Abstract
Background: Inflammation is involved in development, progression, and complications of atherosclerotic disease. Clinical studies have indicated that the level of monocyte chemoattractant protein 1 (MCP-1), IL-18, and adhesion molecules correlates with the severity of atherosclerosis and can predict future cardiovascular events. Experimental studies have shown pentoxifylline (PTX) reduces these factors in animal models. The purpose of the present pilot study was to evaluate effect of PTX on a group of inflammatory biomarkers in patients with coronary artery disease (CAD). Methods: Forty patients with angiographically documented CAD, who fulfilled inclusion and exclusion criteria, were entered in the double-blind, randomized, pilot clinical study. The patients were randomly given PTX (400 mg three times daily) or placebo (3 tab/day) for 2 months. Serum concentrations of MCP-1, IL-18, intercellular adhesion Molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) were measured before and at the end of intervention by enzyme-linked immunosorbant assay. Results: Our study showed that the serum levels of ICAM-1 and VCAM-1 was decreased in the study population after two-month treatment (P<0.05). Conclusion: Based on the results of our pilot study, administration of PTX in CAD patients significantly decreases adhesion molecules levels.
Key Words: Atherosclerosis, Inflammation, Pentoxifylline
INTRODUCTION
Cardiovascular disease (CVD) continues to lead as a principal cause of death in developed countries. The vast majority of CVD-related deaths are attributed to a disease of arterial blood vessels, known as atherosclerosis [1]. The current view of atherosclerosis emphasizes molecular and cellular components of the immune system. The activation of immune system during inflammation decreases plaque stability and provoke its rupture, thus exposes highly pro-coagulant mediators to the blood stream and results in thrombosis and its subsequent clinical symptoms [1]. Hence, immune mechanisms that regulate integrity and stability as well as formation of atherosclerotic plaques have gained considerable attention among vascular biologists [2].
Traditional cardiovascular risk factors include smoking, hypertension, hypercholesterolemia, diabetes and obesity. However, the absence of the traditional risk factors does not completely protect from the disease and new risk factors have been identified, including markers of calcifications such as receptor activator of nuclear factor kappa-B ligand/osteo-protegerin ratio [3] and molecules involved in inflammation such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), monocyte chemoattractant protein 1 (MCP-1) and IL-18 [4]. ICAM-1, CD54 (cluster of differentiation 54), is a transmembrane protein of the immunoglobulin superfamily. The first stage of leukocyte-endothelial interaction is mediated by the weaker nonspecific selectin molecules, including P, E, and L selectins. Inflammatory mediator responses will upregulate the expression of ICAM-1, thereby increasing the adhesive nature of leukocytes and endothelial cells [1, 4, 5].
VCAM-1 is a protein, which mediates the adhesion of lymphocytes, monocytes, eosinophils, and baso-philes to vascular endothelium. It also functions in leukocyte-endothelial cell signal transduction, and it may play a role in the development of atherosclerosis [1, 2, 4-6].
MCP-1 is a chemokine, which plays an important role in the recruitment and activation of monocytes. Indeed, circulating MCP-1 levels are elevated in patients with acute myocardial infarction and in those with unstable angina. Production of MCP-1 and macrophage accumulation are also observed after coronary angioplasty, indicating that MCP-1 expression may be related not only to instability of atheromatous plaques, but also to the formation of restenotic lesions [7].
IL-18 is a member of the IL-1 cytokine family. It was initially discovered as a potent inducer of IFN-γ in TH1 cells and natural killer cells. IL-18 induces the expression of additional inflammatory mediators, including IL-1β, IL-6, TNF-α, IFN-γ, IL-8, MCP-1, MIP-1α, VCAM-1 and ICAM-1 [2, 4-8].
