Abstract
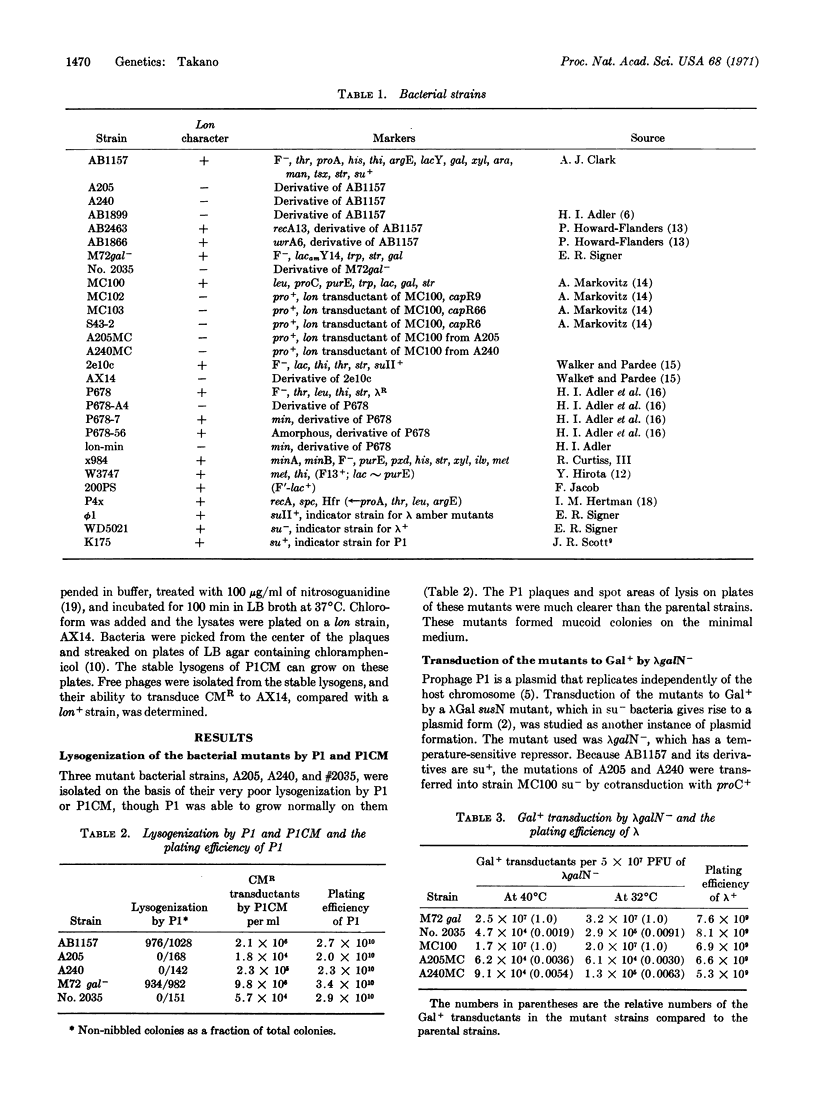
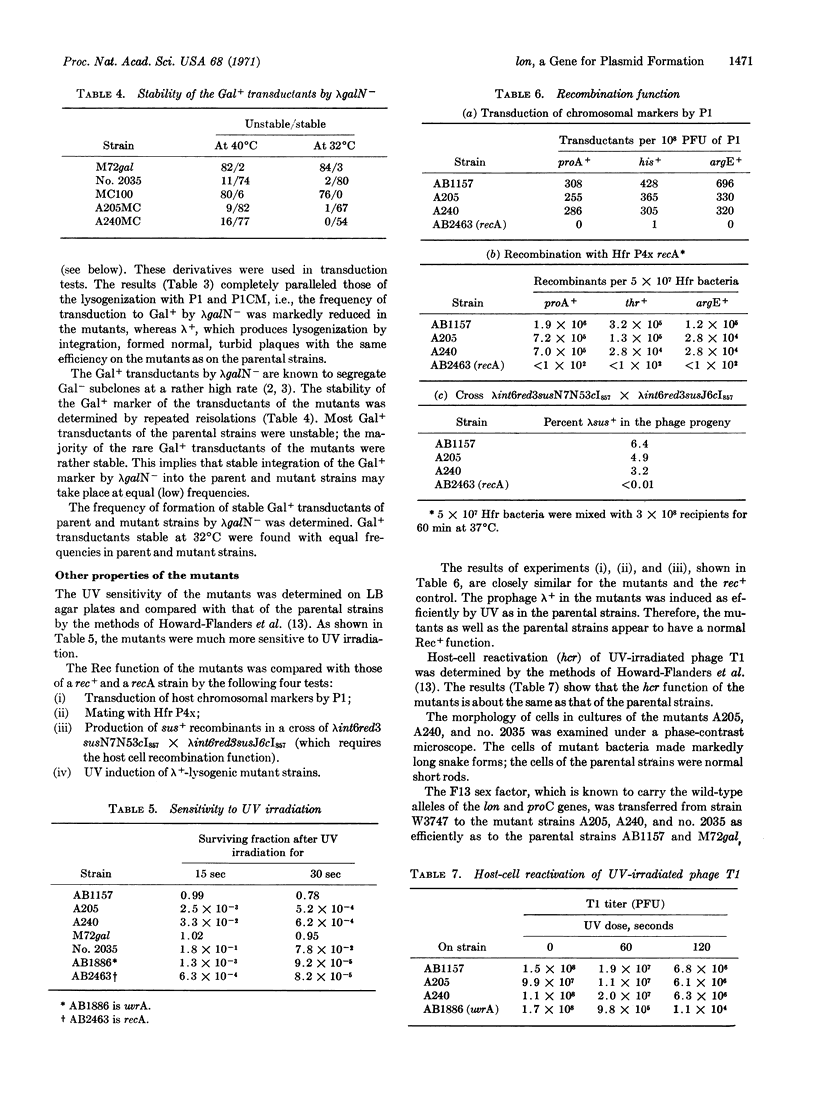
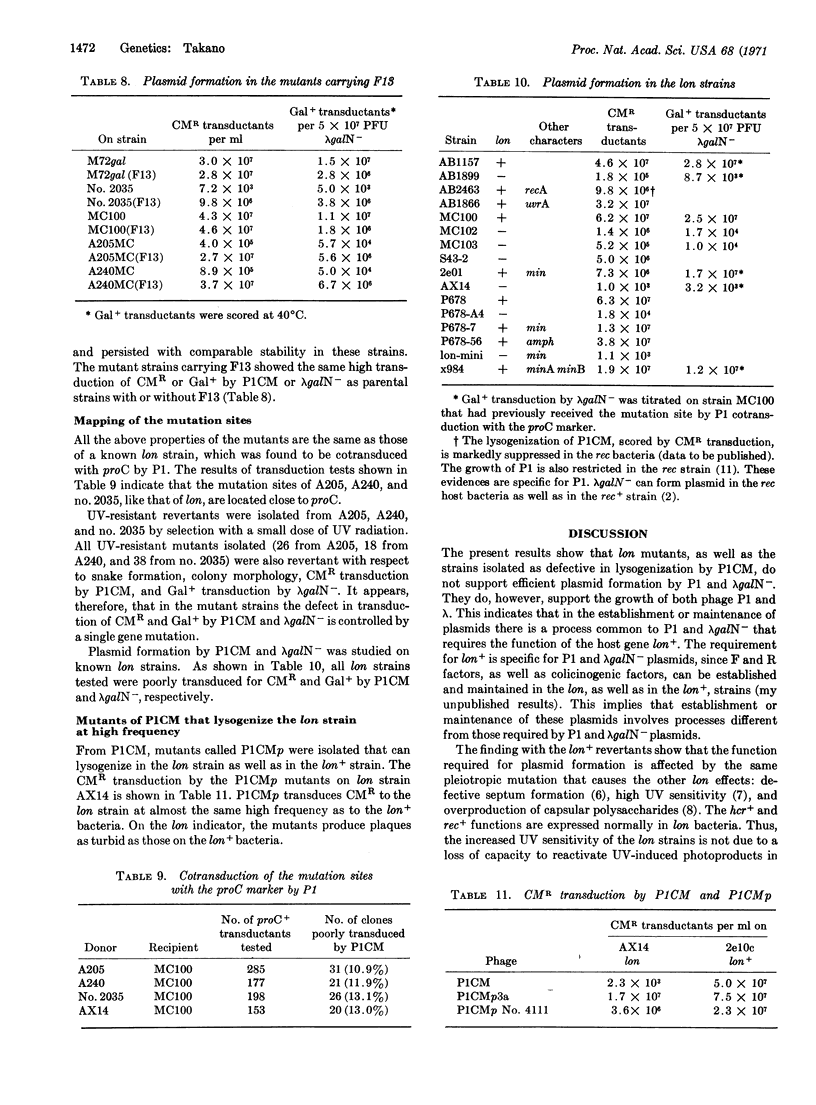
Bacterial mutants defective in plasmid formation were selected for their inability to be transduced to chloramphenicol resistance by bacteriophage PlCM. The mutants isolated are indistinguishable from a lon mutant strain. Both the lon strain and the mutants isolated here show very poor lysogenization by P1 and very low transduction to Gal+ by the plasmid formation of the λgal8-Nam7am53cI857. The lon+ gene function of the host bacteria is indispensable for plasmid formation, even though P1 and λ+ can grow normally on the lon strains and λ+ can be integrated normally in lon as well as in the lon+ bacteria. Phage mutants that can persist as plasmids in lon strains were isolated from P1CM. The function of the lon+ gene is discussed with regard to plasmid formation.
