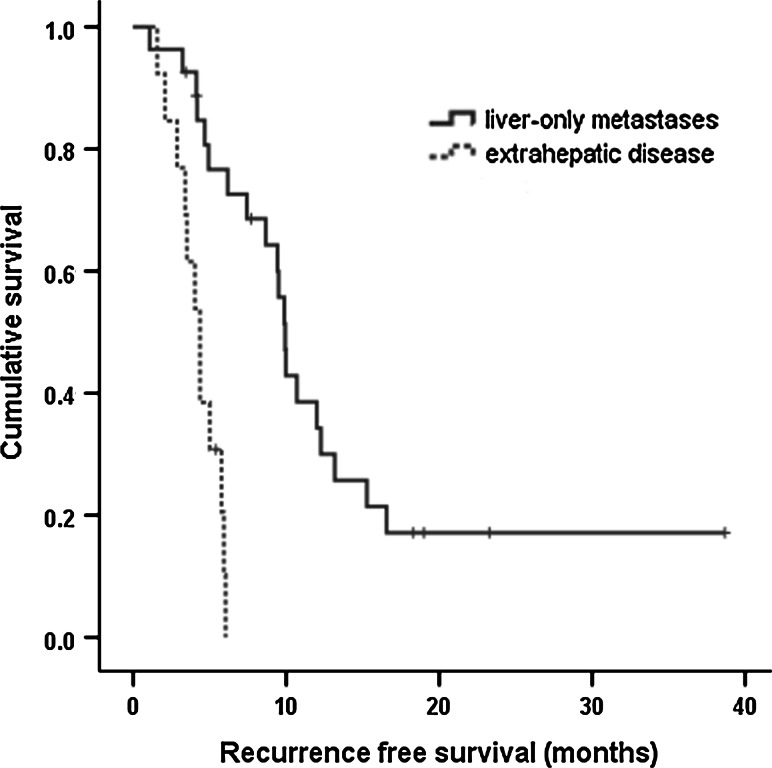
The median OS after RFA in all patients was 14.7 months (95 % CI 10.1–19.2). The median RFS after RFA in all patients was 6.1 months (95 % CI 4.2–8.1). The median OS and RFS of patients with liver-only metastases who underwent RFA and chemotherapy were 20.9 months (95 % CI 18.4–23.4) and 9.8 months (95 % CI 9.2–10.5), respectively. The median OS and RFS of patients with extrahepatic metastatic lesions who underwent RFA and chemotherapy were only 11.2 months (95 % CI 9.8–12.5) and 4.3 months (95 % CI 3.8–4.9), respectively. Patients with liver-only metastases who underwent RFA and chemotherapy had a better median OS and RFS than patients with extrahepatic metastatic lesions who underwent the same treatment. The difference was statistically significant (Table 3) (Figs. 1, 2).
Table 3.
Patients with liver-only metastases who underwent radiofrequency ablation and chemotherapy had a better median overall survival and recurrence-free survival than patients with extrahepatic metastatic lesion who underwent the same treatment (statistically significant)
| Liver-only metastasis with RFA and chemotherapy (n = 27) | Liver and extrahepatic disease with RFA and chemotherapy (n = 13) | p value | |
|---|---|---|---|
| mOS (months) | 20.9 | 11.2 | 0.001 |
| (95 % CI 18.4–23.4) | (95 % CI 9.8–12.5) | ||
| mRFS (months) | 9.8 | 4.3 | 0.001 |
| (95 % CI 9.2–10.5) | (95 % CI 3.8–4.9) |
mOS median overall survival, mRFS median recurrence-free survival, RFA radiofrequency ablation
Fig. 1.
Overall survival curve for patients with liver-only metastases who underwent radiofrequency ablation and chemotherapy (blue line) and patients with extrahepatic metastatic lesion who underwent the same treatment (green line). (Color figure online)
Fig. 2.
Recurrence-free survival curve for patients with liver-only metastases who underwent radiofrequency ablation and chemotherapy (blue line) and patients with extrahepatic metastatic lesion who underwent the same treatment (green line). (Color figure online)
In our study, in spite of the small number of patients (n = 4), patients who did not receive chemotherapy had significantly shorter OS compared with patients receiving chemotherapy (n = 40) [8.1 months (95 % CI 7.6–8.6) vs. 16.7 months (95 % CI 12.0–21.4)]. A total of 18 patients received second-line chemotherapy, however second-line chemotherapy did not affect OS [second-line chemotherapy (+) vs. (−), 18.0 vs. 14.7 months, p = 0.203].
