Abstract
Background: Nonalcoholic steatohepatitis (NASH), a progressive stage of nonalcoholic fatty liver disease (NAFLD), is characterized by steatosis with inflammation. Investigations have suggested that oxidative stress may play an important role in the progress of NAFLD to NASH. To provide further insights into beneficial effects of antioxidants in NASH prevention, we employed two manganese-superoxide dismutase/catalase mimetics, manganese N,N`-bis(salicyldene) ethylene diamine chloride (EUK-8) and manganese-3-methoxy N,N`-bis(salicyldene)ethylenediamine chloride (EUK-134), as two salen representatives and vitamin C as the standard antioxidant. Methods: Experimental NASH was induced in Male N-Mary rats by feeding a methionine/choline-deficient (MCD) diet to rats for 10 weeks. The rats (n = 5, 30 mg/kg/day) were randomly assigned to receive vitamin C, EUK-8, EUK-134 or vehicle orally. Results: Administration of salens together with the MCD diet reduced the serum aminotransferases, glutathione transferase and alkaline phosphatase, cholesterol, and LDL contents. In addition, the EUK-8 and EUK-134 improved NASH pathological features in liver of MCD-fed rats. Conclusion: EUK-8 and EUK-134 supplementation reduces NASH-induced abnormalities, pointing out that antioxidant strategy could be beneficial for prevention of NASH.
Key Words: Fatty liver, Oxidative stress, Antioxidants
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), as a major chronic liver disease in the western countries and Asia [1], is considered as the hepatic manifestation of metabolic syndrome. This clinico-histopathological entity is defined by a hepatic triglyceride content exceeding 5% of liver weight in individuals with no or little alcohol consumption (less than 20 g/d in women and 40 g/d in men) and in the absence of other causes (viral, toxic, or autoimmune) [2]. NAFLD comprises a wide range of liver abnormalities from simple nonalcoholic fatty liver to nonalcoholic steatohepatitis (NASH) and NASH-associated fibrosis and cirrhosis [3].
NASH is the leading cause of liver disease in USA with a prevalence of 3-5% compared to the prevalence of 10-30% for NAFLD. Necroinflammation, with ballooning degeneration of hepatocytes, is the hallmark of NASH [4]. This disease is frequently associated with obesity, hyperlipidemia, hypertention, type II diabetes, and insulin resistance [5]. A “two-hit” hypothesis has been proposed to explain the progression of NAFLD into NASH [, ]. In this hypothesis, insulin resistance induces a reversible deposition of excess triaglycerides within hepatocytes (so called steatosis). Steatosis, as the first hit, sensitizes the hepatic cells to multiple second hits, including a series of complex interactions between hepatocytes, stellate cells, adipose cells, Kupffer cells, inflammatory mediators, and reactive oxygen species (ROS). The second hits could, in turn, result in inflammation, cell death, and fibrosis, which are the histological symptoms of NASH [4, 6, 7].
Improvement of insulin sensitivity, body weight loss, and reduction of hyperlipidemia are the proposed strategies for NAFLD and NASH treatments. Accordingly, insulin-sensitizing agents, such as rosiglitazone, pioglitazone, and metformin have been shown to improve serum alanine transaminase (ALT) level and the histological features of NASH. Furthermore, the hypolipidemic drug, gemfibrozil, has also been reported to reduce serum ALT level [8]. However, histologic improvements were not observed in repeated liver biopsies [8]. The involvement of ROS in the progress of NAFLD into NASH suggests that antioxidants might have beneficial effects on the prevention and/or treatment of NASH [9]. Indeed, it has been shown that vitamin E has a role on the reduction of hepatic steatosis, lobular inflammation, and transaminase levels [10].
Manganese superoxide dismutase (SOD), the first-line defense enzyme of the cells plays a critical role in protecting the cells against oxidative damage [11]. To date, several types of SOD mimics, such as metallo-porphyrins, nitroxides, manganese cyclic polyamines, and salens have been evaluated in various experimental models of oxidative stress [12]. The salen manganese complexes are synthetic and nonproteinaciuos organo-metallic compounds with low molecular weight that exhibit SOD, catalase (CAT), and peroxidase activities [13]. In addition, they show higher stability and better bioavailability compared to proteinaceous antioxidant enzymes [13]. Considering the interesting properties of these catalytic antioxidants, they might be used as potential models for development of useful therapeutic agents for the treatment of the oxidative stress-associated diseases. The protective role of these compounds against oxidative stress has been tested in various in vitro and in vivo disease models, including adult respiratory distress syndrome, autoimmune encephalomyelitis, brain injury, amyotrophic lateral sclerosis, Parkinson’s disease, and prion diseases [14].
