Abstract
Background: Progress in the field of biology and biochemistry has led to the discovery of numerous bioactive peptides and proteins in the last few decades. Delivery of therapeutic proteins/peptides has received a considerable amount of attention in recent years. Methods: In this study, a two-step desolvation method was used to produce biodegradable hydrophilic gelatin nanoparticles (GNP) as a delivery system of protein model (BSA). The size and shape of the nanoparticles were examined by dynamic light scattering and scanning electron microscopy. Results: Particles with a mean diameter of 200-300 nm were produced and the percentage of entrapment efficiency was found to be 87.4. The optimum amount of theoretical BSA loading was obtained, the release of BSA was monitored in vitro, and the mechanism of release was studied. The BSA release profile showed a biphasic modulation characterized by an initial, relatively rapid release period, followed by a slower release phase. Conclusion: Results show that the two-step desolvation is an appropriate method for preparing GNP as a delivery vehicle for BSA.
Key Words: Gelatin, Nanoparticles, Bovine serum albumin
INTRODUCTION
Peptides and proteins play a vital role in all biological processes and have received a growing attention in recent years as drug candidates. Therefore, the ability to deliver these compounds systemically using convenient and effective delivery systems is very important [1, 2]. However, some physicochemical and biological properties of protein and peptide drugs are different from those of conventional ones that cause a number of limitations to delivery of them. These properties may include molecular size, biological half-life, conform-ational stability, solubility, dose requirement and administration [3].
Oral route is the most convenient and popular method for delivery of proteins and peptides, but most peptide drugs show low oral activity. This fact is mainly due to degradation by gastrointestinal tract enzymes and poor permeability of the intestinal mucosa [1, 4]. On the other hand, if protein and peptide drugs administer parenteral, multiple injections are required in order to achieve desirable therapeutic effects because of their short half-lives in vivo. The multiple or frequent injections cause poor compliance. Therefore, designing and manufacturing truly unique protein and peptide drug delivery systems are desired. Recently, there is a significant interest in developing novel delivery systems, which will deliver peptide and protein drugs at a controlled rate throughout pre-determined lengths of time. Various delivery systems, including prolease technology, nano-particulate and microparticulate delivery system, and mucoadhesive delivery of peptides have been developed [5].
In recent years, nanoparticles have received a growing attention as a delivery system of different bioactive molecules. Nanoparticles are solid colloidal particles ranging in size from 1 to 1,000 nm. They can be used to entrap, encapsulate or absorb active agents (drugs or other biologically active agents) [6]. These particles can be prepared from a variety of natural and synthetic materials, such as proteins, polysaccharides, and synthetic polymers. Although various bio-degradable nanoparticles of natural polymers, such as starch, chitosan, liposomes, etc. are largely in use as drug carriers, gelatin nanoparticles (GNP) represent a promising carrier system for controlled drug delivery technology [7].
The interest was based on the facts that gelatin is low cost, biodegradable, biocompatible, non-toxic, and easy to crosslink and to modify chemically and has therefore an immense potential to be used for the preparation of drug delivery systems such as protein-containing nanoparticles [6]. Various methods, including nanoencapsulation [8], water-in-oil emulsion [9, 10], desolvation [11], and coacervation-phase separation [12] have been used to prepare GNP. There are some studies that use these methods to produce GNP containing different bioactive molecules [13-18]. Although all of these methods have several advantages, there are some limitations. In case of water-in-oil emulsion technique, a large amount of surfactant is required to produce the small-sized GNP, which needs a complicated post-process. The coacervation method is a process of phase separation followed by cross-linking step, while the non-homogeneous cross-linking occurs in this method and has unsatisfied loading efficiency [19]. Indeed, GNP prepared by many of these methods was found to be large in size and has a high polydispersity index (PDI) due to heterogeneity in molecular weight of the gelatin polymer. An easier GNP preparation method, two-step desolvation, was developed that enabled the production of GNP with a reduced tendency for aggregation [20]. In this method, after the first desolvation step, the low molecular gelatin fractions presented in the supernatant were removed by decanting, and the high molecular fractions presented in the sediment were redesolved. However, there are some studies that use two-step desolvation to produce GNP containing different bioactive molecules [21-24].
