Abstract
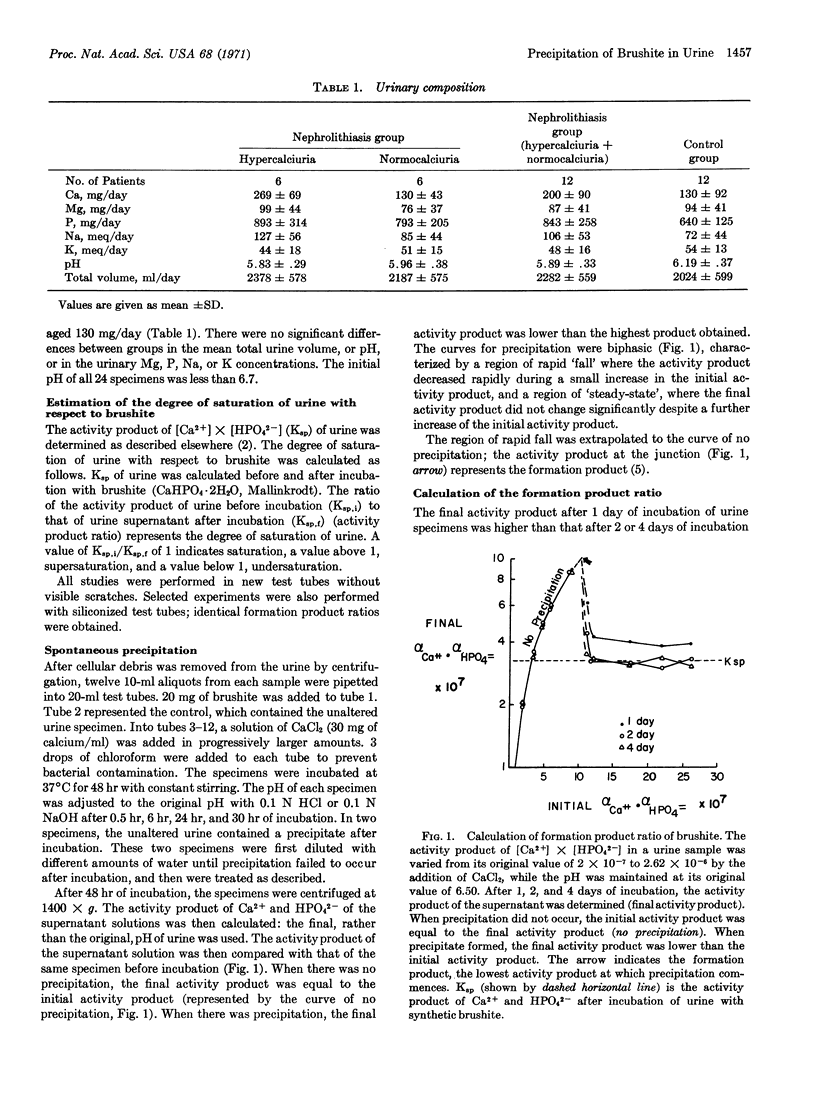
Further evidence that brushite plays a regulatory role in renal stone formation was provided by the identification of brushite as the first precipitate that appears in supersaturated urine by spontaneous precipitation. Calcium chloride was added to induce supersaturation in urine specimens from twelve subjects with and twelve subjects without nephrolithiasis.