Manganese N,N`-bis(salicyldene)ethylenediamine chloride (EUK-8) and manganese-3-methoxy N,N`-bis(salicyldene)ethylenediamine chloride (EUK-134) are salen manganese complex compounds. The structure and catalytic activities of which have been previously described [13]. In the present study we aimed to investigate the possible beneficial activities of antioxidants against diet-induced NASH. We found that EUK-8 and EUK-134 treatments effectively decreased the oxidative stress, improved the sera marker enzyme activities, and also ameliorated the severity of oxidative stress-mediated liver injury. Therefore, antioxidants appear to play important role(s) in prevention of NASH.
MATERIALS AND METHODS
Materials. Bovine serum albumin, reduced nicotinamide adenine dinucleotide, reduced nicotin-amide adenine dinucleotide phosphate (NADPH), ascorbic acid, 5, 5'-dithiobisnitro benzoic acid, nitroblue tetrazolium, hydrogen peroxide, and thiobarbituric acid were obtained from Merck Co. (Darmstadt, Germany). Reduced and oxidized glutathione were obtained from Fluka (Buchs, Switzerland). Glutathione reductase (GR), trichlo-roacetic acid, 2,4-dinitrophenylhydrazine, and Folin-Ciocalteu reagent were obtained from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade.
Animals and diets. Male N-Mary rats, weighing between 200–250 g, were used for the experiment. The animals were housed in cages under ambient temperature (25C) and 60% relative humidity with a 12 h/12 h light/dark cycle. All experiments were carried out according to the guidelines for the care and use of experimental animals approved by the State Veterinary Administration of University of Tehran (Iran). The animals had free access to food and water ad libitum and were allowed to acclimatize to their new conditions for a week before starting the study. NASH was induced by the methionine/choline-deficient (MCD) diet as described by Ustundag et al. [15]. The animals were randomly distributed into five groups of five and were fed orally: the control group was fed a normal diet plus the vehicle (water); the MCD group received a MCD diet plus the vehicle; the MCD + EUK-8 group was fed a MCD diet with EUK-8 (30 mg/kg/day); the MCD + EUK-134 group was fed a MCD diet with EUK-134 (30 mg/kg/day), and MCD + vitamin C group was fed a MCD diet plus vitamin C (30 mg/kg/day). The salen derivates were dissolved in double-distilled water. After 10 weeks, the rats were sacrificed after an overnight fast under diethyl ether anesthesia, and the livers were rapidly removed. Each liver was immediately rinsed with saline, blotted on filter paper, weighed, and kept at -70°C for subsequent biochemical analyses. In addition, blood samples were obtained at the time of sacrifice, and the resulting sera were stored at -70°C until analysis. Manganese salens, EUK-8 and EUK-134, were synthesized as described previously [10] and stored in a desiccator as a dry powder. Fresh solution of each salen was prepared in water at the time of feeding to rats. All rats were weighed in the start of experiments and before sacrifice to calculate body weight variation under the influence of different treatments.
Histopathological examination. Sections from paraffin-embedded tissues were prepared and stained with hematoxylin and eosin for evaluation of necro-inflammatory grading and Masson-trichrome to assess fibrosis. All the specimens were evaluated blindly by an experienced pathologist.
Biochemical analyses. The sera levels of fasting blood glucose (FBS), albumin, total cholesterol, HDL and LDL were quantified using available commercial kits (Pars Azmun, Tehran, Iran). Alkaline phosphatase (ALP), ALT, aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) activities, as a measure of hepatic cell damage, were measured using the relavent commercial kits (Pars Azmun, Tehran, Iran).
Determination of hepatic antioxidant enzymes. The CAT activity was measured by spectophotometric monitoring of hydrogen peroxide decomposition rate using a spectrophotometer (Varian Cary 100 Conc, USA) at 240 nm. The CAT activity was expressed as ×10-1 k/mg protein, where k represents the rate constant of the first order reaction [16]. The SOD activity was measured based on the inhibition of amino blue tetrazolium formazan formation in a reaction mixture composed of nicotinamide adenine dinucleotide, phenazine methosulfate, and nitroblue tetrazolium [17]. One unit of the enzyme activity was defined as the amount of enzyme that caused 50% inhibition of nitroblue tetrazolium reduction/mg protein. The GR activity was determined by monitoring NADPH oxidation at 340 nm. The specific enzyme activity was expressed as nmoles of NADPH oxidized to NADP+/min/mg protein using a molar extinction coefficient of 6.22 × 106 (cm-1 M-1) for NADPH [18]. The glutathione peroxidase (GPx) activity was spectrophotometrically monitored at 340 nm. The GPx activity was expressed as nmoles of NADPH oxidized to NADP+/min/mg protein as described earlier [19].
