Abstract
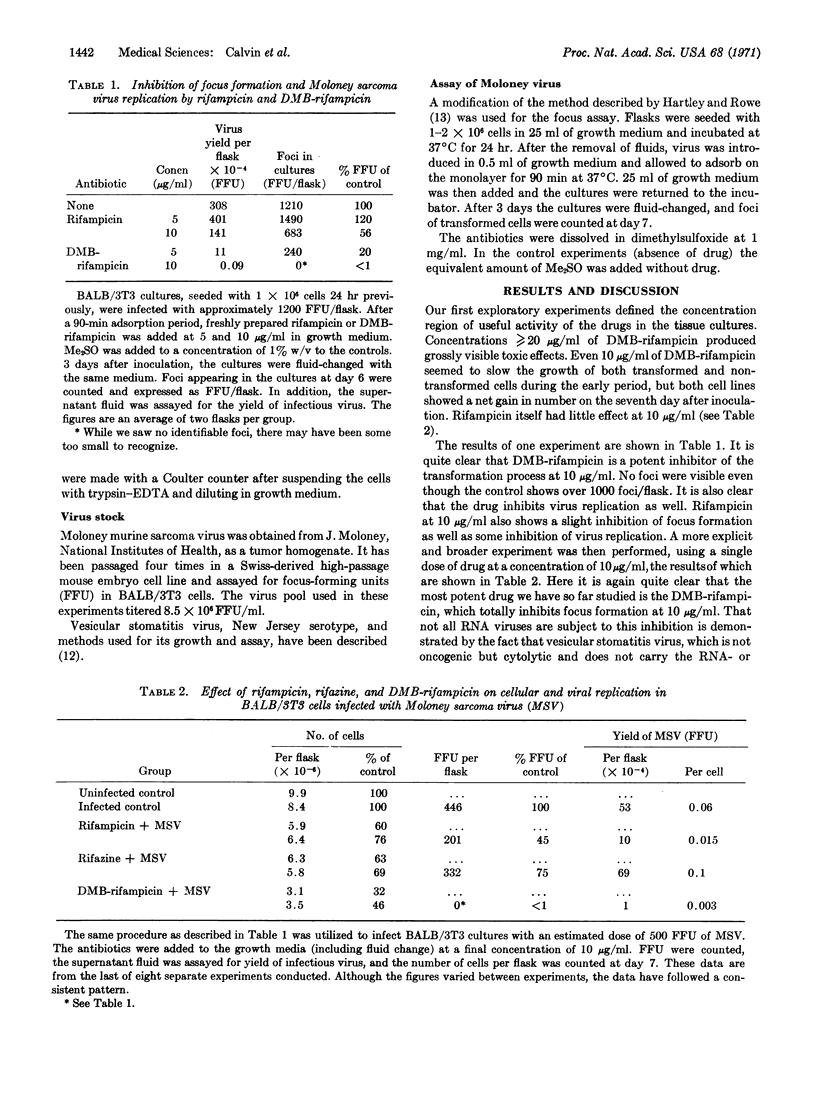
It is shown that rifampicin, and especially the related antibiotic 2′,6′-dimethyl-N(4′)-benzyl-N(4′)- [desmethyl]rifampicin (DMB-rifampicin) can inhibit focus formation by Moloney sarcoma virus on BALB/3T3 tissue cultures. At 10 μg/ml DMB-rifampicin totally inhibits focus formation while reducing virus replication by at least a factor of fifty and cell proliferation by only a factor of three. These observations, taken together with those of others, suggest a role for an RNA-dependent DNA polymerase and the gene for its synthesis both in normal cell processes and in the transformation process.
Keywords: rifazine; 2′,6′-dimethyl-N(4′)-benzyl-N(4′)- [desmethyl]rifampicin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Diggelmann H., Weissmann C. Rifampicin inhibits focus formation in chick fibroblasts infected with Rous sarcoma virus. Nature. 1969 Dec 27;224(5226):1277–1279. doi: 10.1038/2241277a0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Yang S. S., Ting R. C. RNA dependent DNA polymerase of human acute leukaemic cells. Nature. 1970 Dec 5;228(5275):927–929. doi: 10.1038/228927a0. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Ray R. K., Thiry L., Green M. Inhibitors of the RNA and DNA dependent polymerase activities of RNA tumour viruses. Nat New Biol. 1971 Jan 27;229(4):111–114. doi: 10.1038/newbio229111a0. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Schaffer F. L., Madin S. H. The separation of infectious and autointerfering particles in vesicular stomatitis virus preparations. Virology. 1967 Jan;31(1):114–119. doi: 10.1016/0042-6822(67)90014-1. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller E., Argaman M., Levy H., Goldblum N. Selective inhibition of vaccinia virus by the antibiotic rifampicin. Nature. 1969 Apr 19;222(5190):273–274. doi: 10.1038/222273a0. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancini G., Cricchio R., Thiry L. Antiviral activity of rifamycins and N-aminopiperazines. J Antibiot (Tokyo) 1971 Jan;24(1):64–66. doi: 10.7164/antibiotics.24.64. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J., Parks W. P. RNA dependent DNA polymerase activity in mammalian cells. Nature. 1971 Jan 29;229(5283):318–321. doi: 10.1038/229318a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Trávnícek M., Watson K. Synthetic DNA-RNA hybrids and RNA-RNA duplexes as templates for the polymerases of the oncogenic RNA viruses. Nature. 1970 Oct 31;228(5270):430–432. doi: 10.1038/228430a0. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Timbury M. C., Williams J. F. Rifampicin inhibits the growth of some mammalian viruses. Nature. 1969 Apr 26;222(5191):341–345. doi: 10.1038/222341a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]


