Abstract
Background: Oleuropein is a phenolic compound which is present in the olive leaf extract. The purpose of the present study was to investigate the neuroprotective effect of oleuropein as an antioxidant agent on the substantia nigra in aged rats. Methods: Twenty 18-month-old Wistar rats (450-550 g) were randomly divided into control and experimental groups. The experimental group received a daily single dose of 50 mg/kg of oleuropein by oral gavage for 6 months. The control group received only distilled water. All rats were sacrificed two hours after the last gavage and the brains were removed and midbrains were cut. One part of the midbrains were homogenized and centrifuged. The tissue supernatant was assayed for lipid peroxidation (LPO) and antioxidant enzyme activities. The other part of midbrains fixed and embedded in paraffin, then processed for Nissl and immunohistochemistry (IHC) staining. Data was analyzed using SPSS by t-test. Differences were considered significant for P<0.05. Results: The level of LPO in midbrain of the rats was decreased significantly in the experimental group, but superoxide dismutase, catalase and glutathione peroxidase activities were increased in experimental group compared to control group (P<0.05). Morphometric analyses showed significantly that the experimental group had more neurons in substantia nigra pars compacta (SNc) either in Nissl or IHC staining when compared to control (P<0.05). Conclusion: The results of the present study indicate that treatment of the old rats with oleuropein reduces the oxidative damage in SNc by increasing the antioxidant enzyme activities.
Key Words: Oleuropein, Aging, Dopaminergic neurons, Substantia nigra
INTRODUCTION
During aging, accumulation of oxidative damage in tissues and enhancement of oxygen radical production cause cellular damage, which, in turn, alters physiological function and accelerates pathophysiological conditions. Oxidative damage is the result of the release of reactive oxygen species [1-3]. The increased oxidative damage during aging may be due to decline of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [4-7]. Brain is highly susceptible to damage from oxidative stress, because it consumes about 20% of the body’s total oxygen and has a high content of polyunsaturated fatty acids and a low concentration of antioxidant enzymes compared to the other tissues [3, 4]. Dopaminergic neurons in substantia nigra are vulnerable to oxidative damage, because in these neurons, hydrogen peroxidase is produced during dopamine metabolism. Increased hydroxyl radicals cause significant damage to the dopaminergic neurons [4]. Damage or loss of dopaminergic neurons is the main cause of the beginning of neurodegenerative Parkinson’s disease [3-5]. Diets rich in polyphenols protect brain cells against oxidative damage. Studies have shown that poly-phenols in plants and fruits have antioxidant properties and counteract oxidative stress [7, 8]. Olive products constitute a rich source of polyphenols. Oleuropein is the major phenolic composition of the olive leaf, oil, and fruit. Antioxidant capacity of oleuropein is 400% higher than vitamin C and twice of green tea or grape seed extract [6-11]. Studies have indicated that oleuropein attenuates inflammatory response and improves histological and plasma markers of liver damage [12].
There are increasing evidences that suggest oleuropein may prevent the formation of toxic amyloid aggregation [13] and might protect heart arrhythmia by prolonged oral administration [14]. Alirezaei et al. [15] have shown that oleuropein prevents ethanol-induced gastric ulcers via increasing antioxidant enzyme activities. It has been also shown that oral administration of olive leaf extract reduces brain edema and improves neurological damages after transient middle cerebral artery occlusion [16]. In spite of some experimental evidences about the effects of olive phenolic compounds on brain, there is no histological evaluation for the effects of oleuropein on dopaminergic neurons of substantia nigra pars compacta (SNc). Therefore, in the present study, the effect of oleuropein on dopaminergic neurons of SNc was investigated.
MATERIALS AND METHODS
Chemicals . Oleuropein was extracted from olive leaves of Sevillano Variety in Razi Herbal Medicine Research Center (Lorestan, Iran). For this purpose, olive leaves were washed, dried and powdered by an electrical mill, and then 80% (1L) ethyl alcohol was added to 2000 g olive leaf powder. The extract was filtered and evaporated in a rotator evaporator at room temperature under vacuum, and the concentrated extract was then freezed. The compounds of extract were analyzed by using HPLC [17]. Mouse monoclonal antibody to tyrosine hydroxylase (primary antibody, dilution 1:80) and goat polyclonal secondary antibody to mouse IgG + IgM + IgA + horseradish peroxidase (dilution 1:100) were purchased from Abcam (USA) and 3,3’- diaminobenzidine from Dako (Denmark). SOD, CAT, and GPx kits were obtained from Randox, UK.