Keywords: transduction, phage P1, E. coli, lysogeny, UV reduce sensitivity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Terry C. E., Hardigree A. A. Giant cells of Escherichia coli. J Bacteriol. 1968 Jan;95(1):139–142. doi: 10.1128/jb.95.1.139-142.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler B., Echols H. Regulation of bacteriophage lambda development by gene N: properties of a mutation that bypasses N control of late protein synthesis. Virology. 1970 Feb;40(2):212–222. doi: 10.1016/0042-6822(70)90396-x. [DOI] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertman I. M. Isolation and characterization of a recombination-deficient Hfr strain. J Bacteriol. 1967 Feb;93(2):580–583. doi: 10.1128/jb.93.2.580-583.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hopkins N. Bypassing a positive regulator: isolation of a lambda mutant that does not require N product to grow. Virology. 1970 Feb;40(2):223–229. doi: 10.1016/0042-6822(70)90397-1. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Kondo E., Mitsuhashi S. Drug resistance of enteric bacteria. VI. Introduction of bacteriophage P1CM into Salmonella typhi and formation of PldCM and F-CM elements. J Bacteriol. 1966 May;91(5):1787–1794. doi: 10.1128/jb.91.5.1787-1794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. Cell genetics and hereditary symbiosis. Physiol Rev. 1952 Oct;32(4):403–430. doi: 10.1152/physrev.1952.32.4.403. [DOI] [PubMed] [Google Scholar]
- Lieb M. Lambda mutants which persist as plasmids. J Virol. 1970 Aug;6(2):218–225. doi: 10.1128/jvi.6.2.218-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A. REGULATORY MECHANISMS FOR SYNTHESIS OF CAPSULAR POLYSACCHARIDE IN MUCOID MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1964 Feb;51:239–246. doi: 10.1073/pnas.51.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Baker B. Suppression of radiation sensitivity and capsular polysaccharide synthesis in Escherichia coli K-12 by ochre suppressors. J Bacteriol. 1967 Aug;94(2):388–395. doi: 10.1128/jb.94.2.388-395.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Gardinier J. Replication of bacteriophage lambda DNA associated with the host cell membrane. Virology. 1970 May;41(1):38–51. doi: 10.1016/0042-6822(70)90052-8. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Sinsheimer R. L. Intracellular location and number of replicating parental DNA molecules of bacteriophages lambda and phi-X174. J Mol Biol. 1969 Apr 14;41(1):39–65. doi: 10.1016/0022-2836(69)90124-7. [DOI] [PubMed] [Google Scholar]
- Scott J. R. Genetic studies on bacteriophage P1. Virology. 1968 Dec;36(4):564–574. doi: 10.1016/0042-6822(68)90188-8. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Plasmid formation: a new mode of lysogeny by phase lambda. Nature. 1969 Jul 12;223(5202):158–160. doi: 10.1038/223158a0. [DOI] [PubMed] [Google Scholar]
- Uretz R. B., Markovitz A. Dominance of ultraviolet radiation resistance in partial diploids of Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1118–1120. doi: 10.1128/jb.100.2.1118-1120.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


