Abstract
Background: This study was conducted to evaluate fibroblast co-culture and Activin A on in vitro maturation and fertilization of mouse preantral follicles. Methods: The ovaries from 12-14-day-old mice were dissected, and 120-150 μm preantral follicles were cultured individually in α-MEM as based medium for 12 days. A total number of 456 follicles were cultured in four conditions: (i) base medium as control group (n = 113), (ii) base medium supplemented with 30 ng/ml Activin A (n = 115), (iii) base medium co-cultured with mouse embryonic fibroblast (n = 113), and (iv) base medium supplemented with 30 ng/ml Activin A and co-cultured with fibroblast (n = 115). Rate of growth, survivability, antrum formation, ovulation, embryonic development and steroid production were evaluated. Analysis of Variance and Duncan test were applied for analyzing. Results: Both co-culture and co-culture + Activin A groups showed significant difference (P<0.05) in growth (on days 4, 6, and 8 of culture period) and survival rates. However, there was no significant difference in antrum formation, ovulation rate, and embryonic development of ovulated oocytes. There were significant differences (P<0.05) in the estradiol production on days 8, 10, and 12 between co-culture + Activin A and the control group. Progesterone production also was significant (P<0.05) in co-culture + Activin A group on days 6, 8, 10, and 12 compared to control group. Conclusion: Fibroblast co-culture and Activin A promoted growth and survivability of preantral follicles. However, simultaneous use of them was more efficient.
Key Words: Fibroblast, Activin A, Follicle
INTRODUCTION
In vitro growth of follicles and retrieval of immature oocyte have been considered as novel approaches for obtaining mature oocyte and suggested as an additional strategy in preservation of fertility [1, 2]. One of the most important subjects in the techniques of in vitro maturation used in the basic biotechnological research is characterization of optimal conditions [1]. However, establishing a culture system that enables intact preantral follicles to be fully grown is still to be optimized.
It has been suggested that Activins as growth and differentiation factors, which belong to transforming growth factor β superfamily, play key roles in governing oocyte and follicle development [3]. However, it has been reported that Activin A has no effect on in vitro growth of follicles from adult mice [4]. The positive effects of Activin A on in vitro development of follicles have been shown in mice [1, 5], rat [3, 6], sheep [7], cow [8, 9], and human [10]. It has been proved that Activin A promotes follicular growth and differentiation by playing an autocrine/ paracrine role and enhances survival rate via increasing expression of connexin proteins in early follicular development [8, 11]. Cossigny et al. [3] also indicated a stimulatory role for Activin A in the transition of primordial follicles to primary and preantral stages, which is supported by FSH.
Co-culture system is an alternative method used to improve in vitro development of oocyte and embryo [12]. Mouse embryonic fibroblasts as ovarian stroma cells not only support follicles structurally and have reciprocal paracrine signaling with follicles but also are homogenous and easy to produce [13]. They are also commonly used as feeder cells to enhance embryonic stem cell growth [13]. Therefore, the present study was conducted to investigate the synergistic effect of Activin A because of its positive role in mouse embryonic fibroblast co-culture on in vitro development of mouse preantral follicles.
MATERIALS AND METHODS
Chemicals. All the reagents were obtained from Sigma-Aldrich (Germany) unless otherwise specified.
Animals. The NMRI mice were kept in the Central Animal House of Mazandaran University of Medical Sciences (Mazandaran, Iran) under controlled conditions (12 hours light:12 hours dark) and fed water and pellets ad libitum.
Isolation and culture of preantral follicles. A number of 12-14-day old female mice were killed by cervical dislocation and their ovaries were transferred to dissection medium consisted of α-MEM (Gibco, UK) supplemented with 10% FBS (Gibco, UK), 100 μg/ml penicillin, and 50 μg/ml streptomycin under mineral oil. Isolation was carried out by mechanical dissection under a stereomicroscope. Normal preantral follicles (diameter between 120-150 μm and round oocytes) were pooled and randomly divided for further study (Fig. 1A). Culture of preantral follicles was adapted from our previous described method [14]. Briefly, follicles were cultured in α-MEM (Gibco, UK) supplemented with 5% FBS, 100 mIU/ml recombinant follicle stimulating hormone (Gonal-F, Serono, Switzerland), 1% insulin, transferrin, and selenium mix (Gibco, UK), 100 μg/ml penicillin, and 50 μg/ml streptomycin in four culture conditions (control, 30 ng/ml Activin A, co-culture system, and co-culture system supplemented with 30 ng/ml Activin A, a total number of 456 follicles) for 12 days. All sampled media were pooled and stored at -20°C for hormone analysis. Measurement of follicle diameter was assessed using an ocular micrometer at magnification 100× every 48 h during culture period.