Pentoxifylline (PTX) is a methylxanthine derivative and a nonselective phosphodiesterase inhibitor which exhibits its anti-inflammatory properties in many experimental and clinical studies [9-12]. Experimental studies have shown that PTX reduces inflammation in atherosclerosis [13-16]. Clinical studies have demonstrated that it has a possible role in atherosclerosis treatment, and it has been found to be safe [9, 17, 18]. Regarding the anti-inflammatory effect of PTX, we hypothesized that it could suppress inflammatory pathways involved in atherosclerosis progression. Furthermore, because of its documented safety, it could be a reliable choice in a clinical trial. In the present pilot study, we evaluated the effect of PTX on serum levels of ICAM-1, VCAM1, MCP-1, and IL-18 in atherosclerotic patients.
MATERIALS AND METHODS
Patients. This study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Mashhad, Iran). Forty patients with coronary artery disease (CAD), admitted for drug therapy, signed a consent form prior to entry into the study. All patients had established atherosclerosis and enrolled in the study between July 2009 and November 2010. A cardiovascular specialist defined CAD through angiographic findings. The study was double-blinded. Patients were randomized into two equal groups. Blood was obtained from patients before and two months after either PTX (400 mg three times daily) or placebo administration. Demographic data, drug history, post medical history, familial history, and cardiovascular risk factors were recorded for each patient. The inclusion and exclusion criteria were as follow: Patients who were treated with angiotensin converting enzyme inhibitors inhibitors, statins (except few patients who needed these medications in the very late of this study) or immunosuppressive drugs were excluded. Patients who had established chronic disease, including diabetes, renal, or hepatic diseases were also excluded from the study.
Sample collection and storage. Blood sampling was performed before and two months after either PTX or placebo administration. Plasma was collected using EDTA as an anticoagulant and centrifuged at 1,000 ×g at 2-8°C for 15 minutes within 30 minutes of collection. The samples were aliquoted and stored at -70°C. ELISA was chosen as the method for determining the serum level of biomarkers (Human IL-18 ELISA and Quantikine Human sVCAM-1 Immunoassay Kits as well as Quantikine Human CCL2/MCP-1 Immunoassay and Quantikine Human sICAM-1/CD54 Immunoassay [all from R&D systems, USA]). Each assay was calibrated using biomarker standard curves following the manufacturer’s instructions.
Statistical analyses. Results were presented as mean ± SEM. Differences in plasma concentrations between patients and controls were calculated with the independent sample t-test. Chi-square test and paired t test were used in patient characteristics data analysis. P<0.05 were considered significant. All analyses were performed with SPSS software 11.5.
RESULTS
Patient characteristics. Demographics and traditional cardiovascular risk factors and clinical characteristics of the study population are shown in Table 1.
Table 1.
Patient characteristics and drugs administered for them
| Parameters, drugs and intervention (%) | Placebo group (n = 20) | Pentoxifylline group (n = 20) |
|---|---|---|
| Age (year) | 53.62 ± 9.19 | 55.46 ± 7.69 |
| Sex M/F | 85/15 | 75/25 |
| Smoking | 50 | 30 |
| Hypertension | 30 | 30 |
| Hyperlipidemia | 25 | 25 |
| Chest pain | 55 | 25 |
| Involved in CABG | 45 | 30 |
| Involved in PCI | 30 | 55 |
| Plaque in LAD | 75 | 75 |
| Plaque in RCA | 15 | 40 |
| Plaque in Cx | 10 | 45 |
| TVD | 25 | 15 |
| Hypothyroidism | 10 | 0 |
| Ejection fraction | 51.46 ± 8.33 | 52.33 ± 8.63 |