Animals. Twenty 18-month-old male Wistar rats (with an average weight of 450-550 g) were housed in a temperature controlled room at 23°C and 12 hours light and dark cycle. The animals were fed with pellets during the experiment. All animal works were approved by the ethical guidelines for the care of laboratory animals of the Research Center of Iran University of Medical Sciences (Tehran).
Experimental design. The rats were randomized equally into control and experimental groups. The experimental group received daily a single dose of 50 mg/kg [16] of oleuropein by oral gavage for 6 months [18]. The control group received only distilled water. Two hours after the last oral gavage, half of the rats were anesthetized and decapitated then the brains were removed and immersed in cold PBS (0.1 M, pH 7.4) and the midbrains were separated and homogenized in ice-cold 10 nmol/l Tris-HCl. The homogenates were centrifuged at 12000 ×g at 4°C for 20 minutes. The supernatants were collected for antioxidant enzyme activity assay.
Lipid peroxidation level was measured by the Thiobarbituric acid (TBA) using the method of Satoh [19]. This method was used to measure spectro-photometrically the color produced by the reaction of TBA with Malondialdehyde. For this purpose, the supernatant proteins were precipitated with Trichlo-roacetic acid, and the mixture was heated with 3 ml TBA in 2 M sodium sulfate in boiling water for 30 minutes. The colored product was extracted with n-butyl alcohol, and the absorbance of the sample was measured at 530 nm. MDA levels were expressed as nanomol per milligram of protein (nm/mg protein). SOD activity was measured by the method of Misra and Fridovich [20]. In this method, an aliquot of 0.25 ml ice cold chloroform was added to 0.1 ml supernatant, followed by adding 0.15 ml ice cold ethanol. The mixture was centrifuged at 3,000 ×g at 4°C for 10 minutes. Carbonate buffer (1.5 ml), EDTA (0.5 ml), and water (0.8 ml) were added to 0.2 ml supernatant. Reaction was started by adding 0.4 ml epinephrine. Changes in absorbance were read at 480 nm for 30 minutes, and the results were expressed as nmol/minutes/mg protein. CAT activity was measured based on the ability of the enzyme to break down hydrogen peroxide (H2O2). The method was performed according to the modified method of Aebi [21]. For this purpose, the tissues were homogenized and centrifuged at 1000 ×g for 10 minutes. A volume of 20 µl of 100-fold diluted supernatant was added to 980 µl assay mixture, containing 10 mmol/l of H2O2 (900 µl), Tris-HCl buffer (50 µl, pH 8), and distilled water (30 µl). The rate of decomposition of H2O2 was monitored spectrophotometrically at 240 nm. GPx activity was determined spectrophotometrically based on the kit recommendations (Randox, UK) and then was measured based on the oxidation of glutathione, which was coupled with reduced nicotinamide adenin dinucleotide oxidation by glutathione reductase [22]. The reaction was initiated by adding 0.2 mM cumin hydroperoxidase (20 µl) to the reaction mixture (200 µl), containing 20 µl midbrain homogenate, 5 mM glutathione, 0.1 mM reduced nicotinamide adenin dinucleotide phosphate, 50 mM Tris-HCl buffer (pH 7.6), 5 mM EDTA, and 0.1 unit of glutathione reductase at room temperature. The changes in absorbance were recorded at 340 nm. The enzyme activity was calculated as nmol of oxidized nicotinamide adenin dinucleotide phosphate [22].