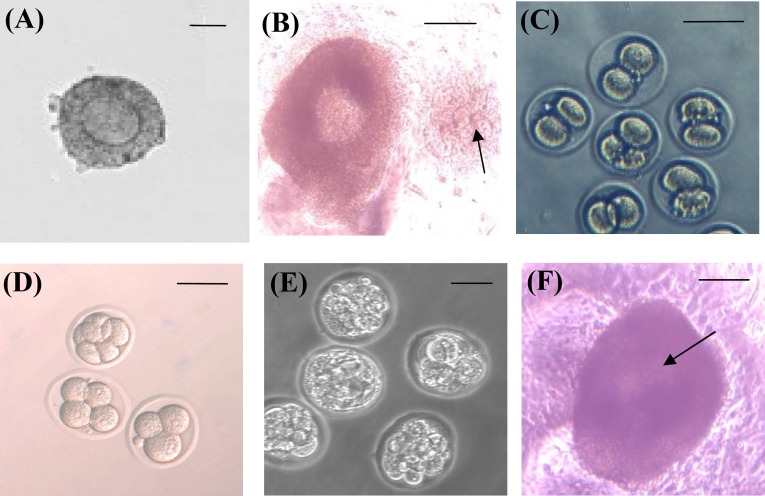
Fig. 1.
I n vitro maturation of preantral follicle from 14-day-old mice. (A) Preantral follicles culture on day 0 (bar: 50 µm), (B) an antral follicle co-cultured with fibroblast cells on day 10 with antrum cavity (arrow, bar: 150 µm), (C) cumulus oocyte complex has released 16-24 hours post human chorionic gonadotropin (arrow, bar: 150 µm), and (D-F) embryonic development after in vitro fertilization of oocyte (bar = 100 µm).
in vitro fertilization and embryo culture. Following ovulation induced by 1.5 IU/ml human chorionic gonadotropin (hCG; Pregnyl, 5,000 IU by Organon) on day 12, released cumulus oocyte complexes were fertilized in vitro (Fig. 1B). The sperm suspension was prepared from cauda epididymis at a concentration of 10 ×106 cell/ml for 90 minutes in 500 µl of human tubal fluid medium supplemented with 4 mg/ml BSA. Fertilization medium was carried out in human tubal fluid medium supplemented with 4 mg/ml BSA in presence of 106/ml spermatozoa. After incubation for 5 h, the oocytes were washed and cultured in T6 medium (containing 4 mg/ml BSA) for 5 days until blastocyst formation (Fig. 1C-E).
Fibroblast feeder layer preparation. Embryonic fibroblast monolayer was prepared according to Hatoya et al. [15] as described previously. Briefly, fetuses from female mice on days 12 and 13 of pregnancy were supplied. Cell suspension was prepared in α-MEM supplemented with 10% FBS, 100 mg/ml streptomycin, and 100 IU/ml penicillin following removing the head, liver, and limb buds and then cut into small pieces. The primary fibroblasts were cultured until confluent and proliferated through two subsequent passages. Inactivation of fibroblast cells was carried out by adding 10 µg/ml mitomycin C (Kyowa, Tokyo, Japan) for 3 h in a humidified atmosphere of 5% CO2 at 37ºC to prevent division of fibroblast cells. Finally, fibroblast layer was plated at a density of 1 × 105 cells/ml one day before use. On the day of culture, fibroblasts had made a feeder layer in the culture droplets.
Assessment of steroid hormones. Radioimmuno-assay kits including IBL (Germany) kit with a sensitivity of 9.7 pg/ml, and a total precision of <10% (% coefficient of variation, CV) and Demeditec (Germany) kit with a sensitivity of 0.04 ng/ml and a total precision of <10% (CV) were used for measuring estradiol and progesterone respectively.
Statistical analysis. Follicular diameter, survival rate, antrum formation, ovulation rate, or cumulus oocyte complex recovery, and embryonic development were analyzed by one-way ANOVA and Duncan’s multiple range tests. P<0.05 was considered to be statistically significant.