Also, few studies have been conducted in production of protein-loaded GNP by this method. In the present study, a two-step desolvation method was used to produce GNP containing a model protein drug, BSA, as protein/peptide delivery system. At the first step, the influence of molecular weight heterogeneity of gelatin on nanoparticle size and PDI was investigated. Then, BSA-loaded GNP was produced and the optimum amount of BSA loading was obtained. Finally, the size, shape, entrapment efficiency (EE%) and drug release kinetic of nanoparticles were investigated.
MATERIALS AND METHODS
Materials. Gelatin from bovine skin, glutaraldehyde (25% v/v aqueous solution), acetone, and BSA were purchased from Merck, Germany.
GNP preparation. GNP was prepared by one- and two-step desolvation method. The mechanism of GNP formation was based on applying a desolvating agent to reduce water available and keep the hydrated gelatin chain in solution, resulting in agglomeration and subsequent formation of particles.
One-step desolvation. Gelatin (200 mg) was dissolved in distilled water (10 mL) under constant heating at 40 ± 1°C, pH 3 (by adding 0.1 M HCL). BSA was added, followed by dropwise addition of acetone (30 mL) to form GNP. At the end of the process, glutaraldehyde solution (25% v/v in distilled water [100 µL]) was added as a cross-linking agent, and the solution was stirred for 30 min. Dispersion was centrifuged at 11790 RCF for 30 min. The particles were purified by threefold centrifugation and ridispersion water. After the last ridispersion, fabricated particles were freeze-dried, and then white freely flowing powder of BSA-loaded GNP was obtained.
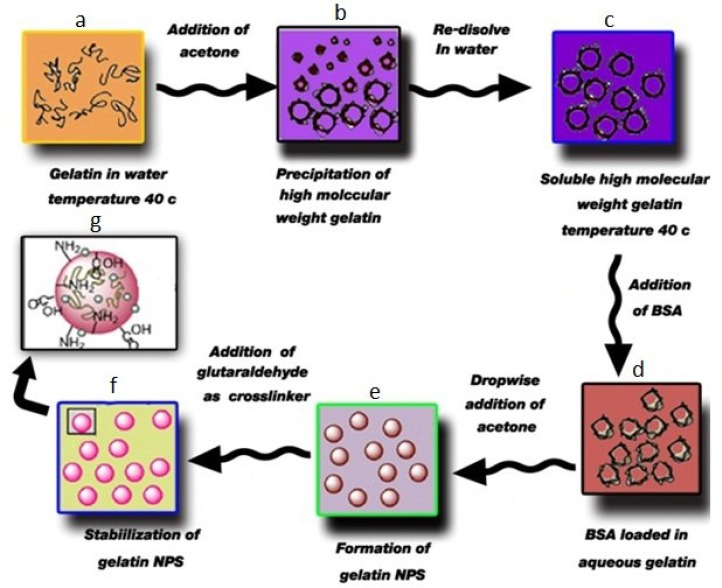
Two-step desolvation. Coester et al. [20] reported a two-step desolvation method to produce GNP. Here, this method was adapted to encapsulate BSA in situ within GNP. Briefly, 200 mg gelatin was dissolved in distilled water (10 mL) under constant heating at 40° ± 1°C. Acetone (10 mL) was added to the gelatin solution as a desolving agent to precipitate the high molecular weight gelatin. The supernatant was discarded, and the high molecular weight gelatin was redissolved by adding distilled water (10 mL) with stirring under constant heating at 40°C, pH 3 (by adding 0.1 M HCL). BSA was added, followed by dropwise addition of acetone (30 mL) to form GNP. At the end of the process, glutaraldehyde solution (100 µL) was added as a cross-linking agent, and the solution was stirred at 40 ± 1°C for 30 min. Solution was centrifuged at 11790 RCF for 30 min. The particles were purified by threefold centrifugation and ridispersion water. After the last ridispersion, fabricated particles were freeze-dried, and then white freely flowing powder of BSA-loaded GNP was obtained. The process has been described schematically in Figure 1.
Fig. 1.