Measurement of hepatic lipid peroxidation and protein carbonyl content (PCO). Hepatic lipid peroxidation was measured spectrophotometrically using thiobarbituric acid assay as previously described. PCO was estimated using a trichloroacetic acid, 2,4-dinitrophenylhydrazine-based procedure [20].
Statistical analyses. All data were expressed as mean ± standard deviation and the statistical analyses of the data were performed using Student’s t-test and analysis of variance (ANOVA). The p value less than 0.05 was considered to be statistically significant.
RESULTS
Effects on body and liver weights, FBS, and albumin. As shown in Table 1, the body weight gains significantly decreased in MCD-fed rats compared to control, whereas EUK and vitamin C increased body weight gains compared to MCD group but reduced to controls. The liver/body weight ratio was reduced under the effects of EUK-8, EUK-134, and vitamin C in MCD group (by 33%, 42%, and 33%, respectively) relative to drug-untreated MCD-fed rats. In addition, the FBS levels were increased in MCD-fed rats (P<0.01) whereas EUK-8, EUK-134, and vitamin C treatments reduced the FBS levels in MCD groups (by 24%, 19% and 11%, respectively). Treatment of the MCD-fed rats with EUK-8, EUK-134, or vitamin C had no significant effect on the reduced albumin level among MCD-fed rats.
Table 1.
Body weight gain, liver weight, FBS, and albumin in rats
| Characteristics | Control | MCD | MCD + EUK-8 | MCD + EUK-134 | MCD + vitamin C |
|---|---|---|---|---|---|
| Average body weight gain (g) | 28.00 ± 5.00 | 9.00 ± 3.00* | 21.00 ± 5.00 ** | 19.00 ± 4.00 ** | 24.00 ± 7.00 ** |
| Liver/body weight gain (g) | 2.70 ± 0.24 | 4.44 ± 0.31* | 2.98 ± 0.17** | 2.60 ± 0.22 ** | 2.98 ± 0.24 ** |
| FBS (mM) | 6.21 ± 0.12 | 9.09 ± 0.18* | 6.88 ± 0.21 ** | 7.32 ± 0.15 ** | 8.11 ± 0.09 ** |
| Albumin (g/dL) | 3.40 ± 0.25 | 2.10 ± 0.34 * | 3.30 ± 0.18 | 3.20 ± 0.21 | 3.40 ± 0.13 |
MCD, methionine/choline-deficient diet; *P<0.01 MCD vs control; **P<0.05 MCD vs EUK-8, EUK-134, and vitamin c
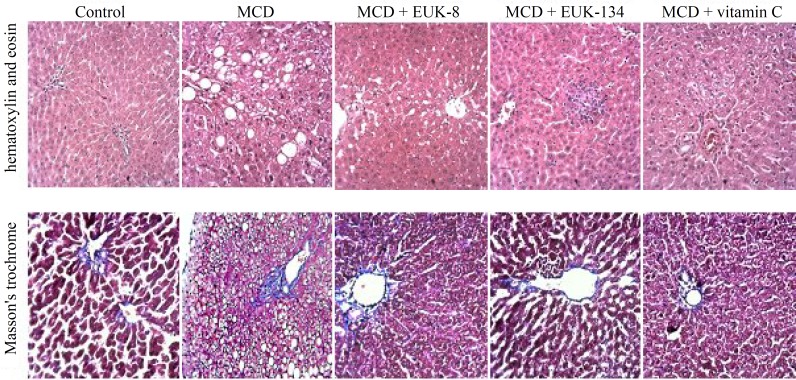
Histopathological analyses of the liver samples. The histological studies of the liver samples from the control and the test groups were performed to investigate the occurrence of steatohepatitis. Treatment of MCD-fed rats with EUK-8, EUK-134, or vitamin C had significant effect on the grade of NASH among MCD-fed rats. As shown in Figure 1, the liver sections from the control animals showed no indication of steatosis, inflammation, or fibrosis. Also, the MCD group indicated liver steatosis (grade 2), lobular inflammation, and ballooning degeneration. Mild ballooning degeneration, focal steatosis (less than 5%), and focal inflammation were observed in EUK-8-treated rats. In the case of MCD + EUK-134 group, the signs of drug-induced hepatocellular injuries, such as ballooning degeneration, single cell apoptosis (apoptotic bodies in lobular areas) as well as lobular inflammation were observed. In addition, this group showed focal microvesicular steatosis. Individual hepatocytes demonstrated mild ballooning degeneration in vitamin C-treated rats. Furthermore, using Masson Trichrome staining of liver sections, no significant fibrosis was identified among the control and the MCD-fed rats.