There is no significant (P>0.05) difference between two groups after statistical analysis (Chi-square/student's t-test) except in case of chest pain (P<0.05); CABG, coronary artery bypass graft surgery; PCI, percutaneous coronary intervention; LAD, left anterior descending-coronary artery; RCA, right coronary artery; Cx, circumflex coronary artery; TVD, triple vessel disease
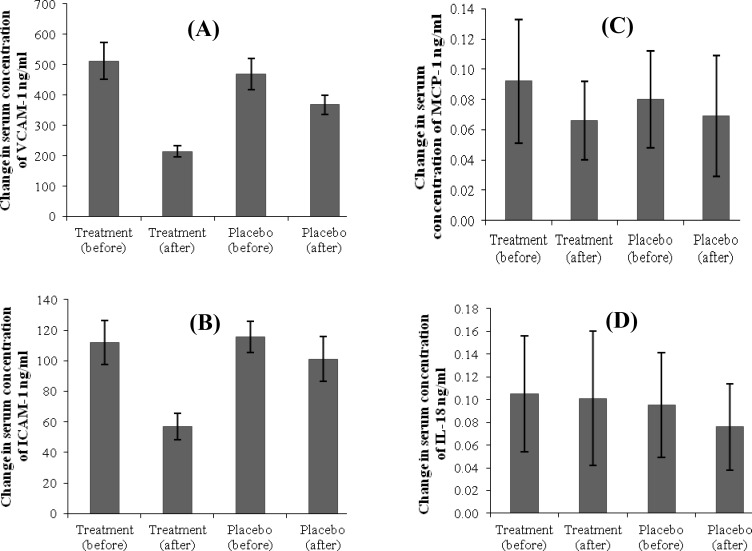
Evaluation of PTX effect on serum concentration of adhesion molecules. The results showed that PTX reduced serum levels of adhesion molecules in the study group compared with those of the placebo group significantly (P<0.0001) (Fig. 1A and 1B and Table 2).
Fig. 1.
Mean variations in measured inflammatory components. Mean variations in (A) VCAM-1 (P = 0.0001), (B) ICAM-1 (P = 0.0001), (C) MCP-1 (P = 0.4), and (D) IL-18 (P = 0.3) concentration before and after treatment in two groups. mean ± SD; VCAM-1, vascular cell adhesion molecule 1; ICAM-1, intercellular adhesion molecule 1, and MCP, monocyte chemoattractant protein 1
Table 2.
Comparison of change in serum concentrations before and after either the drug or placebo administration
|
Inflammatory
component 1 |
Study group
2
(ng/ml) |
Control group
(ng/ml) |
P value 3 |
|---|---|---|---|
| VCAM-1 | -298.27 ± 39.0610 | -100.29 ± 10.8200 | 0.0001 |
| ICAM-1 | -55.13 ± 6.6500 | -14.3 ± 2.9300 | 0.0001 |
| MCP-1 | -0.026 ± 0.0168 | -0.0207 ± 0.0110 | 0.4 |
| IL18 | -0.0375 ± 0.0145 | -0.0194 ± 0.0175 | 0.3 |
1, Serum concentration changes (concentration after administration-concentration before administration); 2, mean + SD; 3, two independent sample test
Evaluation of PTX effect on serum concentration of MCP-1 and IL-18. The results showed that PTX did not reduce serum levels of MCP-1 and IL-18 in the study group compared with those of placebo group significantly (Fig. 1C and 1D and Table 2).
DISCUSSION
Our results indicate that two-month PTX administration decreases both ICAM-1 and VCAM-1 levels in patients with CAD. However, it did not induce any significant changes in expression of IL-18 and MCP-1 proteins.
Without any doubt, inflammation plays a crucial role in virtually all stages of atherosclerotic process. Recent researches have related plaque formation to both dyslipidemia and inflammation [10]. Leukocyte recruitment and inflammatory cytokines expression may be characteristics of early stages of athero-sclerosis. Our results are consistent with other studies suggesting inflammation suppression properties of PTX in various inflammatory disorders. A prior study has reported the inhibitory effect of PTX on ICAM-1 and VCAM-1 [19]. Cultured monocytes isolated after oral application of PTX expressed decreased amounts of ICAM-1 compared with monocytes collected prior to oral PTX administration. Moreover, Northern blot analysis revealed reduced amounts of ICAM-1 mRNA in monocytes derived from volunteers after oral PTX treatment compared with monocytes isolated before oral PTX administration [12]. In our study, the inhibitory effects of PTX administration on ICAM-1 were also confirmed. Other studies have also shown the inhibitory effects of PTX on ICAM-1 and MCP-1 in various pathological conditions. PTX completely inhibited the surface expression of ICAM-1 and the production of IL-8 and MCP-1 by cytokine-activated epithelial cells of airway [10]. In another study [13], continuous intravenous administration of PTX was successful in blunting the increase of soluble adhesion molecules such as VCAM-1 and ICAM-1in critically ill patients undergoing continuous veno-venous hemofiltration. Infusion of PTX decreased the synthesis of ICAM-1 and VCAM-1 in patients with necrotizing pancreatitis and respiratory complications [14]. These findings further support the idea that PTX has inhibitory effects on ICAM-1 and VCAM-1.