Immunohistochemical analyses . The other half of the rats were anesthetized and perfused transcardially with PBS (pH 7.4) followed by 4% paraformaldehyde fixative. The brains were removed and post fixed in the same fixative overnight. Then, the midbrains were cut and dehydrated in alcohol series, cleared in xylene, infiltrated with paraffin and embedded in paraffin. The 5-µm coronal sections were serially collected from bregma -4.52 mm to -6.04 mm of midbrains [23] with a 30-µm interval between each consecutive section. The half of the sections was stained for Nissl (cresyl violet), and the other half of the sections was processed for immunohistochemistry (IHC). In IHC staining, the sections were dehydrated in descending alcohol series, immersed in 10% H2O2/methanol for 10 minutes, and the sections were washed in 0.1 Tris wash buffer. For retrieving the antigens, the sections were kept in citrate buffer (containing sodium citrate and deionized water) in autoclave to boil for 11 minutes. After cooling, the sections were washed in Tris wash buffer and incubated in BSA for 10 minutes. The sections were incubated in the primary antibody at room temperature for 1 hour, then were washed in Tris wash buffer (pH 7.4) and incubated in secondary antibody for 1 hour. To vision the bound antibody, the sections were reacted with 3,3’-diaminobenzidine for 10 minutes, washed in Tris wash buffer (pH 7.4) and counterstained with hematoxylin, then washed in tap water, dehydrated in ascending alcohols, cleared in xylol and covered with cover slip.
Morphometric studies. Coronal sections (n = 5) of SNc of each animal in Nissl and IHC staining were analyzed by a microscope (Olympus AX70) and Olympus DP11 microscope digital camera under magnification of 40×. The number of neurons in Nissl staining including dopaminergic neurons and inter-neurons and TH + neurons in IHC staining were counted using OLYSIA autobioreport software (Olympus Optical Co. Ltd. Japan) [24].