RESULTS
There were significant differences in the follicular diameter (as growth rate) in Activin A-treated group on day 6, in co-culture group on days 4 and 6, and in co-culture + Activin A group on days 4, 6, 8, 10, and 12 when compared to control group (Table 1). Although there was no significant difference in the growth rates of follicles between Activin A-treated and co-culture groups, co-culture + Activin A group showed a significant (P<0.05) growth rate on days 8, 10, and 12 compared to Activin A and co-culture groups alone. All experimental groups also showed significant differences in survival rate compared to control group (Table 2). Interestingly, there was no significant difference in survival rate between Activin A group and co-culture group and between co-culture group and co-culture + Activin A group; however, in co-culture + Activin A group, it was significantly more than Activin A group. Antrum-like cavities (Fig. 1F) were recognized from day 8 onward, and antral rates were between 85.7% (control) and 88.8% (Activin A). There was no significant difference in antrum formation rates among the groups (Table 2). The minimum and maximum quantities of ovulation rate were observed in control (80.2%) and co-culture groups (84.3%), respectively with no significant difference. Rate of 2-cell embryo was between 41.1% (control) and 45.2% (co-culture), morula between 20.4% (co-culture + Activin A) and 22.6 % (co-culture), and blastocyst embryos between 11.9% (co-culture) and 14.1% (Activin A) (Table 2). There was no significant difference in embryonic development as well as antral rates and ovulation rate among all groups.
Table 1.
Comparison of the growth rates of follicles (mean diameters ± SD) in different groups
| Days | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|---|
| Groups | |||||||
| Control | 138 ± 7.44 | 173 ± 12.34 | 233 ± 25.6a | 323 ± 40.3a | 394 ± 44.7a | 460 ± 69.04a | 493 ± 41.6a |
| Activin | 138 ± 9.59 | 179 ± 13.87 | 267 ± 27.1ab | 419 ± 43.3b | 416 ± 59.2a | 472 ± 67.61a | 516 ± 87.5a |
| Co-culture | 137 ± 10.15 | 191 ± 15.97 | 276 ± 15.4b | 408 ± 38.1b | 455 ± 51.7a | 480 ± 64.5a | 526 ± 69.3a |
| Co-culture + Activin | 137 ± 7.02 | 186 ± 16.76 | 289 ± 28.5b | 435 ± 38.5b | 495 ± 44.7b | 597 ± 65.4b | 657 ± 84.2b |
According to Duncan's multiple range test, different and similar letters indicate significant (P<0.05) and non-significant differences, respectively. Data are expressed as mean ± standard deviation (SD).
Table 2.
Effect of Activin A and fibroblast co-culture on in vitro maturation and embryonic development of oocytes derived from preantral follicles
| Groups |
No. (%) of follicles
|
% of oocytes developed embryos
|
||||||
|---|---|---|---|---|---|---|---|---|
| Cultured follicles | Survival rates * |
Antral
rates |
Ovulation rates | 2-cell | morula | blastocyst | ||
| Control | 113 | 91 (80.5)a | 78 (85.7) | 73 (80.2) | 30 (41.1) | 15 (21.7) | 9 (12.4) | |
| Activin | 115 | 98 (85.2)b | 87 (88.8) | 85 (83.2) | 38 (44.7) | 19 (22.3) | 12 (14.1) | |
| Co-culture | 113 | 100 (88.5)bc | 87 (87.0) | 84 (84.3) | 38 (45.2) | 19 (22.6) | 10 (11.9) | |
| Co-culture + Activin | 115 | 103 (89.5)c | 91 (88.3) | 88 (82.2) | 37 (42.0) | 18 (20.4) | 12 (13.7) | |
According to Duncan's multiple range test, different and similar letters indicate significant (P<0.05) and non-significant differences, respectively
There was no significant difference in estradiol production between Activin A and control groups. However, concentration of estradiol in co-culture and co-culture + Activin A groups were significantly higher than control group (P<0.05) on days 4, 6, 8, 10, and 12 (Table 3). All experimental groups produced significantly (P<0.05) more progesterone than control group during days of culture (Table 4). Besides, there were also significant differences (P<0.05) among experimental groups only on day 8, in which 13.4 ng/ml was produced as maximum in co-culture group, 11.1 ng/ml in co-culture + Activin A, and 4.7 ng/ml in Activin A group.