Schematic illustration of BSA-loaded gelatin nanoparticles produced by two-step desolvation. (a) Aqueous solution of gelatin; (b), first step desolvation; (c), aqueous solution of high molecular weight of gelatin; (d), BSA- loaded in aqueous gelatin; (e), dropwise addition of acetone and formation of GNP, and (f), stabilization of GNP by glutaraldehyde [19].
Characterization of nanoparticles. Scanning electron microscopy (EM-3200) and the particle size analyzer (Malvern Zen3600 Zetasizer) were used to study the particle size and morphology of the GNP. The infrared spectrum of GNP was recorded on a Fourier transform infrared spectrophotometer (Thermo Nicolet NEXUS 670, USA). The total quantity of BSA contained in the nanoparticles and in vitro BSA release were determined by a UV-Vis spectrophotometer (LIUV-210 UV/Vis, Lambda Scientific Pty Ltd., Australia).
Determination of entrapment efficiency. The total quantity of BSA contained in the nanoparticles was determined by a ‘direct’ method. Five milligrams of nanoparticles were added to 5 ml PBS (37°C). After complete dissolution, the drug concentration was determined by UV-Vis spectrophotometer at λ = 278 nm.
(1)
In vitro release study. The in vitro release experiment was performed in a shaker bath at 37°C. The BSA-containing GNP was suspended in PBS and shaked in water bath. At predetermined time intervals, 1 ml supernatant was withdrawn. Then, 1 ml fresh PBS was added to the tube and the tube was returned to the shaker bath. The release samples were quantitated using a UV spectrophotometer at 278 nm. The percentage cumulative amount of BSA released was calculated by using the following equation:
(2)
Where Mt is the amount of BSA released at time t.
RESULTS
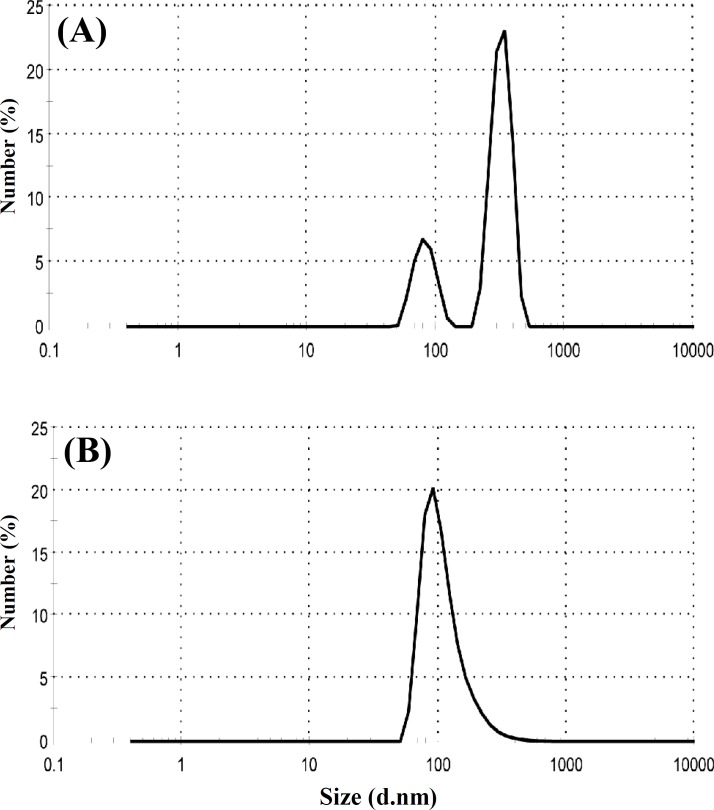
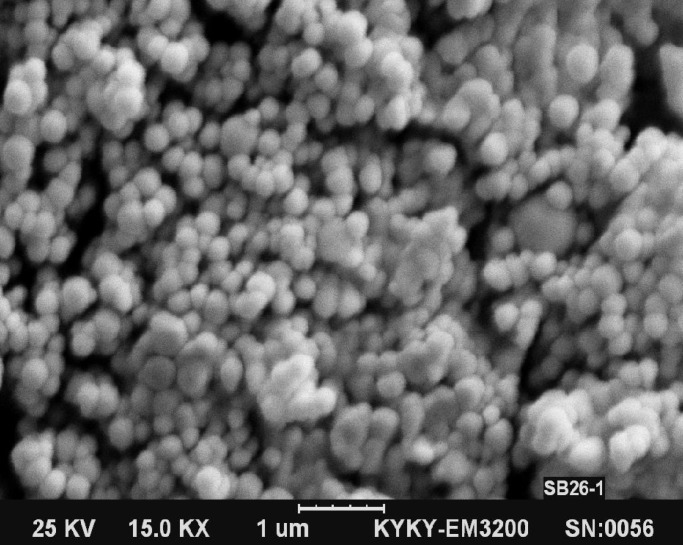
Size and size distribution. The size and size distribution are important characteristics of a nanoencapsulation product used for drug delivery because of the dependence of drug release rate on the size and size distribution. Figure 2A shows size and size distribution of the GNP without BSA loading, produced by one-step desolvation method. The average particle size and PDI of nanoparticles are 360 nm and 0.371, respectively. Figure 2B shows size and size distribution of GNP without