Fig. 1.
Histopathologic features of liver specimens in each group (hematoxylin and eosin and Masson's trichrome staining, original magnifications ×100). Masson's trichrome did not show significant fibrous expansion in portal spaces among control, MCD, and MCD-treated groups. Normal liver from normal (healthy) untreated control rat. Group MCD-fed diet shows ballooning degeneration, pericentral macrovesicular steatosis, and lobular inflammation. Group MCD-fed diet + EUK-8 shows mild ballooning degeneration, focal steatosis, and focal inflammation. Group MCD-fed diet + EUK-134 shows drug induced hepatocellular injuries such as, ballooning degeneration, single cell apoptosis, and lobular inflammation. Group MCD-fed diet + vitamin C shows mild ballooning degeneration. MCD, methionine/choline deficient diet
Effects of EUK-8 and EUK-134 on hepatic enzymes. The sera ALP, AST, ALT, and GGT activities (U/L) were determined to evaluate the EUK-8 and EUK-134 effects on liver function. In the MCD group, the activities of ALT, AST, ALP and GGT were significantly increased (113.31 ± 9.21, 144.53 ± 16.32, 1864.61 ± 12.71, and 14.43 ± 1.72, respectively) relative to the control (65.86 ± 4.23, 108.54 ± 5.02, 859.44 ± 69.12, and 9.72 ± 1.11, respectively). In MCD-fed group, EUK-8 treatment significantly reduced the activities of the aforementioned enzymes to 73.13 ± 6.41, 116.91 ± 8.62, 1204.32 ± 57.63, and 10.54 ± 0.91, respectively. The relevant values in the MCD + EUK-134 group were 65.64 ± 7.83, 130.72 ± 11.65, 1082.45 ± 94.32, and 7.73 ± 1.45, respectively. In the vitamin C-treated MCD rats, the activities of the above enzymes were reduced to 79.64 ± 9.81, 124.42 ± 9.13, 1151.17 ± 88.76, and 8.66 ± 2.13, respectively (Table 2).
Table 2.
Serum lipid profile and levels of AST, ALT, ALP, and GGT in rats fed normal and/or MCD diet for 10 weeks (n = 5)
| Biochemical parameters | Control | MCD | MCD + EUK-8 | MCD + EUK-134 | MCD + vitamin C |
|---|---|---|---|---|---|
| AST (U/L) | 108.54 ± 5.02 | 144.53 ± 16.32* | 116.91 ± 8.62 ** | 130.72 ± 11.65 ** | 124.42 ± 9.13 ** |
| ALT (U/L) | 65.86 ± 4.23 | 113.31 ± 9.21* | 73.13 ± 6.41** | 65.64 ± 7.83 ** | 79.64 ± 9.81** |
| ALP (U/L) | 859.44 ± 69.12 | 1864.61 ± 12.71* | 1204.32 ± 57.63** | 1082.45 ± 94.32** | 1151.17 ± 88.76 ** |
| GGT (U/L) | 9.72 ± 1.11 | 14.43 ± 1.72* | 10.54 ± 0.91** | 7.73 ± 1.45 ** | 8.66 ± 2.13 ** |
| TC (mmol/L) | 3.31 ± 0.21 | 4.25 ± 0.21* | 4.23 ± 0.11** | 3.64 ± 0.12 ** | 3.74 ± 0.11 ** |
| LDL (mmol/L) | 0.92 ± 0.05 | 1.15 ± 0.06* | 0.72 ± 0.09 ** | 0.89 ± 0.08 ** | 0.75 ± 0.10 ** |
| HDL (mmol/L) | 2.01 ± 0.04 | 1.57 ± 0.09* | 2.31 ± 0.06 ** | 2.15 ± 0.14 ** | 2.17 ± 0.13 ** |
Values are mean ± SD (n = 5) of triplicate measurements of each sample. MCD, methionine/choline-deficient diet; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, Alkaline phosphatase; GGT, gamma-glutamyl transferase; TC, total cholesterol. *P<0.05 compared to control; **P<0.05 compared to MCD-fed group
Effects of EUK-8 and EUK-134 on serum lipid profile. Biochemical analyses of the sera were performed to find out any possible changes in the lipid profile of the control and the test animals. The serum cholesterol and LDL levels were significantly higher in the MCD group (4.52 ± 0.21 and 1.15 ± 0.06 mmol/ L, respectively) compared to the normal healthy rats (3.31 ± 0.21 and 0.92 ± 0.05 mmol/ L, respectively), whereas the HDL levels were significantly lower in the MCD group (1.57 ± 0.09 mmol/ L). The cholesterol and LDL concentrations were reduced (14.0%, 30.0%, 26.0%, and 37.5%, 22%, and 34%, respectively) in the MCD rats treated with EUK-8, EUK-134, and vitamin C relative to MCD group. However, a significant increase was observed in the HDL level in the MCD rats treated with EUK-8, EUK-134, and vitamin C by 47.0%, 36% and 38%, respectively (Table 2) with respect to the MCD-fed rats.