Statistical analyses. The data are expressed as mean ± SD, t-test was used to determine significant differences between the control and experimental groups. Differences were considered statistically significant for P<0.05.
RESULTS
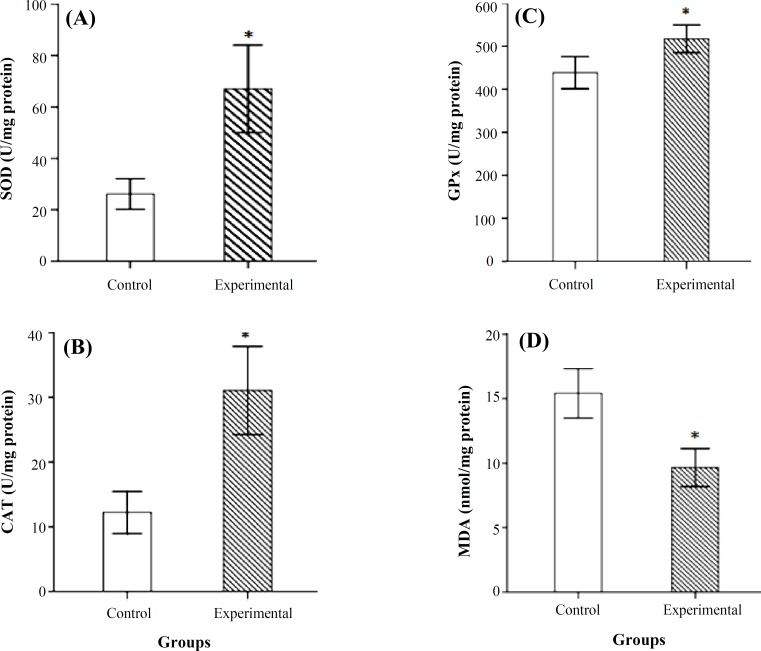
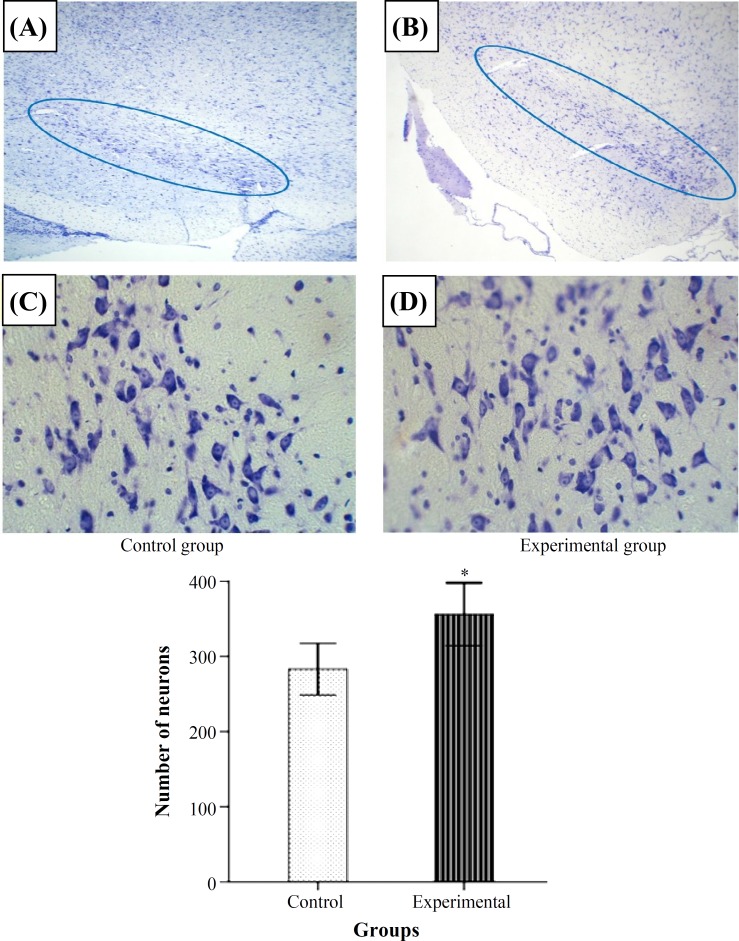
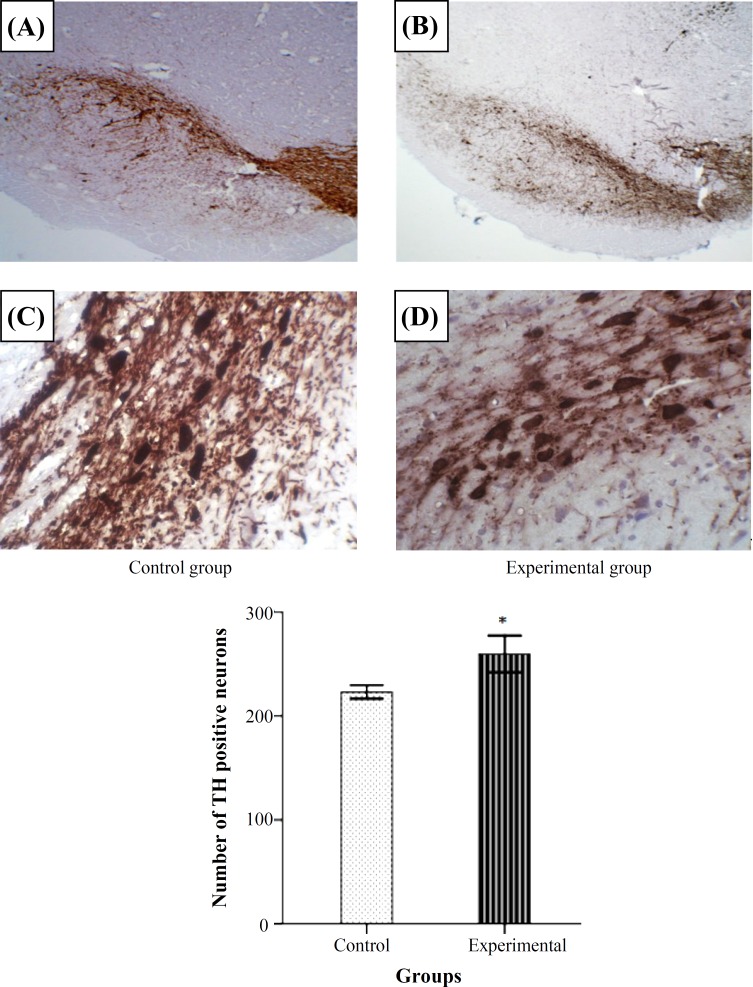
The level of SOD activity was increased significantly in the experimental group when compared to control P<0.05 (Fig. 1A). Figure 1B shows CAT activity in the two groups. A significant increase of CAT activity was observed in the experimental group in comparison to control group (P<0.05).The results indicated that GPx activity was increased in the experimental group and the difference was significant compared to control group (P<0.05) (Fig. 1C). The level of lipid peroxidation in midbrain of rats significantly decreased in the experimental group compared to the control group (P<0.05) (Fig. 1D). The boundaries of SNc in control and experimental groups have been shown in Figure 2A and 2B. The number of Nissl stained neurons inside the boundries was counted (Fig. 2C and 2D). Comparative light microscope analyses demonstrated that population of Nissl-stained neuron number in the experimental group was higher than control group (P<0.05). Figure 3 shows the boundaries of SNc and dopaminergic neurons in IHC staining in the two groups. IHC staining for dopaminergic neurons revealed that the number of dopaminergic neurons in SNc of experimental group were significantly more than the control group (P<0.05).