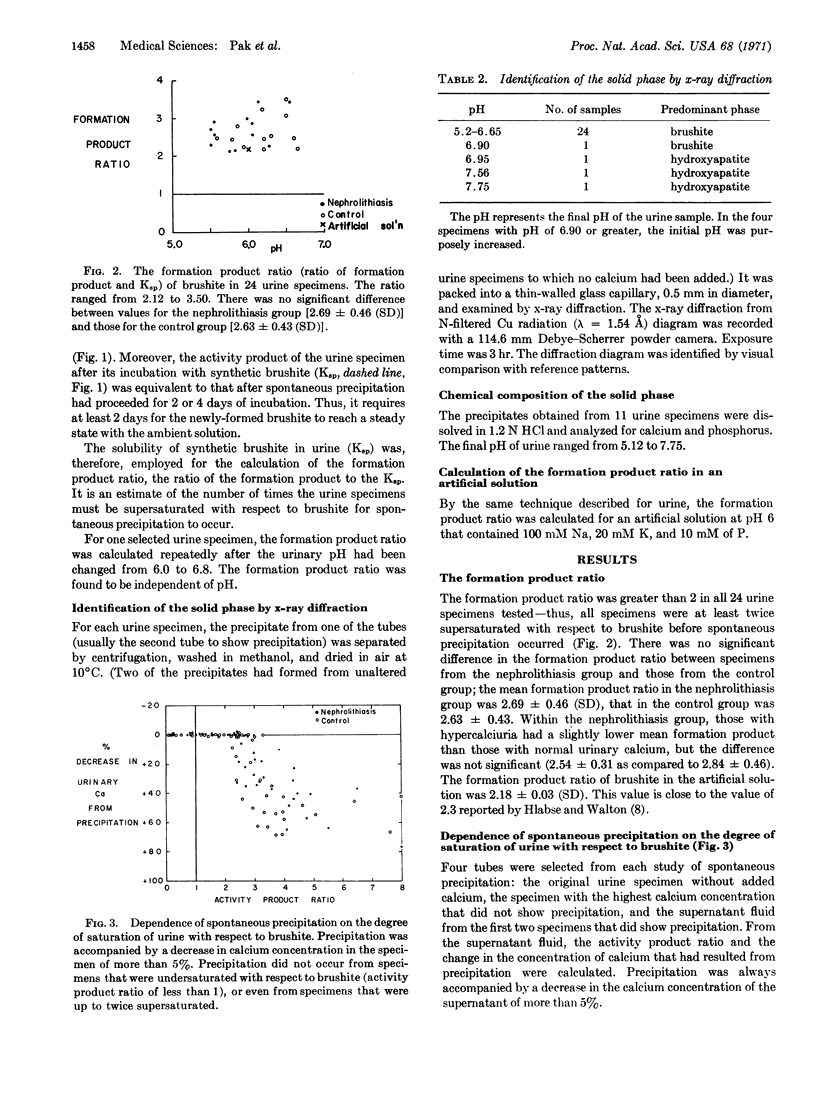
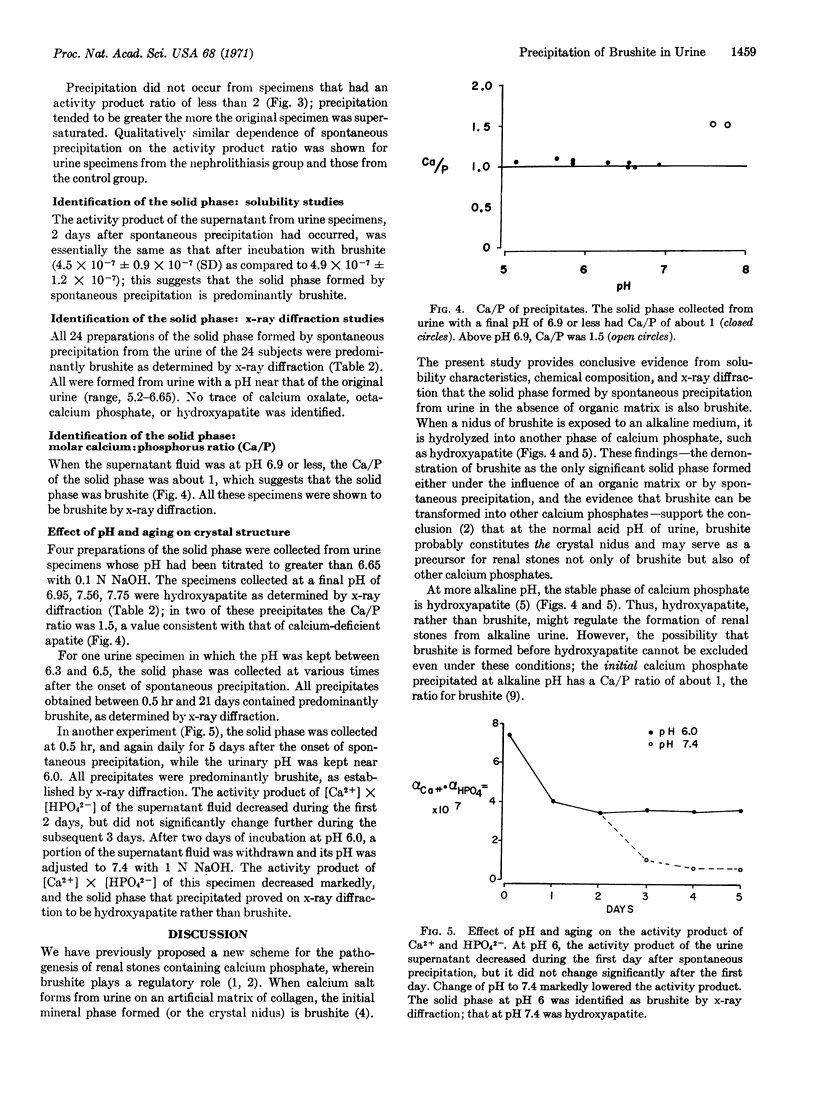
The first precipitate in all specimens with pH below 6.9 was identified as brushite by x-ray diffraction and shown to have a calcium-phosphorus ratio of approximately 1.0.