On the other hand, besides our study, there are several studies on the effects of PTX on inflammatory biomarkers in CVD that support the benefits of PTX administration in CVD. Regarding to the effect of PTX on decreasing MCP-1, Pei et al. [15] have reported that taking 200 mg/day PTX by congestive heart failure patients could reduce the serum levels of MCP-1. A double-blind, prospective, placebo-controlled study on acute coronary syndrome patients have shown that PTX administration (1200 mg/day, for 6 months) decreased TNF-α and C-reactive protein. It also inhibited decrease of IL-10 (an anti-inflammatory cytokine) and increase of IL-12 [16]. TNF-α inhibitory property of PTX has reduced the vascular impairment in insulin resistance [17]. Decreased C-reactive protein level after administration of PTX (800 mg/day, for 2 months) has been reported in hypertensive diabetic patients [18]. Development of hypercholesterolemic atherosclerosis in rats was reduced by PTX probably via decreasing of platelet-activating factor and reactive oxygen species levels [19]. It was suggested that the cardioprotective effects of PTX against ischemia and reperfusion injury may be due to reductions in the activation of NFκB and the production of TNF- α content [20]. An early marker of atherosclerosis, a cross-sectional area of intima media thickness, was decreased after PTX administration in type 1 diabetic patients [21]. These studies have shown PTX administration has a versatile role in inhibition of inflammatory response in CVD.
According to these studies, PTX has an inhibitory effect on ICAM-1, VCAM-1, MCP-1, and IL-18 expression and could suppress other inflammatory steps that may be involved in atherosclerosis. Furthermore, a recent study has shown the inhibitory effect of PTX on CD40/CD40 legands, inflammatory elements involved in atherosclerosis progression, system in patients with atherosclerosis [16]. Our results supported the decreasing effect of PTX on ICAM-1 and VCAM-1 serum levels.
ACKNOWLEDGEMENTS
This study was a part of a thesis of R. Rasooli, and it has been supported financially by Mashhad University of Medical Sciences (Mashhad, Iran).
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes N. The Role of the Pro-Inflammatory Cytokine Interleukin-18, Its Processing Enzyme Caspase-1, and Potential Alternative IL-18-Activating Pathways in Atherosclerosis. Köln: University of Köln; 2005. [Google Scholar]
- 3.Mohammadpour AH, Shamsara J, Nazemi S, Ghadirzadeh S, Shahsavand S, Ramezani M. Evaluation of RANKL/OPG Serum Concentration Ratio as a New Biomarker for Coronary Artery Calcification: A Pilot Study. Thrombosis. 2012;0212:306263. doi: 10.1155/2012/306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels SR. Cardiovascular disease risk factors and atherosclerosis in children and adolescents. Curr Atheroscler Rep. 2001 Nov;3(6):479–85. doi: 10.1007/s11883-001-0038-3. [DOI] [PubMed] [Google Scholar]
- 5.Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005 Dec;78(4):389–97. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 6.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006 Oct;114(14):1504–11. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 7.Martinovic I, Abegunewardene N, Seul M, Vosseler M, Horstick G, Buerke M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 2005 Dec;69(12):1484–9. doi: 10.1253/circj.69.1484. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007 Jan;27(1):98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Ustunsoy H, Sivrikoz MC, Tarakcioglu M, Bakir K, Guldur E, Celkan MA. The effects of pentoxiphylline on the myocardial inflammation and ischemia-reperfusion injury during cardiopulmonary bypass. J Card Surg. 2006 Jan-Feb;21(1):57–61. doi: 10.1111/j.1540-8191.