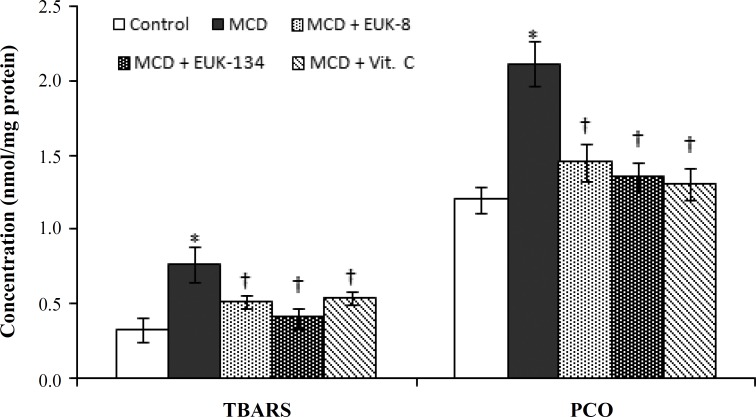
Inhibitory effects of EUK-8 and EUK-134 on lipid peroxidation and protein oxidation. To evaluate the effect of salens on the levels of oxidative stress, we measured the levels of the two widely used markers for lipid peroxidation and protein oxidation in the liver. These markers were the product of thiobarbituric acid reactive substances and PCO, respectively. As shown in Figure 2, the MCD diet increased the extent of thiobarbituric acid reactive substances formation (0.76 ± 0.12 nmol/mg protein) relative to the control (0.33 ± 0.08 nmol/mg protein). However, EUK-8, EUK-134, and vitamin C reduced lipid peroxidation to 0.51 ± 0.04, 0.41 ± 0.07, and 0.54 ± 0.04 nmol/mg protein, respectively in MCD-fed rats. Although the effects of EUK-8 and vitamin C were almost the same, the highest percent of inhibition of lipid peroxidation was observed for EUK-134. The MCD diet also increased the extent of PCO formation (2.11 ± 0.15 nmol/mg protein) relative to the normal diet (1.2 ± 0.09 nmol/mg protein) (P<0.01). However, in the presence of EUK-8, EUK-134, and vitamin C, the extent of PCO reduced to 1.45 ± 0.13, 1.36 ± 0.09, and 1.31 ± 0.11 nmol/mg protein, respectively. Significant difference between the effects of EUK-8, EUK-134, and vitamin C were not recorded (Fig. 2).
Fig. 2.