BSA loading produced by two-step desolvation method. The average particle size and PDI of nanoparticles are 199 nm and 0.250, respectively. These results demonstrate that two-step desolvation is more suitable to get GNP with desirable size and PDI. Therefore, we used two-step desolvation method for producing of BSA-loaded gelatin nanoparticles. Scanning electron microscopy images of GNP produced by two-step desolvation method have been shown in Figure 3. As shown in this Figure, smooth and spherical GNP was produced. The photograph clearly indicates that no hairline cracks or heterogeneity appear on the nanoparticles surface. These data present morphological evidence for the solid and the smooth nanoparticles.
Fig. 2.
Particle size distribution of gelatin nanoparticles produced by (A) one-step and (B) two-step desolvation methods by number
Fig. 3.
Scanning electron microscopy images of gelatin nanoparticles (1-μm scale).
The effect of theoretical BSA loading on size and entrapment efficiency of GNP. Table 1 shows the effect of theoretical BSA loading on particle size, PI, and EE%. The results revealed that BSA loading in GNP had a significant effect on particle size. There was a considerable increase in EE% between 1% and
Table 1.
Effect of theoretical BSA loading on particle size, PI, and EE%
| Theoretical BSA loading (%) | Amount of glutaraldehyde (µL) |
Temperature
(°C) |
Size (nm)
polydispersity index |
Entrapment
efficiency (%) |
|---|---|---|---|---|
| 0 | 200 | 40 | 199.02 (0.250) | 0 |
| 1 | 200 | 40 | 275.44 (0.257) | 61.3 |
| 2 | 200 | 40 | 290.52 (0.264) | 87.4 |
| 3 | 200 | 40 | 498.04 (0.481) | 89.1 |
| 4 | 200 | 40 | … | … |
| 5 | 200 | 40 | … | … |
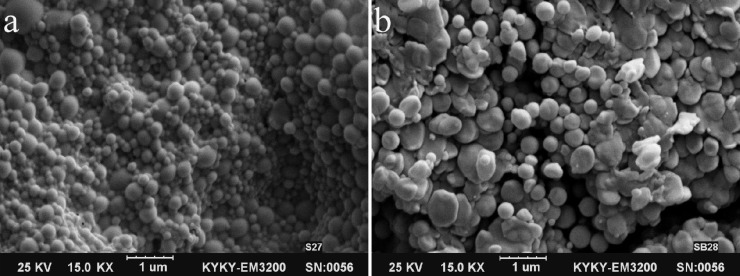
2% w/w of theoretical BSA loading. Figure 4 shows GNP containing 2% and 3% w/w of BSA. By using 3% w/w of BSA, the particle size and PDI was increased significantly and some aggregation was observed (Fig. 4b), whereas no important effect was observed on EE%. In 4% and 5% w/w of BSA, some precipitate was formed after addition of acetone and desirable nanoparticles could not be obtained at these percentages of theoretical BSA loading. Therefore, 2% of theoretical BSA loading was selected as optimum for producing nanoparticles with a desirable size, PDI, and EE%.
Fig. 4.