Table 3.
Production of estradiol (pg/ml) in pooled media during culture period of preantral follicles
| Days | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|
| Groups | |||||
| Control | 31.6 ± 4.7a | 226 ± 8.4a | 868 ± 34.4a | 1642 ± 53.3a | 3449 ± 121.9a |
| Activin | 38.6 ± 3.4ab | 246 ± 15.3a | 908 ± 26.7 ab | 1681 ± 67.2a | 3378 ± 193.4a |
| Co-culture | 47 ± 5.2b | 243 ± 8.5a | 934 ± 23.3b | 1882 ± 73.9b | 3708 ± 134.7b |
| Co-culture + Activin | 41.7 ± 4.6b | 262.6 ±17.1b | 1133 ± 46.5c | 2078 ± 83.3c | 3873 ± 88.8b |
According to Duncan's multiple range test, different and similar letters indicate significant (P<0.05) and non-significant differences, respectively. Data are expressed as mean ± standard deviation (SD).
Table 4.
Production of progesterone (ng/ml) in pooled media during culture period of preantral follicles
| Days | 6 | 8 | 10 | 12 |
|---|---|---|---|---|
| Groups | ||||
| Control | ---a | ---a | 12.7 ± 0.8a | 16.7 ± 2.2a |
| Activin | ---a | 4.7 ± 0.9b | 16.4 ± 1.7b | 22.6 ± 2.5b |
| Co-culture | 7.2 ± 0.3b | 13.4 ± 0.6d | 15.0 ± 1.1b | 22.0 ± 2.3b |
| Co-culture + Activin | 6.4 ± 0.8b | 11.1 ± 1.5c | 17.4 ±1.4b | 24.2 ± 2.8b |
According to Duncan's multiple range test, different and similar letters indicate significant (P<0.05) and non-significant differences, respectively. Data are expressed as mean ± standard deviation (SD).
DISCUSSION
It may imply a synergistic role for Activin A and fibroblast co-culture in follicular growth rate. In co-culture + Activin A group, significant growth rate was observed from middle to the end of culture period due to a pronounced granulosa cell proliferation leading to large preantral follicles followed by antrum formation. Different studies have shown that Activin A increases follicle diameter in domestic cat [16], cow [8], and mice [17]. Recently, Xia and Schneyer [18] have shown a mode of Activin action, in which Activin only in accompany with FSH can promote G1/S transition and cell proliferation of primary granulosa cells in rats. Interestingly, maximum survival rates were observed in co-culture + Activin A group, which may enhance the synergistic role of Activin A and fibroblast co-culture in follicular development. It has been demonstrated that Activin A promotes follicular survival rate via increasing expression of connexin proteins, such as Cx43, Cx37, and Cx32 in oocyte and granulosa cells [8]. On the other hand, different cytokines such as steel factor, leukemia inhibitory factor, and basic fibroblast growth factor secreted from fibroblast cells in co-culture system [15] may also enhance survival rate directly or via synergistic effect of Activin A.
The results of this study showed no significant difference in antral and ovulation rates. It has long been accepted that presence of FSH receptors despite absence of FSH is enough for activation of follicle growth [8]. These findings suggest that fibroblast co-culture and Activin could not probably increase FSH receptors on the surface of granulosa cells. Rodgers and Irving-Rodgers [18] declared that follicular antrum can be formed by cell death in preantral follicles as the dead granulosa cells are observed in normal healthy follicles as well as cavity formation in normal blastocyst [19]. However, some studies have shown that Activin can promote antrum formation in bovine and mice preantral follicles [8, 10].
No significant difference was observed in embryonic development among all groups, indicating that Activin A has no remarkable effect on embryonic development of oocytes from in vitro matured preantral follicles. These results are consistent with the findings of some researchers in which Activin A had no beneficial effect on the cytoplasmic maturation [20] and early embryonic development of bovine and mice oocytes [21]. Fibroblast co-culture system used in this study also did not affect embryonic development. A recent study has demonstrated that cumulus co-culture can not affect embryonic development of oocytes of in vitro matured follicles [12]. Since oocytes have to reach their competence to undergo both nuclear and cytoplasmic maturation to support further development [22], it could be argued that despite its positive effect on nuclear maturation, co-culture system could not induce cytoplasmic maturation in oocytes, probably due to decreased potential of co-culture system during 12 days of culture.