Effects of EUK-8 or EUK-134 treatments on hepatic lipid peroxidation and protein oxidation. Data represent means ± SD (n = 5) of triplicate measurement of each sample. *P<0.05 compared to control. †P<0.05 compared to MCD-fed group. MCD, methionine/choline deficient diet, TBARS, thiobarbituric acid reactive substances; PCO, protein carbonyl content
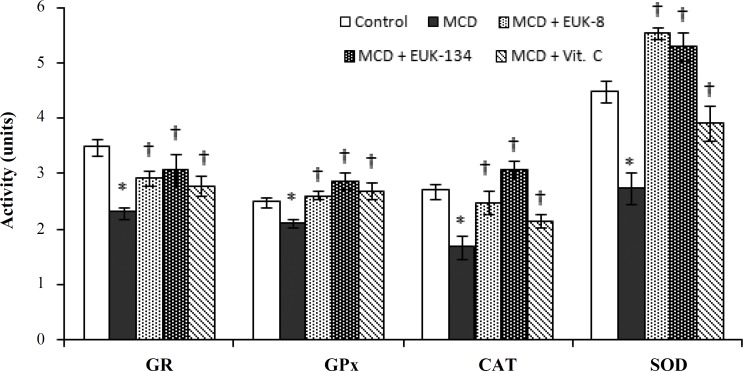
Effects of EUK-8 and EUK-134 on hepatic anti-oxidant enzymes. The activities of the main antioxidant enzymes of the liver (GR, GPx, CAT, and SOD) were evaluated following treatments with the antioxidants. As shown in Figure 3, significant suppression in the hepatic GR, GPx, CAT and SOD activities was recorded among MCD-fed rats (2.31 ± 0.11, 2.12 ± 0.08, 1.69 ± 0.21, and 2.75 ± 0.31 units, respectively) relative to the control (3.50 ± 0.15, 2.51 ± 0.11, 2.7 1 ± 0.13, and 4.51 ± 0.21 units, respectively). GR activity was increased by EUK-8, EUK-134, and vitamin C (2.94 ± 0.14, 3.08 ± 0.28, and 2.82 ± 0.18 units, respectively) in MCD-fed rats. EUK-8, EUK-134, and vitamin C had stimulating effects on SOD and CAT activities in the MCD-fed rats (5.56 ± 0.11, 5.31 ± 0.26, 3.92 ± 0.31, and 2.48 ± 0.22, 3.09 ± 0.15, 2.16 ± 0.13 units, respectively). A significant increase was also observed in the activity of GPx among MCD rats treated by EUK-8, EUK-134, and vitamin C (2.62 ± 0.07, 2.87 ± 0.15, and 2.71 ± 0.16 units, respectively).
Fig. 3.
Effects of EUK-8 or EUK-134 treatments on the activities of hepatic level of glutathione reductase (GR, nmol min-1 mg-1 protein), glutathione peroxidase (GPx, nmol min-1 mg-1 protein), catalase (CAT, ×10-1 k/mg protein), and superoxide dismutase (SOD, U/mg protein). Data represent means ± SD (n = 5) of trpiplicate measurement of each sample. *P<0.05 compared to control. †P<0.05 compared to MCD-fed group. MCD, methionine/choline deficient diet
DISCUSSION
NASH is considered as the extreme form of NAFLD and represents the highest liver-related mortality among the chronic liver diseases [21]. Previous studies have indicated that oxidative stress is a major factor in NAFLD progression into NASH [5, 7]. It has been suggested that overproduction of superoxide anions from mitochondrial and cytoplasmic origin has a crucial role in NASH development [21]. Current strategy to prevent NASH is therefore to use antioxidants and hepatoprotective drugs such as betaine and vitamin E [22].
It has been previously demonstrated that MCD diet causes oxidative stress, lipid peroxidation, and protein oxidation [23]. In the current study, steatohepatitis was induced by a MCD diet and the effects of manganese salens were for the first time assessed for the prevention of MCD-induced NASH. Our results showed increased lipid peroxidation, protein oxidation, and inflammation in MCD-fed rats. In addition, our results indicated that simultaneous treatment with either EUK-8 or EUK-134 along with MCD feeding significantly lowered the extent of hepatic lipid peroxidation and PCO production. Hepatoprotective effects of EUK-8 and EUK-134 might be related to the dismutation of superoxide anions and therefore prevention of the radical initiation reaction through scavenging of oxygen radicals. Our results are in line with Xu`s findings [14]. In addition to the direct antioxidative effects of the salens, their supplementation also elevated the expression and/or activity of GPx and GR, where increased GPx activity would favor the reduction of lipid peroxides into hydroxylipids [24].
A previous study has shown that ROS or lipid peroxides could inhibit SOD and CAT activities [25]. Our data indicated that SOD, CAT, and GR activities were reduced in MCD-fed rats. These findings were in accordance to observation of Nosrati et al. [26]. Our present study indicated that salens enhanced hepatic antioxidant enzymes, confirming the high antioxidative activity of these catalytic antioxidants.
Our results also revealed that EUK-8 and EUK-134 supplementation significantly reduced the serum transferases (ALT and AST) activity levels. This finding is in agreement with Laurent et al. [21] results showing that SOD mimics reduced the serum abnormalities in ob/ob mice. The results also showed that salen treatments effectively decreased the serum GGT and ALP activities, where the GGT and ALP elevation are considered as an index of liver injury. Furthermore, a considerable decrease in steatotic lesions was observed.