In univariate analysis, the OS after RFA was significantly shorter for patients with the following clinical factors: no systemic chemotherapy, the presence of extrahepatic metastatic lesions, ≥2 liver metastases, and bilobar intrahepatic distribution. Univariate analysis also demonstrated that the presence of extrahepatic metastatic lesions, ≥2 liver metastases, and bilobar intrahepatic distribution were significantly associated with a shorter RFS after RFA (Table 4).
Table 4.
Univariate analysis of clinical factors influencing median overall survival and median recurrence-free survival (n = 44)
| Clinical factors | mOS | mRFS | ||
|---|---|---|---|---|
| Months (95 % CI) | p value | Months (95 % CI) | p value | |
| Age (years) | ||||
| <64 | 13.1 (7.1–19.1) | 0.473 | 5.9 (4.4–7.3) | 0.642 |
| ≥64 | 18.0 (9.0–26.9) | 7.4 (3.0–11.8) | ||
| Gender | ||||
| Male | 13.1 (11.1–15.0) | 0.827 | 7.3 (4.8–9.7) | 0.269 |
| Female | 16.1 (12.1–20.2) | 6.0 (0.7–11.3) | ||
| ECOG PS | ||||
| 0 | 13.8 (11.3–16.3) | 0.761 | 7.3 (4.2–10.3) | 0.504 |
| 1 | 17.1 (7.6–26.6) | 5.9 (4.5–7.2) | ||
| Primary tumor pathologic stage | ||||
| II | 16.1 (9.9–22.4) | 0.602 | 7.3 (4.2–10.4) | 0.464 |
| III | 14.7 (8.8–20.5) | 6.0 (2.5–9.4) | ||
| Histologic grade | ||||
| Well differentiated | 13.8 (9.0–18.7) | 0.811 | 5.9 (3.2–8.5) | 0.925 |
| Poorly differentiated | 20.6 (11.3–29.8) | 9.4 (5.7–13.0) | ||
| Chemotherapy | ||||
| Yes | 16.7 (12.0–21.4) | 0.001 | 7.4 (3.5–11.3) | 0.379 |
| No | 8.1 (7.6–8.6) | 5.4 (2.5–8.3) | ||
| Tumor size | ||||
| <2 cm | 16.1 (5.8–26.4) | 0.187 | 7.4 (2.8–1.9) | 0.297 |
| ≥2 cm | 13.1 (7.6–18.6) | 5.9 (2.3–9.5) | ||
| Tumor number | ||||
| 1 | 20.9 (11.4–30.3) | 0.006 | 9.9 (8.1–11.7) | 0.005 |
| ≥2 | 11.8 (8.9–14.6) | 4.6 (3.5–5.7) | ||
| Extrahepatic metastatic lesion | ||||
| No (liver-only metastasis) | 19.6 (14.5–24.7) | 0.001 | 9.5 (7.9–11.0) | 0.001 |
| Yes | 11.2 (9.8–12.5) | 4.3 (3.8–4.9) | ||
| Intrahepatic distribution | ||||
| Unilobar | 20.9 (13.9–27.9) | 0.001 | 9.8 (9.1–10.6) | 0.001 |
| Bilobar | 11.2 (8.6–13.7) | 4.1 (3.5–4.7) | ||
mOS median overall survival, mRFS median recurrence-free survival, ECOG PS Eastern Cooperative Oncology Group performance status
Multivariate regression analysis identified the independent negative prognostic factors for OS and RFS (Table 5). The independent negative prognostic factors for OS were the presence of extrahepatic metastatic lesions (HR 12.6, 95 % CI 3.7–42.9; p = 0.001), ≥2 liver metastases (HR 2.6, 95 % CI 1.2–5.9; p = 0.015), and no systemic chemotherapy (HR 43.3, 95 % CI 7.4–251.3; p = 0.001). The independent negative prognostic factors for RFS were the presence of extrahepatic metastatic lesions (HR 3.6, 95 % CI 1.6–7.8; p = 0.003), and intrahepatic bilobar distribution (HR 3.9, 95 % CI 1.5–9.9; p = 0.001).
Table 5.
Multivariate analysis of factors influencing overall survival and recurrence-free survival
| Factors | Hazard ratio (95 % CI) | p value |
|---|---|---|
| OS | ||
| Extrahepatic metastatic lesion | 12.6 (3.7–42.9) | 0.001 |
| Number of liver metastasis (≥2) | 2.6 (1.2–5.9) | 0.015 |
| No chemotherapy | 43.3 (7.4–251.3) | 0.001 |
| RFS | ||
| Extrahepatic metastatic lesion | 3.6 (1.6–7.8) | 0.003 |
| Intrahetpatic distribution (bilobar) | 3.9 (1.5–9.9) | 0.001 |
OS overall survival, RFS recurrence-free survival
Discussion
Recurrent or metastatic gastric cancer, including the presence of liver metastases has a very poor prognosis, and most oncologists recommend systemic chemotherapy. According to recent reports, the median OS of patients treated with combination chemotherapy, with or without a targeted agent such as trastuzumab, is about 13 months [6, 10, 11].