Results of the present study revealed that Activin A has no significant effect on estradiol production. Controversial roles have been attributed to Activin A in estradiol production: (i) affecting granulosa cell in culture positively [23], (ii) increasing estradiol production only in early stages of follicle culture but not during the late stages [7, 8], and (iii) inhibiting estradiol production irrespective of maturity of antral follicles, which serves as a negative modulator of steroidogenesis [24]. These controversial roles seem to suggest that Activin A function may be changed according to the use of different compounds in culture conditions and their subsequent interaction with Activin A. However, follicles of the co-culture and the co-culture + Activin A groups significantly produced more estradiol, which implies promoting effect of co-culture but not Activin A on estradiol production. This result confirms findings of Wu et al. [25] that porcine follicles co-cultured with cumulus cells increase estradiol production.
Progesterone production remained below the sensitivity range in radioimmunoassay technique up to days 10, 8 and 6 for control, Activin A, and other groups, respectively. This finding has been reflected in Table 4, which shows significant differences between experimental groups and the control group. It was interesting that Activin A and fibroblast co-culture induced progesterone production 2 and 4 days earlier than control group respectively, which may indicate that fibroblast co-culture could enhance progesterone production more than Activin A. Despite a previous observation that progesterone production is not affected by Activin A [26], our result is in agreement with the findings of Mukasa et al. [26] in which Activin A has positive effect on aromatase activity of granulosa cells, thereby affect follicular steroid-genesis.
Finally, the results of the present study demonstrated that although Activin A and fibroblast co-culture did not have a remarkable effect on embryonic development, fibroblast co-culture was more efficient than Activin A in the growth and survival rate of follicles in vitro. Besides, using both of them simultaneously is more effective than alone, indicating a synergistic role for fibroblast co-culture and Activin A. However, a comprehensive evaluation of co-culture system effect on in vitro maturation of preantral follicles needs to be assessed by more detailed molecular and biochemical investigations.
ACKNOWLEDGMENTS
We gratefully thank Dr. Alireza Vafaeipour from Islamic Azad University, Gorgan Branch (Gorgan) for editing the manuscript.
References
- 1.Tavana S, Eimani H, Azarnia M, Shahverdi A, Eftekhari-Yazdi P. Effects of saffron (Crocus sativus L) aqueous extract on in vitro maturation, fertilization and embryo development of mouse oocytes. Cell J. 2012;13(4):259–64. [PMC free article] [PubMed] [Google Scholar]
- 2.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011 Apr;84:689–97. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cossigny DA, Findlay JK, Drummond AE. The effects of FSH and Activin A on follicle development in vitro. Reproduction. 2012 Feb;143(2):221–9. doi: 10.1530/REP-11-0105. [DOI] [PubMed] [Google Scholar]
- 4.Yokota H, Yamada K, Liu X, Kobayashi J, Abe Y, Mizunuma H, et al. Paradoxical action of activin A on folliculogenesis in immature and adult mice. Endocrinology. 1997 Nov;138(11):4572–6. doi: 10.1210/endo.138.11.5526. [DOI] [PubMed] [Google Scholar]
- 5.Javed A, Jamil A, Rezaei-Zarchi S, Kalantar SM, Anvari M, Nazem H. An in vitro comparative study of follicle stimulating hormone (FSH) and activin A effects on the maturation of preantral follicle-enclosed oocytes from immature Syrian mice. Iran Biomed J. 2008 Apr;12(2):85–92. [PubMed] [Google Scholar]
- 6.Zhao J, Taverne MA, van der Weijden GC, Bevers MM, van den Hurk R. Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biol Reprod. 2001 Sep;65(3):967–77. doi: 10.1095/biolreprod65.3.967. [DOI] [PubMed] [Google Scholar]