Scanning electron microscopy images of gelatin nanoparticles (1-μm scale). (a) Gelatin nanoparticles with 2% w/w BSA, (b) gelatin nanoparticles with 3% w/w BSA fabricated by two-step desolvation method
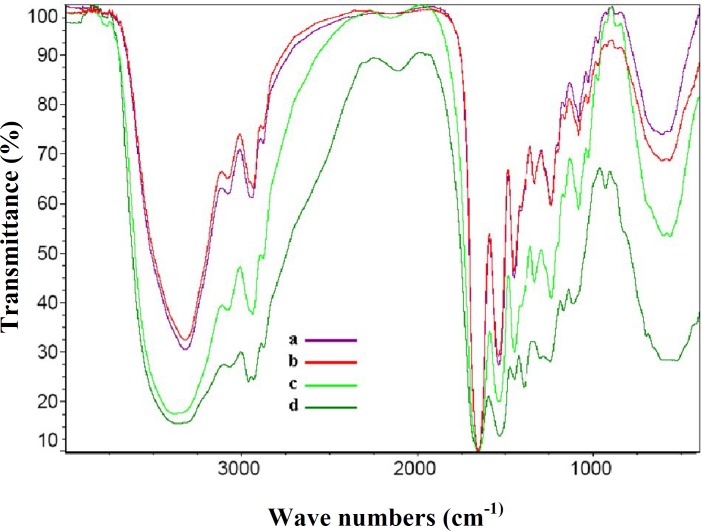
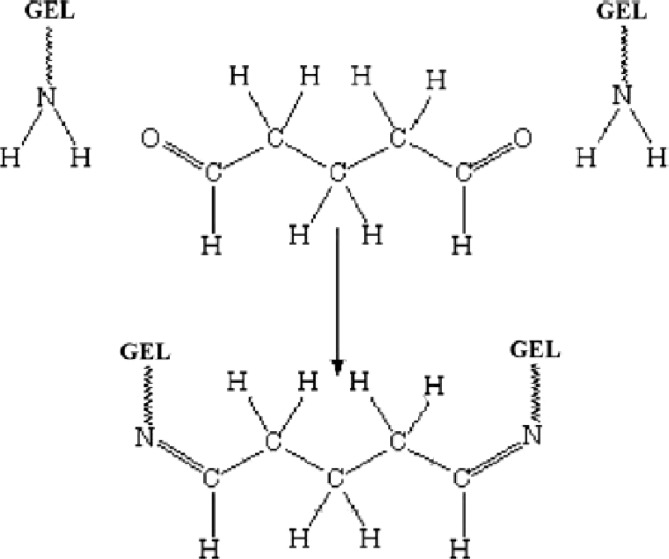
Infrared spectral analysis. The gelatin powder, blank GNP, BSA, and BSA-loaded GNP were characterized by Fourier transform infrared spectrophotometer spectra (Fig. 5). The strong band observed at 3373 cm−1 was due to N-H stretching of amine group, 2930 cm−1 due to C-H stretching of methyl and methylene group, 1653 due to C=O stretching of amide group, 1545 cm−1 due to C-N stretching vibration, and 1450 cm−1 due to C=N ring stretching. Glutaraldehydes have an aldehyde group (-CHO) that reacts with the amino group of gelatin, resulted in the formation of aldimine linkage (CH=N). A stronger peak at 1450 cm-1 in the BSA-loaded gelatin nanoparticles is related to this linkage. Figure 6 shows the formation of bond between gelatin and glutaraldehyde as crosslinking agent into gelatin chains.
Fig. 5.
The Infrared spectra of different materials. (a) Gelatin powder, (b) blank gelatin nanoparticles, (c) BSA-loaded gelatin nanoparticles, and (d) BSA
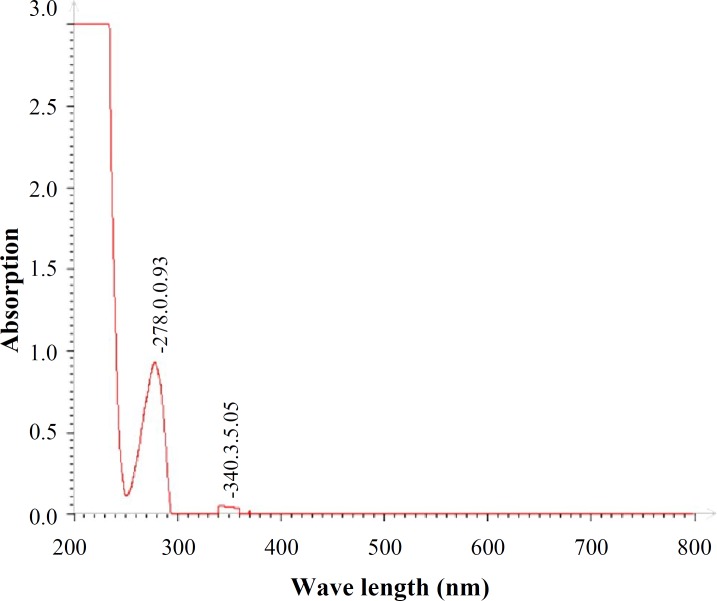
Fig. 6.