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 10.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009 Jan;335(1):191–203. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 11.de Campos T, Deree J, Martins JO, Loomis WH, Shenvi E, Putnam JG, et al. Pentoxifylline attenuates pulmonary inflammation and neutrophil activation in experimental acute pancreatitis. Pancreas. 2008 Jul;37(1):42–9. doi: 10.1097/MPA.0b013e3181612d19. [DOI] [PubMed] [Google Scholar]
- 12.Neuner P, Klosner G, Pourmojib M, Knobler R, Schwarz T. Pentoxifylline in vivo and in vitro down-regulates the expression of the intercellular adhesion molecule-1 in monocytes. Immunology. 1997 Mar;90(3):435–9. doi: 10.1111/j.1365-2567.1997.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boldt J, Heesen M, Padberg W, Martin K, Hempelmann G. The influence of volume therapy and pentoxiphylline infusion on circulating adhesion molecules in trauma patients. Anaesthesia. 1996 Jun;51(6):529–35. doi: 10.1111/j.1365-2044.1996.tb12557.x. [DOI] [PubMed] [Google Scholar]
- 14.Hyun YM, Chung HL, McGrath JL, Waugh RE, Kim M. Activated integrin VLA-4 localizes to the lamellipodia and mediates T cell migration on VCAM-1. J Immunol. 2009 Jul;183(1):359–69. doi: 10.4049/jimmunol.0803388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei Z, Kai-zheng G, Hong-guang SUN, Xiao-lei LV, Shun-di W, Xiao-ning SUN, et al. Long-term effects of pentoxiphylline in patients with congestive heart failure. Chinese Heart J. 2008;51:51–4. [Google Scholar]
- 16.Fernandes JL, de Oliveira RT, Mamoni RL, Coelho OR, Nicolau JC, Blotta MH, et al. Pentoxifylline reduces pro-inflammatory and increases anti-inflammatory activity in patients with coronary artery disease- a randomized placebo-controlled study. Atherosclerosis. 2008 Jan;196(1):434–42. doi: 10.1016/j.atherosclerosis.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 17.El-Bassossy HM, El-Moselhy MA, Mahmoud MF. Pentoxifylline alleviates vascular impairment in insulin resistance via TNF-alpha inhibition. Naunyn Schmiedebergs Arch Pharmacol. 2011 Sep;384(3):277–85. doi: 10.1007/s00210-011-0669-z. [DOI] [PubMed] [Google Scholar]
- 18.Maiti R, Agrawal NK, Dash D, Pandey BL. Effect of Pentoxifylline on inflammatory burden, oxidative stress and platelet aggregability in hypertensive type 2 diabetes mellitus patients. Vascul Pharmacol. 2007 Aug-Sep;47(2-3):118–24. doi: 10.1016/j.vph.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Prasad K, Lee P. Suppression of hypercholesterolemic atherosclerosis by pentoxiphylline and its mechanism. Atherosclerosis. 2007 Jun;192(2):313–22. doi: 10.1016/j.atherosclerosis.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R, Bharadwaj U, Li M, Chen C, Yao Q. Effects of pentoxiphylline on differentiation, maturation, and function of human CD14+ monocyte-derived dendritic cells. J Immunother. 2007 Jan;30(1):89–95. doi: 10.1097/01.cji.0000211323.53396.38. [DOI] [PubMed] [Google Scholar]
- 21.Atabek ME, Kurtoglu S, Selver B, Baykara M. Effectiveness of pentoxiphylline on the cross-sectional area of intima media thickness and functions of the common carotid artery in adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2011;24(11-12):945–51. doi: 10.1515/jpem.2011.199. [DOI] [PubMed] [Google Scholar]
- 22.Shamsara J, Mohammadpour AH, Behravan J, Falsoleiman H, Ramezani M. Pentoxifylline decreases soluble CD40 ligand concentration and CD40 gene expression in coronary artery disease patients. Immunopharmacol Immunotoxicol. 2012 Jun;34(3):523–9. doi: 10.3109/08923973.2011.621435. [DOI] [PubMed] [Google Scholar]