Fig. 1.
The activities of superoxide dismutase (SOD) (A), catalase (CAT) (B), glutathione peroxidase (GPx) (C) and the levels of malondialdehyde (MDA) (D) in control and experimental groups. Values are expressed as mean ± SD. *Significant (P<0.05
Fig. 2.
Photomicrographs of boundaries of the midbrain in coronal sections in the control and experimental groups (A and B) (magnification 10×). C and D show neurons in the SNc (Nissl staining, magnification 40×). The histogram shows the difference of the number of neurons between control and experimental groups which was significant (P<0.05). *Significant
Fig. 3.
Photomicrographs of IHC staining for dopaminergic neurons of SNc in control and experimental groups (A and B, magnification 10×). C and D show 40× magnification of dopaminergic neurons in SNc. Columns represent the number of dopaminergic neurons. The number of dopaminergic neurons in control group was less than experimental group significantly (P<0.05). All figures are right side of the SNc. *Significant
DISCUSSION
Brain has high content of polyunsaturated fatty acids, which is considered very vulnerable to oxidative stress. The excessive production of free radicals in aging brain and the imbalance between reactive oxygen species and antioxidant system are related to the pathogenesis of neurodegenerative diseases such as Parkinson’s disease [25]. Dopaminergic neurons of substantia nigra are more sensitive to oxidative stress compared to the other neurons in brain because of iron ions and large amount of free radicals. The free radicals are produced during dopamine metabolism in dopaminergic neurons [4]. Iron, which plays a major role in lipid peroxidation, is mediated in the formation of hydroxyl radicals that react with lipid hydro-peroxidase and produce peroxyl and alkoxyl radicals that could initiate lipid peroxidation [2, 5]. The lipid peroxidation process is involved in oxidative damage at cellular level. Increased level of free radicals and lipid peroxidation has been reported in nigral tissue of Parkinson's patients [5, 25].
In the current study, the protection role of oleuropein against oxidative stress changes in midbrain of aged rats was investigated. The changes were evaluated by measuring the level of lipid peroxidation (LPO) as well as SOD, CAT, GPx enzyme activities and morphometric analyses of dopaminergic neurons in SNc. LPO by reactive oxygen species is involved in the damaging mechanism in brain disorders. Assay of LPO products, TBA assay, is based on the reactivity of end product of LPO (MDA) with TBA to produce a red color [26]. In this research, MDA level was decreased significantly in the experimental group compared to control. This finding show that LPO level was decreased after oleuropein treatment in the experimental group which can be due to antioxidant effect of oleuropein phenolic compounds. Researchers have suggested that the MDA levels as a result of LPO in damaging brain, are overestimated and one of the beneficial effects of oleuropein is reducing MDA in these patients [8, 26]. Researchers have also reported that oleuropein declines the expression of TNF-α activation to inhibit LPO [27].
Our experimental results suggest that oleuropein, which is rich in antioxidants, primarily induces the antioxidant enzyme activities in midbrain during aging; therefore, modulates free radical oxidative damage to dopaminergic neurons of SNc [7, 28]. SOD is an antioxidant enzyme of defense mechanism against oxidative stress. Superoxide radical formation is increased with age, and SOD activity is decreased [29]. There is a relation between decrease of SOD and increase of LPO level in aged rat brain [30]. The results of our study indicated that administration of oleuropein to aged rats for 6 months induced the increase of SOD activity compared to control group, which is in agreement with the results of McCord's research [31]. In the present study, quantification of CAT showed a significant increase in CAT activity in midbrain of the experimental group. Studies have indicated that CAT activity is decreased with age [30]. These findings revealed that oleuropein has properties against age-related decrease in antioxidant enzyme activities [8].