Liver resection for metastatic tumors is performed mainly in patients with primary colorectal cancer, with resection rates of 20–30 % and a 5-year survival rate of 25–85 % [12, 13]. In contrast, liver resection for LMGC is a rarely-performed procedure. The rate of hepatic resection in LMGC ranges from only 0.5 to 2.3 % [2, 14]. Most patients with LMGC remain incurable due to bilobar, multinodular tumor spread, gross peritoneal dissemination, diffuse metastases to distant lymph nodes, or unresectable local recurrences [4, 15–17]. In a recently published review, 19 studies were analyzed to compare survival periods following hepatic resection for LMGC. The median survival for all 436 patients was 17 months, and the 5-year survival was 26.5 % [18]. Several authors have reported better OS in patients with metachronous liver metastasis than in those with synchronous disease after hepatic resection [19–21]. Ambiru et al. [21] reported significantly longer survival in patients with metachronous metastasis (5-year survival 29 %) than in those with synchronous disease (5-year survival 6 %). However, Sakamoto et al. [2] reported no significant difference in survival between synchronous and metachronous metastasis after hepatic resection (19.2 vs. 21.4 months, p = 0.84). In our study, we found a median OS of 20.9 months for those patients who underwent RFA and chemotherapy for liver-only metastases; this is comparable to the results for surgical resection.
After detecting a liver recurrence, oncologists have to be extremely cautious about patient selection for surgical resection, so as to avoid an inadequate or extensive operation in a high-risk patient whose disease is incurable by resection. Sakamoto et al. [1] reported that unilobar metastases and/or a tumor <4 cm in diameter may be indications for surgical resection. The number of liver metastases can be another significant prognostic factor. Gannon et al. [22] reported that, from a tumor biology and behavior perspective, RFA would not be effective for more than five metastases. Shirabe et al. [23] also reported that the presence of more than three liver metastases was an independent poor prognostic factor, based on observations in patients after liver resection of LMGC. In our study, the independent negative prognostic factors for OS were presence of extrahepatic metastatic lesions, number of liver metastases (≥2), and no systemic chemotherapy.
Recurrent liver tumors are common after curative hepatic resection for gastric metastases, occurring in about two-thirds of patients [7, 24]. This high recurrence rate might suggest the presence of occult intrahepatic metastases, even at the time of the hepatectomy [1]. In our study, a total of 27 patients (61.4 %) developed intrahepatic recurrence after RFA.
The efficacy of adjuvant chemotherapy after liver resection of LMGC has not been fully evaluated [25, 26]. Chemotherapy after liver resection of LMGC may be beneficial in terms of the high risk of recurrence, and the same may apply to RFA. In our study, in spite of the small number of patients (n = 4) who did not receive chemotherapy, these patients had significantly shorter OS compared with those who did undergo chemotherapy.
In Korea, it has been common practice for patients who fail first-line palliative chemotherapy to receive second-line chemotherapy and Kang et al. [27] reported that salvage chemotherapy (second- or third-line chemotherapy) in advanced gastric cancer resulted in significant prolongation of survival when compared with best supportive care. In our study, second-line chemotherapy did not affect OS. We propose that this was due to the small number of patients of this study.
Radiofrequency ablation has been used for the treatment of primary and secondary hepatic malignancies, particularly in hepatocellular carcinoma and colorectal cancer, and its use is steadily increasing. Compared with hepatic resection, RFA is relatively less invasive, resulting in fewer complications, a lower mortality rate, a shorter hospital stay, and a lower treatment cost [8, 28]. The role of RFA in LMGC is not clearly defined. Several reports have demonstrated the beneficial local control effect of RFA and the potential additional benefit of chemotherapy, however, these reports featured a very small and heterogeneous patient population [14, 25, 29].
Considering the high recurrence rate of this disease and the early clinical recovery after RFA compared with surgery, the combined treatment of systemic chemotherapy and RFA may be a potential therapeutic strategy for metachronous liver metastases after resection of the primary gastric cancer. More early administration of chemotherapy may be possible after RFA rather than after surgery.
Conclusion
Combination of percutaneous RFA and systemic chemotherapy may be effective treatment modalities for patients with a single, unilobar metachronous LMGC without extrahepatic metastatic disease.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Jun-Eul Hwang and Seung-Hun Kim have contributed equally to this work as co-first authors.