It has been demonstrated that NASH is associated with elevated plasma cholesterol and LDL levels. EUK-8, EUK-134, and vitamin C treatments decreased the plasma cholesterol and LDL levels while increased the HDL level. These observations are consistent with Oliveira et al. [27] findings. It has been reported that vitamin C and other antioxidant deficiencies could enhance the potential of LDL oxidation, leading to apolipoprotein B100 modifications [28, 29]. Similar to the ligand for the LDL receptor, apolipoprotein B100 is responsible for clearance of the LDL from plasma by the LDL receptor pathway. Therefore, oxidative modification of apolipoprotein B100 is expected to result in the loss of recognition by LDL receptor [30]. Antioxidants might protect free radical oxidation of LDL. In addition, HDL has an antioxidant property that directly or indirectly protects LDL from oxidation [31, 32]. Accordingly, our results indicated that salens and vitamin C treatments increase HDL-C concentration, and prevent LDL oxidation. This result is consistent with Okamoto [33] findings. It is likely that these antioxidant compounds also protect HDL particles from oxidative modification.
Our histopathological observations showed that manganese salens have protective effects against MCD diet-induced liver damage. Inflammation was reduced in the EUK-8 and vitamin C-treated MCD-fed rats, whereas some cytotoxicity was observed in the EUK-134 group. These findings might be due to the dose and duration of drug exposure and further work is in progress to resolve the case. The phagocytic leukocytes, such as neutrophils, that infiltrate the tissues, are important sources of oxidizing agents [34]. It has been shown that ROS upregulate several different genes involved in the inflammatory responses and proinflammatory mediators. Antioxidants attenuate the production of proinflammatory mediators and prevent activation of phagocytic leukocytes through suppression of oxidative stress [35].
ACKNOWLEDGMENTS
The authors appreciate the financial support of this investigation by the Research Council of University of Tehran.
References
- 1.Wong VW. Nonalcoholic fatty liver disease in Asia: A story of growth. J Gastroenterol Hepatol. 2013 Jan;28(1):18–23. doi: 10.1111/jgh.12011. [DOI] [PubMed] [Google Scholar]
- 2.Begriche K, Knockaert L, Massart J, Robin M, Fromenty B. Mitochondrial dysfunction in nonalcoholic steatohepatitis (NASH): are there drugs able to improve it? Drug Discov Today Dis Mech. 2009;6(1):e11–e23. [Google Scholar]
- 3.Persico M, Masarone M. Indirect markers of non-alcoholic fatty liver disease: Another piece of the puzzle? Dig Liver Dis. 2010 Dec;42(12):846–7. doi: 10.1016/j.dld.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Kirpich IA, Gobejishvili L, Homme M, Waigel S, Cave M, Arteel G, et al. Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J Nutr Biochem. 2011 Jan;22(1):38–45. doi: 10.1016/j.jnutbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mito-chondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006 Feb;6(1):1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: A review and update. Dig Dis Sci. 2010 Mar;55(3):560–78. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 7.Rolo A, Teodoro J, Palmeira C. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012 Jan;52(1):59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010 May;42(5):320–30. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Amini R, Nosrati N, Yazdanparast R, Molaei M. Teucrium polium in prevention of steatohepatitis in rats. Liver Int. 2009 Sep;29(8):1216–21. doi: 10.1111/j.1478-3231.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 10.Le T, Loomba R. Management of non-alcoholic fatty liver disease and steatohepatitis. J Clin Exp Hepatol. 2012;2(2):156–73. doi: 10.1016/S0973-6883(12)60104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bafana A, Dutt S, Kumar A, Kumar S, Ahuja PS. The basic and applied aspects of superoxide dismutase. J Mol Cat B Enzym. 2011;68(2):129–38. [Google Scholar]
- 12.Batinić-Haberle I, Rebouças J, Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010 Sep;13(6):877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998 Jan;284(1):215–21. [PubMed] [Google Scholar]