The results of this study have led us to know the significant increase of GPx in the experimental group compared to control group. The biological function of GPx is to reduce conversion of lipid hydroperoxidase to their corresponding alcohol and also eliminates H2O2 [30], but some authors have observed unchanged or decreased GPx activity in old subjects [32]. Morphometric analyses revealed that the experimental group had more Nissl-stained neurons and also contained more dopaminergic neurons in IHC staining compared to control group. Aging is accompanied with dopaminergic neuron loss in SNc. Gao et al. [28] and Emborg et al. [33] have shown the differences of the number of dopaminergic neurons in SNc of young and old rats and primates. Rehman and Masson [34] have demonstrated that old people show a decrease in the number of dopaminergic neurons in SNc of midbrain that is consistent with our findings. The findings of our research demonstrate that oral administration of oleuropein may protect the dopaminergic neuron loss and, to our knowledge, this is the first study of oleuropein effect against neuron loss in SNc.
The results of the present investigation indicated that treatment of old rats with oleuropein for 6 months improved the antioxidant enzyme activities and decreased LPO level in midbrains of experimental group in comparison to control group. On the other hand, oleuropein protected dopaminergic neuron loss in SNc of old rats. Therefore, oleuropein consumption in daily diet may useful to reduce the oxidative stress damage and scavenging free radicals by increasing the antioxidant enzyme activities.
ACKNOWLEDGEMENTS
This research was supported by Iran university of Medical Sciences (Tehran). The authors wish to thank Dr. Rasoulian for providing oleuropein.
References
- 1.Poon HF, Calabrese V, Scapagnini G, butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004 May;20(2):329–59. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Barja G. Free radicals and aging. Trends neurosci. 2004 Oct;27(10):595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity Implications for neuroimaging. Trends Neurosci. 2004 Aug;27(8):489–95. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Floyd RA, Hensley K. Oxidative stress in brain aging implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002 Sep-Oct;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 5.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53 (Suppl 3):S26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 6.Buterfield D, Castegna A, Pocernich C, Drake J, Scapaqunini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem. 2002 Aug;13(8):444. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 7.Yanishlieva NV, Marianova E, Pokomy J. Natural antioxidants from herbs and spices. Eur J Lipid Sci Tecnol. 2006;108:776–93. [Google Scholar]
- 8.Benavente Garcia O, Castillo J, Lorente J, Ortuno A, Del Rio JA. Antioxidant activity of phenolic extracted from olea europaea L. leaves. Food Chem. 2000;68:457–562. [Google Scholar]
- 9.Andreadou I, Iliodromitis Ek, Mikros E, Constantinou M, Agalias A, Magiatis P, et al. The olive constituent oleuropein exhibits anti-ischemic, antioxidative and hypolipidemic effects in anesthetized rabbits. J Nutr. 2006 Aug;136(8):2213–9. doi: 10.1093/jn/136.8.2213. [DOI] [PubMed] [Google Scholar]
- 10.Andreadou I, Sigala F, Iliodromitis Ek, Papaefthimiou M, Sigalas C, Aligiannis N, et al. Acute doxorubicin cadiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol. 2007 Mar;42(3):549–58. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Durlu Ozkaya F, Ozkaya MT. Oleuropein using as additive for feed and products used for human. J food process Technol. 2011;2(3):113. [Google Scholar]
- 12.Domitrovic R, Jakovac H, Marchesi VV, Sain I, Romic Z, Rahelic D. Prevention and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol Res. 2012 Apr;65(4):451–64. doi: 10.1016/j.phrs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Diomede L, Rigacci S, Romeo M, Stefani M, Salmona M. Oleuropein aglycone protects transgenic celegans strains expressing Aβ 42 by reducing plaque load and motor deficit. PLoS one. 2013;8(3):e58893. doi: 10.1371/journal.pone.0058893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmailidehaj M, Mirhosseini S, Rezvani ME, Rasoulian B, Mosaddeghmehrjardi MH, Haghshenas D. Prolonged oral administration of oleuropein might protect heart against aconitine induced arrhythmia. IJPR. 2012;11(4):1255–63. [PMC free article] [PubMed] [Google Scholar]