References
- 1.Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, Katai H, Kosuge T, Sasako M. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95:534–539. doi: 10.1002/jso.20739. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, Kokudo N, Yamaguchi T, Muto T, Makuuchi M. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003;133:507–511. doi: 10.1067/msy.2003.147. [DOI] [PubMed] [Google Scholar]
- 3.Okuyama K, Isono K, Juan IK, Onoda S, Ochiai T, Yamamoto Y, Koide Y, Satoh H. Evaluation of treatment for gastric cancer with liver metastasis. Cancer. 1985;55:2498–2505. doi: 10.1002/1097-0142(19850515)55:10<2498::AID-CNCR2820551032>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrelli D, Roviello F, De Stefano A, Fotia G, Giliberto C, Garosi L, Pinto E. Risk factors for liver metastases after curative surgical procedures for gastric cancer: a prospective study of 208 patients treated with surgical resection. J Am Coll Surg. 2004;198:51–58. doi: 10.1016/j.jamcollsurg.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takakeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 7.Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today. 2010;40:287–294. doi: 10.1007/s00595-009-4152-0. [DOI] [PubMed] [Google Scholar]
- 8.Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5:550–560. doi: 10.1016/S1470-2045(04)01567-0. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK, Investigators TT. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 12.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg. 2009;394:973–983. doi: 10.1007/s00423-009-0530-8. [DOI] [PubMed] [Google Scholar]
- 14.An JY, Kim JY, Choi MG, Noh JH, Choi D, Sohn TS, Kim S. Radiofrequency ablation for hepatic metastasis from gastric adenocarcinoma. Yonsei Med J. 2008;49:1046–1051. doi: 10.3349/ymj.2008.49.6.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, Yaguchi Y, Hatsuse K, Yamamoto J, Hase K. Outcomes for patients following hepatic resection of metastatic tumors from gastric cancer. Hepatol Int. 2010;4:406–413. doi: 10.1007/s12072-009-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, Götz M, Scheuerlein H, Jandt K, Settmacher U. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer. 2012;15:131–136. doi: 10.1007/s10120-011-0080-y. [DOI] [PubMed] [Google Scholar]
- 17.Thelen A, Jonas S, Benckert C, Lopez-Hänninen E, Neumann U, Rudolph B, Schumacher G, Neuhaus P. Liver resection for metastatic gastric cancer. Eur J Surg Oncol. 2008;34:1328–1334. doi: 10.1016/j.ejso.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford) 2010;12:589–596. doi: 10.1111/j.1477-2574.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, Kitamura H, Ikegami T, Miyagawa SI, Kawasaki S. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001;96:3178–3184. doi: 10.1111/j.1572-0241.2001.05278.x. [DOI] [PubMed] [Google Scholar]
- 20.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, Shimizu Y, Nakajima N. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–283. doi: 10.1016/S0002-9610(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 22.Gannon CJ, Curley SA. The role of focal liver ablation in the treatment of unresectable primary and secondary malignant liver tumors. Semin Radiat Oncol. 2005;15:265–272. doi: 10.1016/j.semradonc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, Ikeda Y, Ezaki T, Fukuzawa S, Takenaka K, Kishikawa K, Ikeda T, Taguchi K, Maehara Y, Sugimachi K. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepatogastroenterology. 2003;50:1560–1563. [PubMed] [Google Scholar]
- 24.Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, Ahn JB, Roh JK, Noh SH, Chung HC. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146–1153. doi: 10.1093/annonc/mdn026. [DOI] [PubMed] [Google Scholar]
- 25.Kim HO, Hwang SI, Hong HP, Yoo CH. Radiofrequency ablation for metachronous hepatic metastases from gastric cancer. Surg Laparosc Endosc Percutan Tech. 2009;19:208–212. doi: 10.1097/SLE.0b013e3181a033d7. [DOI] [PubMed] [Google Scholar]
- 26.Yamakado K, Nakatsuka A, Takaki H, Mori Y, Tonouchi H, Kusunoki M, Kida H, Takeda K. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol. 2005;16:1747–1751. doi: 10.1097/01.RVI.0000188738.84911.3B. [DOI] [PubMed] [Google Scholar]
- 27.Kang JH, Lee SI, Lim do H, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 28.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SE, Yang DH, Kim CY. Prognostic factors for survival in patients with hepatic recurrence after curative resection of gastric cancer. World J Surg. 2009;33:1468–1472. doi: 10.1007/s00268-009-0034-2. [DOI] [PubMed] [Google Scholar]