- 14.Xu ZS, Jung CW, Rong YQ, Doctrow S, Baudry M, Malfroy B. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett. 2001 May;304(3):157–60. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 15.Ustundag B, Bahcecioglu IH, Sahin K, Duzgun S, Koca S, Gulcu F, et al. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steato-hepatitis model. Dig Dis Sci. 2007 Aug;52(8):2006–14. doi: 10.1007/s10620-006-9251-9. [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Nafees S, Sultana S. Perillyl alcohol protects against ethanol induced acute liver injury inWistar rats by inhibiting oxidative stress, NFκ-B activation and proinflammatory cytokine production. Toxicology. 2011 Jan;279(1-3):108–14. doi: 10.1016/j.tox.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Rashid K, Das J, Sil P. Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol. 2013 Jan;51:317–29. doi: 10.1016/j.fct.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Waseem M, Parvez S. Mitochondrial dysfunction medi-ated cisplatin induced toxicity: Modulatory role of curcumin. Food Chem Toxicol. 2013 Mar;53:334–42. doi: 10.1016/j.fct.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 19.Łuczaj W, Stankiewicz-Kranc A, Milewska E, Roszkowska-Jakimiec W, Skrzydlewska E. Effect of sweet grass extract against oxidative stress in rat liver and serum. Food Chem Toxicol. 2012 Feb;50(2):135–40. doi: 10.1016/j.fct.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achilleasantolina extracts. Food Chem. 2007;104(1):21–9. [Google Scholar]
- 21.Laurent A, Nicco C, Tran Van Nhieu J, Borderie D, Chereau C, Conti F, et al. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology. 2004 May;39(5):1277–85. doi: 10.1002/hep.20177. [DOI] [PubMed] [Google Scholar]
- 22.Ariz U, Mato JM, Lu SC, Chantar ML. Nonalcoholic steatohepatitis, animal models and biomarkers: what is new? Methods Mol Biol. 2010;593:109–36. doi: 10.1007/978-1-60327-194-3_6. [DOI] [PubMed] [Google Scholar]
- 23.Pelz S, Stock P, Brückner S, Christ B. A methionine-choline-deficient diet elicits NASH in the immunodeficient mouse featuring a model for hepatic cell transplantation. Exp Cell Res. 2012 Feb;318(3):276–87. doi: 10.1016/j.yexcr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Kuiper HC, Bruno RS, Traber MG, Stevens JF. Vitamin C supplementation lowers urinary levels of 4-hydroperoxy-2-nonenal metabolites in humans. Free Radic Biol Med. 2011 Apr;50(7):848–53. doi: 10.1016/j.freeradbiomed.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazdanparast R, Bahramikia S, Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact. 2008 Apr;172(3):176–84. doi: 10.1016/j.cbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Nosrati N, Aghazadeh S, Yazdanparast R. Effects of Teucriumpoliumon insulin resistance in nonalcoholic steatohepatitis. J Acupunct Meridian Stud. 2010 Jun;3(2):104–10. doi: 10.1016/S2005-2901(10)60019-2. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira CP, Gayotto LC, Tatai C, Della Nina BI, Lima ES, Abdalla DS, et al. Vitamin C and vitamin E in prevention of nonalcoholic fatty liver disease (NAFLD) in choline deficient diet fed rats. Nutr J. 2003 Oct;2:9. doi: 10.1186/1475-2891-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez ML, Vega S, Ayala MT, Shen H, Conde K, Vergara Jimenez M, et al. Vitamin C level and dietary fat saturation alter hepatic cholesterol homeostasis and plasma LDL metabolism in guinea pigs. J Nutr Biochem. 1997;8(7):414–24. [Google Scholar]
- 29.Samsam Shariat SZ, Mostafavi SA, Khakpour F. Antioxidant effects of vitamins C and E on the low-density lipoprotein oxidation mediated by yeloperoxidase. Iran Biomed J. 2013;17(1):22–8. doi: 10.6091/ibj.1092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clin Chim Acta. 2010 Dec;411(23-24):1875–82. doi: 10.1016/j.cca.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 31.Ali W, Ahmad I, Usman K, Kumar S. Anti-atherogenic functions of high density lipoporoteins. IJPMBS. 2013 Jan;2(1):17–26. [Google Scholar]
- 32.Tsompanidi EM, Brinkmeier MS, Fotiadou EH, Giakoumi SM, Kypreos KE. HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis. 2010 Jan;208:3–9. doi: 10.1016/j.atherosclerosis.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto K. Vitamin C intake and apolipoproteins in a healthy elderly Japanese population. Prev Med. 2002 Mar;34(3):364–9. doi: 10.1006/pmed.2001.0993. [DOI] [PubMed] [Google Scholar]
- 34.Segal AW. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol. 2008;40(4):604–18. doi: 10.1016/j.biocel.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sam C, Lu H. The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. J Dent Sci. 2009;4(2):45–54. [Google Scholar]