The activity product of [Ca2+] × [HPO42-] necessary to produce a precipitate ranged from 2.2 to 3.5 times the solubility product of brushite, but the range and mean were the same for both groups of subjects.
The activity product of [Ca2+] × [HPO42-] in the supernatant (after spontaneous precipitation) was not significantly different from that obtained after incubation of the same urine specimen with synthetic brushite.
These results provide conclusive evidence that brushite constitutes the solid phase formed by spontaneous precipitation from acidic urine supersaturated with respect to calcium and phosphorus; they suggest that the nidus for calcium-containing renal stones is brushite as well.
Keywords: nephrolithiasis, acid urine, x-ray diffraction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE W. H., KING J. S., Jr Present concepts concerning the origin of matrix and stones. Ann N Y Acad Sci. 1963 Mar 5;104:563–578. doi: 10.1111/j.1749-6632.1963.tb17693.x. [DOI] [PubMed] [Google Scholar]
- Boyce W. H. Organic matrix of human urinary concretions. Am J Med. 1968 Nov;45(5):673–683. doi: 10.1016/0002-9343(68)90203-9. [DOI] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol. 1962 Oct;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- HENNEMAN P. H., BENEDICT P. H., FORBES A. P., DUDLEY H. R. Idiopathic hypercaicuria. N Engl J Med. 1958 Oct 23;259(17):802–807. doi: 10.1056/NEJM195810232591702. [DOI] [PubMed] [Google Scholar]
- Howard J. E., Thomas W. C., Jr, Barker L. M., Smith L. H., Wadkins C. L. The recognition and isolation from urine and serum of a peptide inhibitor to calcification. Johns Hopkins Med J. 1967 Mar;120(3):119–136. [PubMed] [Google Scholar]
- NEMAN W. F., TORIBARA T. Y., MULRYAN B. J. Synthetic hydroxyapatite crystals. 1. Sodium and potassium fixation. Arch Biochem Biophys. 1962 Sep;98:384–390. doi: 10.1016/0003-9861(62)90202-3. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Cox J. W., Powell E., Bartter F. C. Effect of the oral administration of ammonium chloride, sodium phosphate, cellulose phosphate and parathyroid extract on the activity product of brushite in urine. Am J Med. 1971 Jan;50(1):67–76. doi: 10.1016/0002-9343(71)90206-3. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Diller E. C., Smith G. W., 2nd, Howe E. S. Renal stones of calcium phosphate: physicochemical basis for their formation. Proc Soc Exp Biol Med. 1969 Mar;130(3):753–757. doi: 10.3181/00379727-130-33648. [DOI] [PubMed] [Google Scholar]
- Pak C. Y. Physicochemical basis for formation of renal stones of calcium phosphate origin: calculation of the degree of saturation of urine with respect to brushite. J Clin Invest. 1969 Oct;48(10):1914–1922. doi: 10.1172/JCI106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C. Y., Ruskin B. Calcification of collagen by urine in vitro: dependence on the degree of saturation of urine with respect to brushite. J Clin Invest. 1970 Dec;49(12):2353–2361. doi: 10.1172/JCI106454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson W. G., Hambleton J., Hodgkinson A. Peptide inhibitors of calcium phosphate precipitation in the urine of normal and stone-forming men. Clin Chim Acta. 1969 Aug;25(2):247–253. doi: 10.1016/0009-8981(69)90262-9. [DOI] [PubMed] [Google Scholar]
- Robertson W. G., Peacock M., Nordin B. E. Activity products in stone-forming and non-stone-forming urine. Clin Sci. 1968 Jun;34(3):579–594. [PubMed] [Google Scholar]
- VERMEULEN C. W., LYON E. S., FRIED F. A. ON THE NATURE OF THE STONE-FORMING PROCESS. J Urol. 1965 Aug;94:176–186. doi: 10.1016/S0022-5347(17)63596-1. [DOI] [PubMed] [Google Scholar]
- VERMEULEN C. W., LYON E. S., GILL W. B. ARTIFICIAL URINARY CONCRETIONS. Invest Urol. 1964 Jan;1:370–386. [PubMed] [Google Scholar]