- 7.Thomas FH, Armstrong DG, Telfer EE. Activin promotes oocyte development in ovine preantral follicles in vitro. Reprod Biol Endocrinol. 2003;1:76. doi: 10.1186/1477-7827-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin M, Bromfield JJ, Albertini DF, Telfer EE. Activin promotes follicular integrity and oogenesis in cultured pre-antral bovine follicles. Mol Hum Reprod. 2010 Sep;16(9):644–53. doi: 10.1093/molehr/gaq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction. 2010 Jun;139(6):971–8. doi: 10.1530/REP-10-0025. [DOI] [PubMed] [Google Scholar]
- 10.Telfer EE, Mclaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008 May;23(5):1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 11.Mather JP, Moore A, Li RH. Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Proc Soc Exp Biol Med. 1997 Jul;215(3):209–22. doi: 10.3181/00379727-215-44130. [DOI] [PubMed] [Google Scholar]
- 12.Haidari K, Salehnia M, Rezazadeh Valojerdi M. The effect of leukemia inhibitory factor and co-culture on the in vitro maturation and ultrastructure of vitrified and nonvitrified isolated mouse preantral follicles. Fertil Steril. 2008 Dec;90(6):2389–97. doi: 10.1016/j.fertnstert.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, et al. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A. 2012 Jun;18(11-12):1229–38. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidari M, Karimpour Malekshah A, Parivar K, Khanbabaei R, Rafiei A. Effect of fibroblast co-culture on in vitro maturation and fertilization of mouse preantral follicles. Int J Fertil Steril. 2011;5(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Wongbandue G, Jewgenow K, Chatdarong K. Effects of thyroxin (T4) and activin A on in vitro growth of preantral follicles in domestic cats. Theriogenology. 2013 Mar;79(5):824–32. doi: 10.1016/j.theriogenology.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, Mizunuma H. A comparative study on transforming growth factor-beta and activin A for preantral follicles from adult, immature, and diethylstilbestrol-primed immature mice. Endocrinology. 1999 Jun;140(6):2480–5. doi: 10.1210/endo.140.6.6827. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Schneyer AL. The biology of activin: recent advances in structure, regulation and function. J Endocrinol. 2009 Jul;202(1):1–12. doi: 10.1677/JOE-08-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010 Jun;82(6):1021–9. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 19.Van Tol HT, de Loos FA, Vanderstichele HM, Bevers MM. Bovine actvin A does not affect the in vitro maturation of bovine oocytes. Theriogenology. 1994 Feb;41(3):673–9. doi: 10.1016/0093-691x(94)90176-j. [DOI] [PubMed] [Google Scholar]
- 20.Izadyar F, Zeinstra E, Colenbrander B, Vanderstichele HMJ, Bevers MM. In vitro maturation of bovine oocytes in the presence of bovine Activin A does not affect the number of embryos. Anim Reprod Sci. 1996 Dec;2;45(1-2):37–45. doi: 10.1016/s0378-4320(96)01574-6. [DOI] [PubMed] [Google Scholar]
- 21.Ryle M. The growth in vitro of mouse ovarian follicles of different sizes in response to purified gonadotrophins. J Reprod Fertil. 1972 Sep;30(3):395–405. doi: 10.1530/jrf.0.0300395. [DOI] [PubMed] [Google Scholar]
- 22.Shidaifat F, Khamas W, Hailat N. Activin-A differentially regulates steroidogenesis by sheep granulosa cells. Res Vet Sci. 2001 Aug;71(1):23–5. doi: 10.1053/rvsc.2001.0479. [DOI] [PubMed] [Google Scholar]
- 23.Ford JJ, Howard HJ. Activin inhibition of estradiol and progesterone production in porcine granulosa cells. J Anim Sci. 1997 Mar;75(3):761–6. doi: 10.2527/1997.753761x. [DOI] [PubMed] [Google Scholar]
- 24.Wu MF, Huang WT, Tsay C, Hsu HF, Liu BT, Chiou CM, et al. The stage-dependent inhibitory effect of porcine follicular cells on the development of preantral follicles. Anim Reprod Sci. 2002 Sep;73(1-2):73–88. doi: 10.1016/s0378-4320(02)00119-7. [DOI] [PubMed] [Google Scholar]
- 25.Smitz J, Cortvrindt R, Hu Y, Vanderstichele H. Effects of recombinant activin A on in vitro culture of mouse preantral follicles. Mol Reprod Dev. 1998 Jul;50(3):294–304. doi: 10.1002/(SICI)1098-2795(199807)50:3<294::AID-MRD5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Mukasa C, Nomura M, Tanaka T, Tanaka K, Nishi Y, Okabe T, et al. Activin signaling through type IB activin receptor stimulates aromatase activity in the ovarian granulosa cell-like human granulosa (KGN) cells. Endocrinology. 2003 Apr;144(4):1603–11. doi: 10.1210/en.2002-220978. [DOI] [PubMed] [Google Scholar]