UV-Vis absorption spectrum of BSA
In vitro protein release. The release profiles of BSA from GNP were measured by using UV-Vis spectrophotometer. First, UV-Vis absorption spectrum of BSA was plotted (Fig. 7). The BSA had the maximum absorption centering at 278 nm. For the calibration curve, five different concentrations of the drugs were prepared and the absorbance was plotted against the concentrations to get the standard curve.
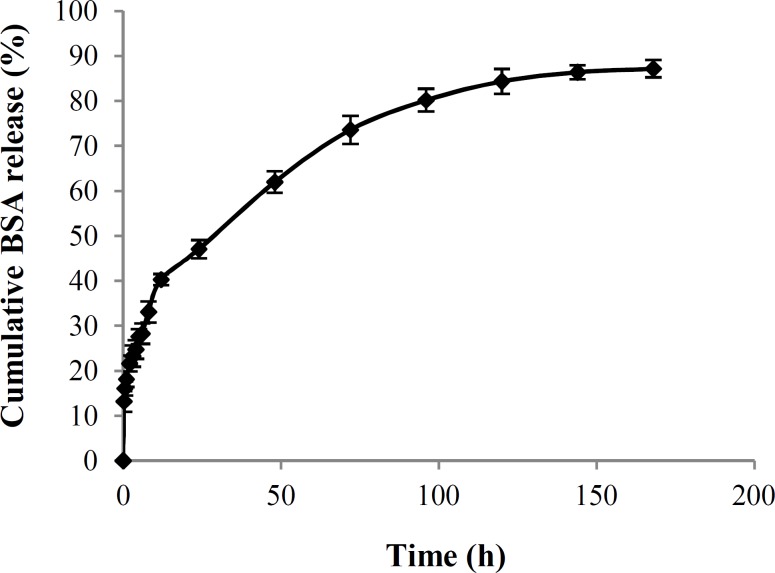
Fig. 7.
Cumulative percentage of BSA released from gelatin nanoparticles as a function of time.
Figure 8 illustrates the BSA release profile from the GNP (containing 2% BSA) at 37°C over 7 days of incubation. Over 70% of BSA was released when the release time (t) was less than 80 h. When t>90 h, the release rate was quite slow. The burst release phase may be due to the BSA molecules imbedded in the surface of GNP. Reduction in release rate may be attributed to the reduced concentration of BSA in the gelatin matrix.
DISCUSSION
The rapid advances in peptide and protein pharmacology along with the large-scale production of these compounds have fueled enormous interest in these compounds. Development of peptide and protein has been more rapidly than the ability to deliver these components effectively. In recent years, nanoparticles have received a growing attention as a delivery system of different bioactive molecules such as proteins and peptides. Gelatin as a nanoparticle material has a number of advantages owing to its biocompatibility, biodegradability and low antigenicity [22, 25]. In this study, one- and two-step desolvation methods were used to produce gelatin nanoparticles as the delivery system of BSA as a protein model. In particle size distribution curve of GNP produced by one-step desolvation method, two peaks can be observed (Fig. 2A). The second peak is related to the low-molecular-weight gelatin chains in solution, which results in a higher particle size and PDI. These particles frequently encounter some problems. They not only may form irreversible aggregates during cross-linking but also additionally tend to be aggregated more extensively after cross-linking [20]. Also, variation in size and PDI of nanoparticulate carrier systems may affect drug release, drug loading, and alternation in in vivo parameters such as uptake by macrophages. Therefore, heterogeneity in molecular weight of the gelatin is one major obstacle for formulating GNP. In two-step desolvation method, low-molecular-weight gelatin chains were discarded in the first desolvation step, and subsequently the obtained high- and uniform-molecular-weight gelatin was used for the GNP formulation, which ensured nanometric particles with lower PDI (Fig. 2B). These results demonstrate that two-step desolvation is necessary to get more desirable GNP. Therefore, we used two-step desolvation method for producing of BSA-loaded gelatin nanoparticles. In this method, different processing parameters such as pH, the amount of glutaraldehyde, and temperature may affect the size, PDI, and EE% of drug-loaded GNP [26].