- 15.Alirezaei M, Dezfoulian O, Neamati S, Rashidipour M, Tanideh N, Kheradmand A. Oleuropein prevents ethanol induced gastric ulcers via elevation of antioxidant enzyme activities in rats. J Physiol Biochem. 2012 Dec;68(4):583–92. doi: 10.1007/s13105-012-0177-8. [DOI] [PubMed] [Google Scholar]
- 16.Mohagheghi F, Bigdeli Mr, Rasoulian B, Hashemi P, Rashidpour M. The neuroprotective effect of olive leaf extract is related to improved blood brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine. 2011 Jan;18(2-3):170–5. doi: 10.1016/j.phymed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi P, Delfan B, Ghiasvand AR, Alborzi M, Riasi F. A study of the effects of cultivation variety, collection time and climate on the level of oleuropein in olive leaves. Acta Chromatographica. 2010;22:131–138. [Google Scholar]
- 18.Kimura Y, Sumiyoshi M. Olive leaf extract and its main component oleuropein prevent chronic ultraviolet B radiation- induced skin damage and carcinogenesis in hairless mice. J Nutr. 2009 Nov;139(11):2079–86. doi: 10.3945/jn.109.104992. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chem Acta. 1978 Nov;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 20.Misra HP, Fridovich I. The role of superoxide anion the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May;247(10):3170–5. [PubMed] [Google Scholar]
- 21.Aebi H. Catlase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–69. [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The brain in the stereotaxis coordinates. San Diago: New York: Academic Press; 2006. [Google Scholar]
- 24.Mehraein F, Talebi R, Jameie B, Joghataie Mt, Madjd Z. Neuroprotective effect of exogenous melatonin on dopaminergic neurons of the substantia nigra in ovariectomized rats. Iran Biomed J. 2011;15(1-2):44–50. [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000 Aug;267(16):4904–11. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 26.Garcia YJ, Rodriguez-Malavar AJ, Penaloza N, Penaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods. 2005 May;144(1):127–35. doi: 10.1016/j.jneumeth.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Geng C, Jiang L, Gong D, Lin D, Yoshimura H, et al. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur J Nutr. 2008 Aug;47(5):235–43. doi: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Miao H, Xiao CH, Sun Y, Du X, Yuan HH, et al. Influence of aging on the dopaminergic neurons in the substantia nigra pars compacta of rats. Curr Aging Sci. 2011 Feb;4(1):19–24. doi: 10.2174/1874609811104010019. [DOI] [PubMed] [Google Scholar]
- 29.Fki I, Bouaziz M, Sahnoun Z, Sayadi S. Hypercholesterolemic effects of phenolic rich extracts of chemlali olive cultivar in rats fed a cholesterol-rich diet. Bioorg Med Chem. 2005 Sep;13(18):5362–70. doi: 10.1016/j.bmc.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 30.Dittmar M, Knuth M, Beineke M, Epe B. Role of oxidative DNA damage and autooxidative enzymatic defense systems in human aging. The open anthropology journal. 2008;1:38–45. [Google Scholar]
- 31.McCord JM. Superoxid dismutase in aging and disease: an overview. Methods Enzymol. 2002;349:331–41. doi: 10.1016/s0076-6879(02)49348-2. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Martinez MA, Ruiz-Torres A. Homeostasis between lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech Aging Dev. 1992 Nov;66(2):213–22. doi: 10.1016/0047-6374(92)90137-3. [DOI] [PubMed] [Google Scholar]
- 33.Emborg ME, Ma SY, Mufson EJ, Levey AL, Taylor MD, Brown WD, et al. Age related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J comp Neurol. 1998 Nov;401(2):253–65. [PubMed] [Google Scholar]
- 34.Rehman HU, Masson EA. Neuroendocrinology of aging. Age Aging. 2001;30(4):279–87. doi: 10.1093/ageing/30.4.279. [DOI] [PubMed] [Google Scholar]