Theoretical drug loading is also significant to obtain GNP of desired size and EE%. Therefore, BSA loading was varied from 1% to 5% w/w to observe the effect on size, PDI , and EE% (at a temperature of 40°C, 200 μL glutaraldehyde, pH 3). Our results show that at higher concentration of BSA (4% and 5% w/w), some precipitate was formed after the addition of acetone. It was probably because of the formation of irreversible aggregation during cross-linking of BSA molecules with glutaraldehyde happening at higher percentage of theoretical BSA loading.
Fig. 8.
Formation of bond between gelatin and glutaraldehyde as cross-linking agent into GEL chains [27].
As previously noted, designing and manufacturing a truly unique protein and peptide drug delivery systems are important to preserve the biological activity of them. The results of FTIR showed that the spectra of the BSA, gelatin and BSA-loaded GNP are approximately the same, and no new peak or migration was observed. A stronger peak at 1450 cm-1 in spectra of BSA-loaded GNP was due to the formation of bond between gelatin and glutaraldehyde as crosslinking agent into gelatin chains. It can be concluded that this method is safe, and there is no risk for changing the conformation of the protein during GNP production. The BSA release profiles showed a biphasic modulation characterized by an initial relatively rapid release period, followed by a slower release phase. The mechanism of release of hydrophilic protein from GNP may involve the following aspects: (1) water permeation through the hydrogel matrix and absorption by the GNP, (2) GNP swelling, and (3) diffusion of BSA molecules through the swollen GNP. The release was continuously monitored for 7 days and it demonstrated that these GNP are appropriate as delivery system for BSA.
It can be concluded from this study that desirable BSA-loaded GNP, can be prepared using two-step desolvation method. By using 2% w/w of theoretical drug loading as optimum, particles with a mean diameter of 200-300 nm and EE% of 87.4% were produced. The BSA release profiles showed that these protein-containing nanoparticles could sustain the release of protein drugs and consequently prolong the bioavailability of the drugs.
References
- 1.Al-Tahami K, Singh J. Smart polymer based delivery systems for peptides and proteins. Recent Pat Drug Deliv Formul. 2007;1(1):65–71. doi: 10.2174/187221107779814113. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today. 2003 Mar;8(6):259–66. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 3.Shaji J, Patole V. Protein and peptide drug delivery: oral approaches. Indian J Pharm Sci. 2008 May-Jun;70(3):269–77. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morishita M, Nicholas NA. Peppas, Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 2006 Oct;11(19-20):905–10. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv. 2007 Apr;4(2):141–51. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 6.Jahanshahi M, Babaei Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7:4926–34. [Google Scholar]
- 7.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001 Jun;221(1-2):1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 8.Li JK, Wang N, Wu XS. Gelatin nanoencapsulation of protein/peptide drugs using an emulsifier-free emulsion method. J Microencapsul. 1998 Mar-Apr;15(2):163–72. doi: 10.3109/02652049809006846. [DOI] [PubMed] [Google Scholar]
- 9.Cascone MG, Lazzeri L, Carmignani C, Zhu Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J Mater Sci Mater Med. 2002 May;13(5):523–6. doi: 10.1023/a:1014791327253. [DOI] [PubMed] [Google Scholar]
- 10.Bajpai AK, Choubey J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J Mater Sci Mater Med. 2006 Apr;17(4):345–58. doi: 10.1007/s10856-006-8235-9. [DOI] [PubMed] [Google Scholar]
- 11.Ofokansi K, Winter G, Fricker G, Coester C. Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur J Pharm Biopharm. 2010 Sep;76(1):1–9. doi: 10.1016/j.ejpb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Yeh TK, Lu Z, Wientjes MG, Au JL. Formulating paclitaxel in nanoparticles alters its disposition. Pharm Res. 2005 Jun;22(6):867–74. doi: 10.1007/s11095-005-4581-4. [DOI] [PubMed] [Google Scholar]
- 13.Mladenovska K, Kumbaradzi E, Dodov G, Makraduli L, Goracinova K. Biodegradation and drug release studies of BSA loaded gelatin microspheres. Int J Pharm. 2002 Aug;242(1-2):247–9. doi: 10.1016/s0378-5173(02)00167-9. [DOI] [PubMed] [Google Scholar]
- 14.Bajpai AK, Choubey J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J Mater Sci Mater Med. 2006 Apr;17(4):345–58. doi: 10.1007/s10856-006-8235-9. [DOI] [PubMed] [Google Scholar]
- 15.Ofokansi K, Winter G, Fricker G, Coester C. Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur J Pharm Biopharm. 2010 Sep;76(1):1–9. doi: 10.1016/j.ejpb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bajpai AK, Choubey J. In vitro release dynamics of an anticancer drug from swellable gelatin nanoparticles. J Appl Polym Sci. 2006 Aug;101(4):2320–32. [Google Scholar]
- 17.Lee EJ, Khan SA, Park JK, Lim KH. Studies on the characteristics of drug-loaded gelatin nanoparticles prepared by nanoprecipitation. Bioprocess Biosyst Eng. 2012 Jan;35(1-2):297–307. doi: 10.1007/s00449-011-0591-2. [DOI] [PubMed] [Google Scholar]
- 18.Vijaya Kumar Naidu B, Paulson AT. A New method for the preparation of gelatin nanoparticles: encapsulation and drug release characteristics. J Appl Polym Sci. 2011 Sep;121(6):3495–3500. [Google Scholar]
- 19.Akhter KF, Zhu J, Zhang J. Nanoencapsulation of protein drug for controlled release. J Physic Chem Biophysic. 2012 doi:10.4172/2161-0398.S11-001. [Google Scholar]
- 20.Coester CJ, Langer K, van Briesen H, Kreuter J. Gelatin nanoparticles by two step desolvation--a new preparation method, surface modifications and cell uptake. J Microencapsul. 2000 Mar-Apr;17(2):187–93. doi: 10.1080/026520400288427. [DOI] [PubMed] [Google Scholar]
- 21.Saxena A, Sachin K, Bohidar HB, Verma AK. Effect of molecular weight heterogeneity on drug encapsulation efficiency of gelatin nano-particles. Colloids Surf B Biointerfaces. 2005 Sep;45(1):42–8. doi: 10.1016/j.colsurfb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Nahar M, Mishra D, Dubey V, Jain NK. Development, characterization, and toxicity evaluation of amphotericin B–loaded gelatin nanoparticles. Nanomedicine. 2008 Sep;4(3):252–61. doi: 10.1016/j.nano.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Saragi GK, Gupta P, Gupta UD, Jain NK, Agrawal GP. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int J Pharm. 2010 Jan;385(1-2):143–9. doi: 10.1016/j.ijpharm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Qazvini NT, Zinatloo S. Synthesis and characterization of gelatin nanoparticles using CDI/NHS as a non-toxic cross-linking system. J Mater Sci Mater Med. 2011 Jan;22(1):63–9. doi: 10.1007/s10856-010-4178-2. [DOI] [PubMed] [Google Scholar]
- 25.Jahanshahi M, Sanati MH, Hajizadeh S, Babaei Z. Gelatin nanoparticle fabrication and optimization of the particle size. Physica Status Solidi. 2008;205(12):2898–2902. [Google Scholar]
- 26.Jahanshahi M, Sanati MH, Babaei Z. Optimization of parameters for the fabrication of gelatin nanoparticles by the Taguchi robust design method. J Appl Stat. 2008;35(12):1345–53. [Google Scholar]
- 27.Azami M, Rabiee M, Moztarzadeh F. Glutaraldehyde crosslinked gelatin/hydroxyapatite nanocomposite scaffold, engineered via compound techniques. Polym compos. 2010 Dec;31(12):2112–20. [Google Scholar]